AMMI Analysis of the Effects of Different Insecticidal Treatments against Agrotis spp. on the Technological Yield from Sugar Beet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Site
2.2. Plant Material
2.3. Trial Design
- −
- Pest alert (S)—the first adult insects (moths) captured in traps/signs of caterpillar feeding,
- −
- Phenology (F)—calculation of the sum of effective temperatures (F1) and heat sums (F2),
- −
- Control plots, K (no pest control), K2 (plots were sprayed with water) [12].
2.4. Data Collection
2.5. Statistical Analysis
3. Results
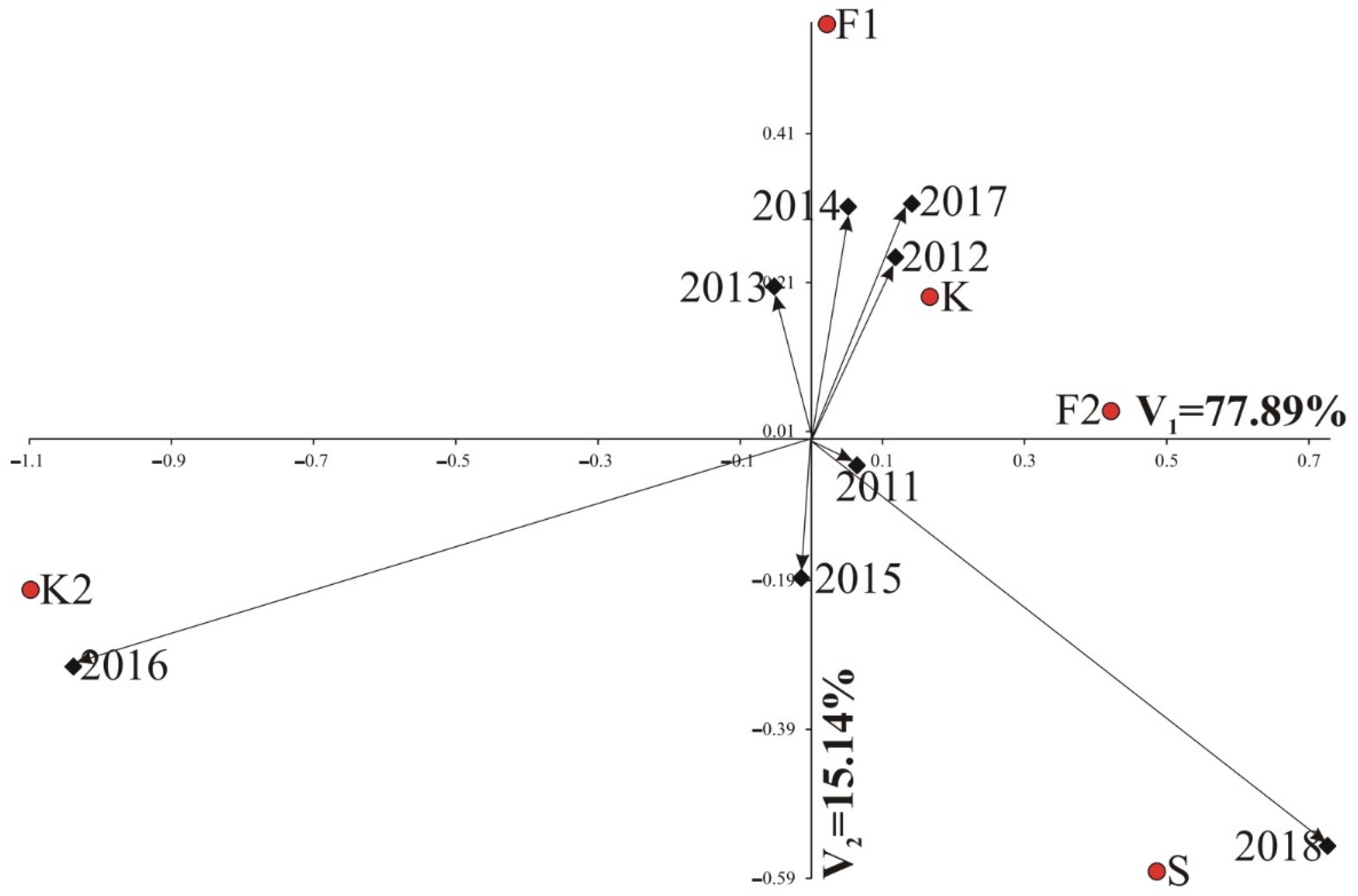
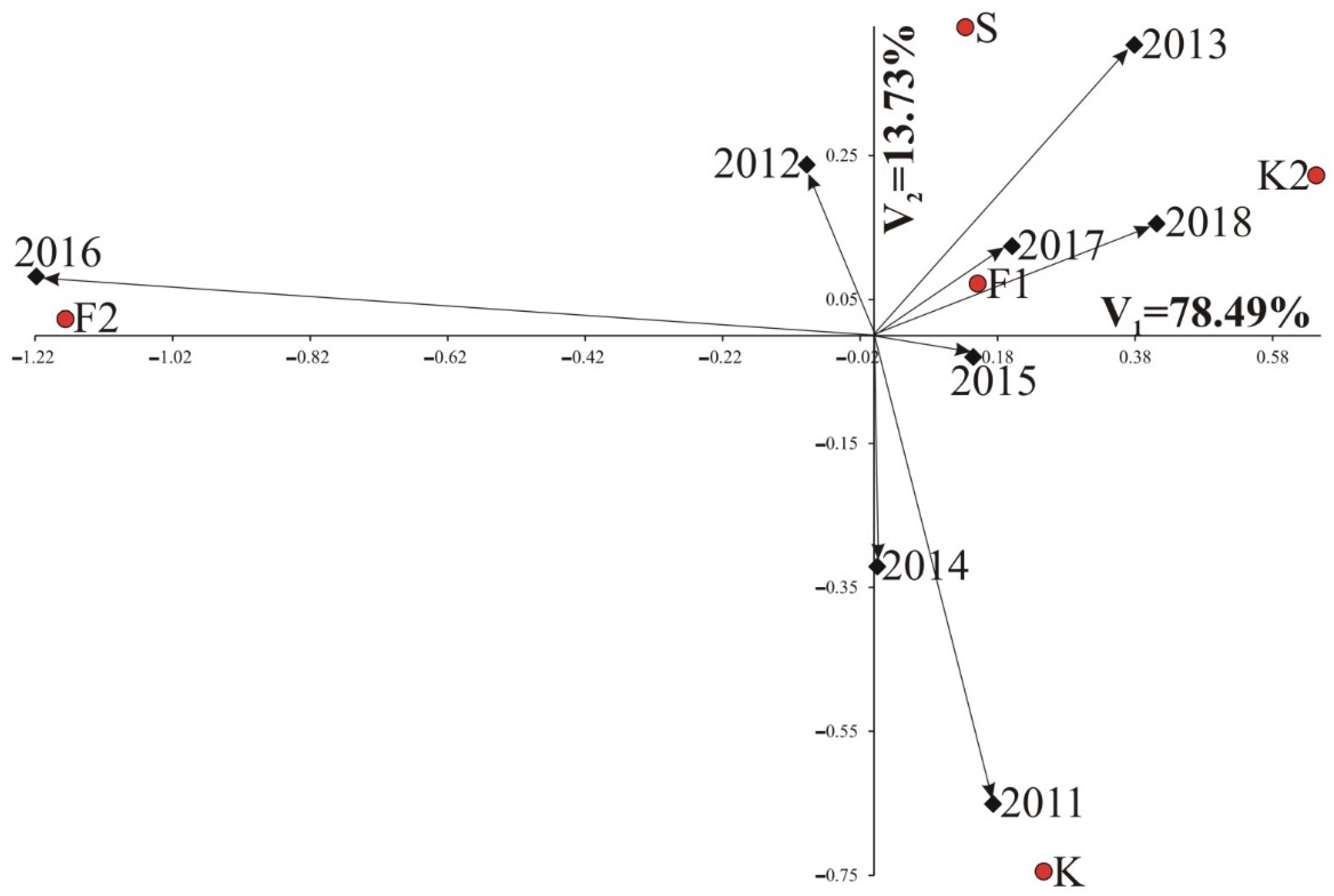
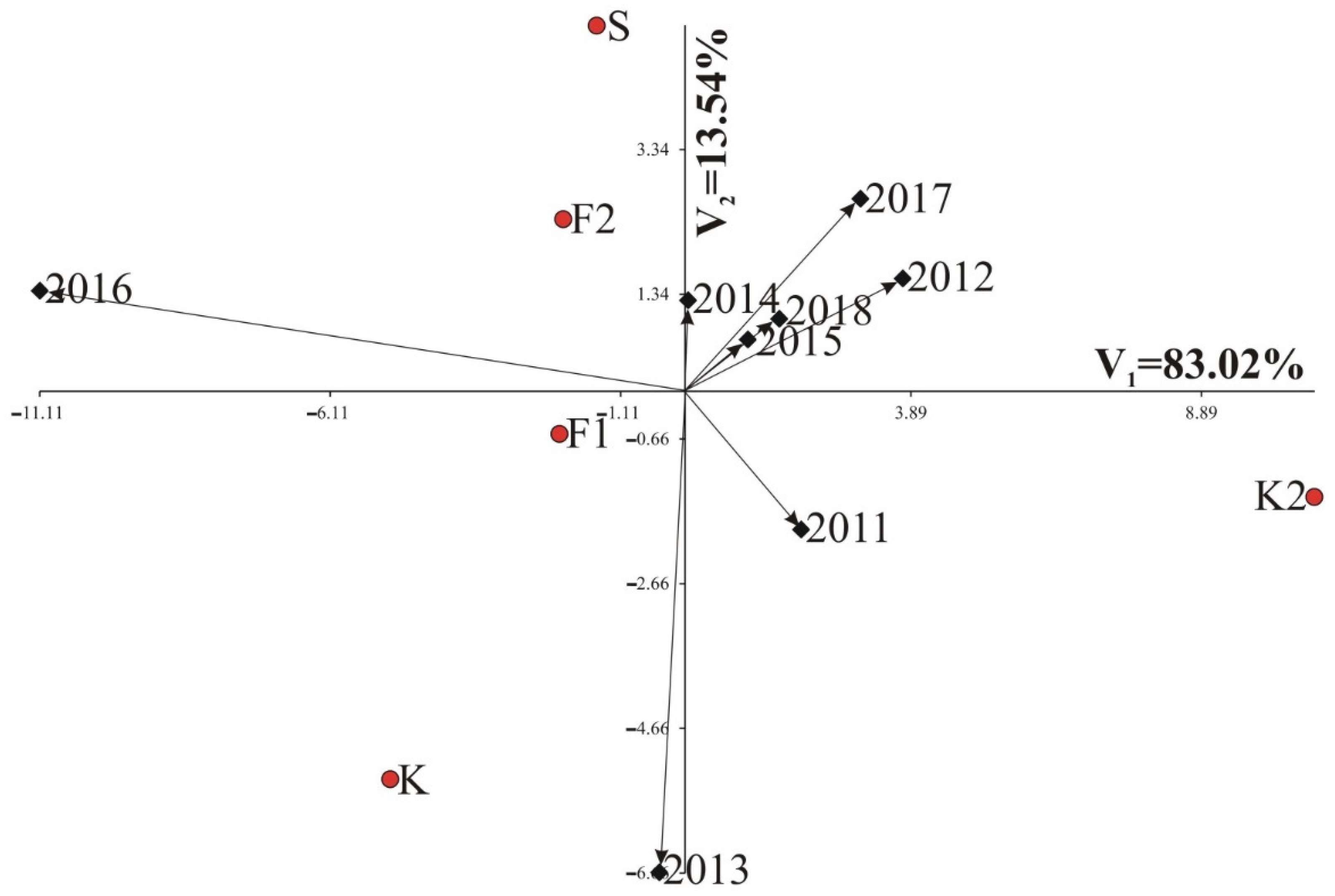
3.1. Results of AMMI Analysis
3.1.1. Root Weight
3.1.2. Polarization
3.1.3. Potassium (K) Molasses
3.1.4. Sodium (Na) Molasses
3.1.5. α-Amino-Nitrogen Molasses (N-Amino)
3.1.6. Technological Yield—White Sugar Yield
4. Discussion
5. Conclusions
- The AMMI model can be a useful tool for detecting these interactions (TYI) and improving estimation accuracy. In the AMMI model, the obtained qualitative and quantitative parameters of yield can be grouped based on the similarity of the analysed trait and the identification of potential trends observed in the study years.
- The results of the analysis of variance of our study indicated that significant treatment × year interaction for all considered physiological traits in the experiment were occurred.
- Findings from this study indicate that environmental conditions, e.g., soil fertility, crop variety, and abiotic factors (such as temperature and rainfall), are very important parameters with a wide range of variability between the applied treatment variants, years, and their interactions. These significant interactions (TYI) suggest that it is possible to select stable variants of treatments over time.
- AMMI analysis used to estimate the interaction of treatments based on environmental conditions showed the additive effect of the applied treatments on the quality parameters of white sugar yield from sugar beet. These effects were demonstrated for polarization and the content of Na in molassigenic substances.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Artyszak, A.; Podlaska, J.; Mądry, W. Analiza współczynników ścieżek technologicznego plonu cukru buraka cukrowego i cech łanu ujawniający się w trakcie ontogenezy. Rocz. Nauk. Rol. Ser. A 1999, 114, 41–54. [Google Scholar]
- Hoffmann, C.M.; Huijbregts, T.; Van Swaaij, N.; Jansen, R. Impact of different environments in Europe on yield and quality of sugar beet genotypes. Eur. J. Agron. 2009, 30, 17–26. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Kenter, C. Yield potential of sugar beet–Have we hit the ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Řezbová, H.; Belová, A.; Škubna, O. Sugar beet production in the European Union and their future trends. Agris -Line Pap. Econ. Inform. 2013, 5, 165–178. [Google Scholar]
- Jakubowska, M.; Cyplik, A.; Bocianowski, J.; Wielkopolan, B. Wpływ wybranych cech chemicznych na wartość technologiczną plonu buraka cukrowego po zastosowaniu zabiegów na szkodniki glebowe [Effect of selected chemical features on the technological value of sugar beet yield after application of treatments on soil pests]. Prog. Plant Prot. 2020, 60, 275–282. [Google Scholar] [CrossRef]
- Scott, R.K.; English, S.D.; Wood, D.W.; Unsworth, M.H. The yield of sugar beet in relation to weather and length of growing season. J. Agric. Sci. 1973, 81, 339–347. [Google Scholar] [CrossRef]
- Klotz, K.L.; Finger, F.L. Impact of temperature, length of storage and postharvest disease on sucrose catabolism in sugar beet. Postharvest Biol. Technol. 2004, 34, 1–9. [Google Scholar] [CrossRef]
- Worral, D.; Holroyd, G.H.; Moore, J.P.; Głowacz, M.; Croft, P.; Taylor, J.E.; Paul, N.D.; Roberts, M.R. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol. 2012, 193, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Cipollini, D.; Purrington, C.B.; Bergelson, J. Costs of induced responses in plants. Basic Appl. Ecol. 2003, 4, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Heil, M.; Hilpert, A.; Kaiser, W.; Linsenmair, K.E. Reduced growth and seed set following chemical induction of pathogen defence: Does systemic acquired resistance (SAR) incur allocation costs? J. Ecol. 2000, 88, 645–654. [Google Scholar] [CrossRef]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Jakubowska, M.; Bocianowski, J.; Nowosad, K.; Kowalska, J. Decision support system to improve the effectiveness of chemical control against cutworms in sugar beet. Sugar Tech. 2020, 22, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Abedi, Z.; Saber, M.; Vojoudi, S.; Mahdavi, V.; Parsaeyan, E. Acute, sublethal, and combination effects of azadirachtin and Bacillus thuringiensis on the cotton bollworm, Helicoverpa armigera. J. Insect Sci. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazazo, K.G.I.; Mashaal, R.E.F. Pests attacking post-harvest sugar beet roots, and their adverse effects on sugar content. J. Plant Prot. Pathol. 2014, 5, 673–678. [Google Scholar] [CrossRef]
- Bazok, R.; Drmic, Z.; Cacija, M.; Mrganic, M.; Viric Gasparic, H.; Lemic, D.A. Moths of Economic Importance in the Maize and Sugar Beet Production. Intech Publ. 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Perveen, F.K. (Ed.) Moths—Pests of potato, maize and sugar beet. Intech Publ. 2018, 1, 18. [Google Scholar] [CrossRef]
- Betz, A.; Andrew, N.R. Influence of non-lethal doses of natural insecticides spinetoram and azadirachtin on Helicoverpa punctigera (native budworm, Lepidoptera: Noctuidae) under laboratory conditions. Front. Physiol. 2020, 11, 1089. [Google Scholar] [CrossRef]
- Allahvaisi, S.; Hassani, M.; Heidari, B. Bioactivity of azadirachtin against Scrobipalpa ocellatella Boyd. (Lepidoptera: Gelechidae) on sugar beet. J. Plant Protec. Res. 2021, 61, 280–289. [Google Scholar] [CrossRef]
- Bucholtz, K.; Märländer, B.; Puke, H.; Glattkowski, H.; Thielecke, H. Neubewertung des technischen Wertes von Zuckerrüben. Zuckerindustre 1995, 120, 113–121. [Google Scholar]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of yield trial. J. Agron. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Gauch, H.G.; Zobel, R.W. Imputing missing yield trial data. Theor. Appl. Genet. 1990, 79, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, K.; Liersch, A.; Popławska, W.; Bocianowski, J. Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica 2016, 208, 187–194. [Google Scholar] [CrossRef]
- Purchase, J.L.; Hatting, H.; van Deventer, C.S. Genotype × environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. S. Afr. J. Plant Soil. 2000, 17, 101–107. [Google Scholar] [CrossRef]
- Nowosad, K.; Liersch, A.; Poplawska, W.; Bocianowski, J. Genotype by environment interaction for oil content in winter oilseed rape (Brassica napus L.) using additive main effects and multiplicative interaction model. Indian J. Genet. Plant Breed. 2017, 77, 293. [Google Scholar] [CrossRef] [Green Version]
- Paderewski, J.; Gauch, H.G.; Mądry, W.; Gacek, E.S. AMMI analysis of Four-Way Genotype × Location × Management × Year Data from a wheat trial in Poland. Crop Sci. 2016, 56, 2157–2164. [Google Scholar] [CrossRef]
- Rosenkranz, H.; Vogel, R.; Greiner, S.; Rausch, T. Wounded sugar beet (Beta vulgaris L.) tap-root, hexose accumulation correlates with the induction of a vascular invertase isoform. J. Exp. Bot. 2001, 52, 2381–2385. [Google Scholar] [CrossRef] [Green Version]
- Fotso, A.K.; Hanna, R.; Kulakow, P.; Parkes, E.; Iluebbey, P.; Ngome, F.A.; Suh, C.; Massussi, J.; Choutnji, I.; Wirnkar, V.L. AMMI analysis of cassava response to contrasting environments: Case study of genotype by environment effect on pests and diseases, root yield, and carotenoids content in Cameroon. Euphytica 2018, 214, 155. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica 2018, 214, 79. [Google Scholar] [CrossRef]
- Bocianowski, J.; Księżak, J.; Nowosad, K. Genotype by environment interaction for seeds yield in pea (Pisum sativum L.) using additive main effects and multiplicative interaction model. Euphytica 2019, 215, 191. [Google Scholar] [CrossRef] [Green Version]
- Bocianowski, J.; Niemann, J.; Nowosad, K. Genotype-by-environment interaction for seed quality traits in interspecific cross-derived Brassica lines using additive main effects and multiplicative interaction model. Euphytica 2019, 215, 7. [Google Scholar] [CrossRef] [Green Version]
- Bocianowski, J.; Nowosad, K.; Szulc, P. Soil tillage methods by years interaction for harvest index of maize (Zea mays L.) using additive main effects and multiplicative interaction model. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 75–81. [Google Scholar] [CrossRef]
- Bocianowski, J.; Tratwal, A.; Nowosad, K. Genotype by environment interaction for main winter triticale varieties characteristics at two levels of technology using additive main effects and multiplicative interaction model. Euphytica 2021, 217, 26. [Google Scholar] [CrossRef]
- Bocianowski, J.; Szulc, P.; Nowosad, K. Soil tillage methods by years interaction for dry matter of plant yield of maize (Zea mays L.) using additive main effects and multiplicative interaction model. J. Integr. Agric. 2018, 17, 2836–2839. [Google Scholar] [CrossRef] [Green Version]
- Podlaski, S.; Chołuj, D.; Wiśniewska, A. Kształtowanie się plonu buraka cukrowego w zależności od wybranych czynników środowiskowych. Adv. Agric. Sci. Probl. 2017, 590, 59–71. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Kluge-Severin, S. Growth analysis of autumn and spring sown sugar beet. Eur. J. Agron. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Märländer, B.; Hoffmann, C.M.; Koch, H.J.; Ladening, E.; Merkes, R.; Petersen, J.; Stockfisch, N. Environmental situation and yield performance of the sugar beet crop in Germany: Heading for sustainable development. J. Agron. Crop Sci. 2003, 189, 201–226. [Google Scholar] [CrossRef]
- Kenter, C.; Hoffmann, C.M.; Märländer, B. Effects of weather variables on sugar beet yield development (Beta vulgaris L.). Eur. J. Agron. 2006, 24, 62–69. [Google Scholar] [CrossRef]
- Bzowska-Bakalarz, M.; Banach, M. Właściwości technologiczne surowca buraczanego produkowanego w zmodyfikowanej technologii nawożenia. Acta Agrophys. 2009, 14, 31–40. [Google Scholar]
- Moliszewska, E. Cechy morfologiczne buraka cukrowego a jakość plonu. Adv. Agric. Sci. Probl. 2015, 582, 43–51. [Google Scholar]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. The Plant Cell. 2000, 12, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Draycott, A.P. Introduction. In Sugar Beet; Draycott, A.P., Ed.; Blackwell Publishing Ltd: Oxford, UK, 2006; pp. 1–8. [Google Scholar]
- Webb, C.R.; Werke, A.R.; Gilligan, C.A. Modelling the dynamical components of sugar beet crops. Ann. Bot. 1997, 80, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Musolf, R.; Grzebisz, W.; Szczepaniak, W. Wpływ nawożenia potasem na tle zróżnicowanych warunków wodnych na plon i jakość korzeni buraka cukrowego (Beta vulgaris L.) Część II. Jakość technologiczna korzeni i plony cukru. [Effect of potassium fertilization under diversified water conditions on yield and quality of sugar beets (Beta vulgaris L.) Part II. Quality of taproots and yield of sugar]. Biul. Inst. Hod. I Aklim. Roślin 2004, 234, 115–121. [Google Scholar]
- Lafta, A.M.; Fugate, K.K. Dehydration accelerate respiration in post-harvest sugar beet roots. Postharvest Biol. Physiol. 2009, 54, 32–37. [Google Scholar] [CrossRef]
- Maurya, A.K.; Patel, R.C.; Frost, C.J. Acute toxicity of the plant volatile indole depends on herbivore specialization. J. Pest Sci. 2020, 93, 1107–1117. [Google Scholar] [CrossRef]
- Lima, A.F.; Ribeiro, L.P.; Gonçalves, G.L.P.; Maimone, N.M.; Gissi, D.S.; de Lira, S.P.; Vendramim, J.D. Searching for bioactive compounds from Solanaceae: Lethal and sublethal toxicity to Spodoptera frugiperda and untargeted metabolomics approaches. J. Pest Sci. 2021. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in content of polyphenols and ascorbic acid in leaves of white cabbage after pest infestation. Molecules 2019, 45, 2622. [Google Scholar] [CrossRef] [Green Version]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A. Tackling the threat to food security caused by crop pests in the new millennium. Food Sec. 2010, 2, 133–141. [Google Scholar] [CrossRef]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.d.; Vachova, P.; Hejnak, V. Betacyanins and betaxanthins in cultivated varieties of Beta vulgaris L. compared to weed beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef]



| Source of Variation | d.f. | Root Weight | Polarization | Potassium Molasses | Sodium Molasses | α-Amino-Nitrogen | Technological Yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m.s. | ve | m.s. | ve | m.s. | ve | m.s. | ve | m.s. | ve | m.s. | ve | ||
| Total | 159 | 34.26 | 1.86 | 103.91 | 1.98 | 72.11 | 6900 | ||||||
| Treatments, T | 4 | 20.66 * | 1.5 | 0.55 | 0.7 | 8.52 | 0.2 | 0.42 | 0.5 | 22.86 | 0.8 | 5526 * | 2.0 |
| Years, Y | 7 | 560.15 *** | 72.0 | 34.12 *** | 80.8 | 2032.30 *** | 86.1 | 35.99 *** | 80.1 | 1051.71 *** | 64.2 | 103,216 *** | 65.9 |
| Block | 24 | 7.93 | 3.5 | 0.72 *** | 5.8 | 48.71 *** | 7.1 | 0.45 | 3.4 | 59.64 *** | 12.5 | 1988 | 4.4 |
| TY Interactions | 28 | 16.35 ** | 8.4 | 0.50 ** | 4.7 | 12.27 * | 2.1 | 0.66 ** | 5.8 | 30.22 * | 7.4 | 4111 ** | 10.5 |
| IPCA 1 | 10 | 37.30 *** | 81.4 | 1.09 *** | 78.0 | 19.90 ** | 57.9 | 1.44 *** | 78.5 | 49.19 ** | 58.2 | 9556 *** | 83.0 |
| IPCA 2 | 8 | 8.06 * | 14.0 | 0.26 * | 15.1 | 10.89 * | 25.3 | 0.32 | 13.7 | 26.57 * | 25.2 | 1949 * | 13.5 |
| IPCA 3 | 6 | 2.03 | 2.6 | 0.15 | 6.2 | 7.11 | 12.5 | 0.17 | 5.5 | 14.71 | 10.4 | 444 | 2.3 |
| Residuals | 4 | 2.02 | 1.8 | 0.02 | 0.7 | 3.72 | 4.4 | 0.11 | 2.3 | 12.92 | 6.2 | 323 | 1.1 |
| Error | 96 | 8.28 | 0.24 | 7.84 | 0.33 | 18.08 | 1976 | ||||||
| Year | Treatment | Mean | IPCAe1 | IPCAe2 | ||||
|---|---|---|---|---|---|---|---|---|
| F1 # | F2 | K | K2 | S | ||||
| 2011 | 15.10 ± 0.65 | 14.46 ± 0.89 | 15.51 ± 2.46 | 15.01 ± 0.56 | 13.19 ± 1.21 | 14.65 ± 1.57 | −0.444 | −0.407 |
| 2012 | 23.80 ± 5.91 | 24.86 ± 5.12 | 22.77 ± 2.27 | 25.91 ± 5.56 | 23.38 ± 4.25 | 24.14 ± 4.91 | −1.099 | 0.589 |
| 2013 | 15.58 ± 2.72 | 15.32 ± 0.96 | 19.76 ± 3.38 | 14.98 ± 4.68 | 13.49 ± 1.38 | 15.82 ± 3.62 | 0.225 | −1.697 |
| 2014 | 9.93 ± 1.95 | 12.47 ± 2.61 | 12.40 ± 2.20 | 10.13 ± 1.47 | 11.15 ± 2.87 | 11.22 ± 2.52 | 0.053 | 0.247 |
| 2015 | 6.78 ± 1.21 | 6.78 ± 1.74 | 7.43 ± 0.48 | 6.49 ± 0.72 | 6.56 ± 1.38 | 6.81 ± 1.24 | −0.258 | 0.127 |
| 2016 | 16.90 ± 1.50 | 17.60 ± 2.26 | 19.25 ± 2.12 | 6.89 ± 1.23 | 16.27 ± 2.12 | 15.38 ± 4.75 | 2.716 | 0.487 |
| 2017 | 12.34 ± 0.85 | 12.50 ± 0.81 | 12.48 ± 1.52 | 13.74 ± 1.34 | 13.42 ± 0.90 | 12.89 ± 1.26 | −0.744 | 0.545 |
| 2018 | 8.60 ± 1.18 | 8.84 ± 0.24 | 9.14 ± 1.77 | 8.97 ± 3.49 | 8.29 ± 2.37 | 8.77 ± 2.14 | −0.448 | 0.109 |
| Mean | 13.63 ± 5.69 | 14.10 ± 5.70 | 14.84 ± 5.52 | 12.76 ± 6.62 | 13.22 ± 5.34 | 13.71 ± 5.83 | - | - |
| ASV | 2.800 | 3.379 | 7.665 | 15.618 | 2.291 | - | - | - |
| IPCAg1 | 0.484 | 0.574 | 1.301 | −2.699 | 0.340 | - | - | - |
| IPCAg2 | 0.034 | 0.630 | −1.437 | −0.405 | 1.178 | - | - | - |
| Year | Treatment | Mean | IPCAe1 | IPCAe2 | ||||
|---|---|---|---|---|---|---|---|---|
| F1 # | F2 | K | K2 | S | ||||
| 2011 | 18.0 ± 0.4 | 17.9 ± 0.6 | 18.0 ± 0.2 | 18.1 ± 0.3 | 18.2 ± 0.3 | 18.1 ± 0.4 | 0.064 | −0.036 |
| 2012 | 14.4 ± 0.4 | 14.3 ± 0.5 | 14.9 ± 0.1 | 14.5 ± 0.6 | 14.5 ± 0.4 | 14.5 ± 0.5 | 0.119 | 0.244 |
| 2013 | 16.4 ± 0.9 | 16.0 ± 0.8 | 16.1 ± 0.6 | 16.4 ± 0,7 | 16.3 ± 0.7 | 16.2 ± 0.8 | −0.052 | 0.204 |
| 2014 | 16.4 ± 0.1 | 16.3 ± 0.4 | 16.2 ± 0.1 | 16.3 ± 0.7 | 16.2 ± 0.8 | 16.3 ± 0.5 | 0.052 | 0.312 |
| 2015 | 18.5 ± 0.8 | 18.4 ± 0.2 | 18.6 ± 0.3 | 18.8 ± 0.3 | 18.9 ± 0.5 | 18.7 ± 0.5 | −0.014 | −0.187 |
| 2016 | 17.3 ± 0.2 | 16.9 ± 0.6 | 17.3 ± 0.3 | 18.9 ± 0.6 | 17.3 ± 0.1 | 17.5 ± 0.8 | −1.038 | −0.306 |
| 2017 | 17.0 ± 0.5 | 16.8 ± 0.4 | 16.7 ± 0.7 | 16.7 ± 0.2 | 16.8 ± 0.2 | 16.8 ± 0.4 | 0.142 | 0.316 |
| 2018 | 17.4 ± 0.4 | 17.9 ± 0.7 | 17.7 ± 0.2 | 17.2 ± 0.3 | 18.5 ± 0.8 | 17.7 ± 0.7 | 0.726 | −0.547 |
| Mean | 16.9 ± 1.3 | 16.8 ± 1.4 | 16.9 ± 1.2 | 17.1 ± 1.5 | 17.1 ± 1.5 | 17.0 ± 1.4 | - | - |
| ASV | 0.569 | 2.169 | 0.88 | 5.647 | 2.567 | - | - | - |
| IPCAg1 | 0.022 | 0.422 | 0.167 | −1.098 | 0.486 | - | - | - |
| IPCAg2 | 0.557 | 0.037 | 0.191 | −0.203 | −0.581 | - | - | - |
| Year | Treatment | Mean | IPCAe1 | IPCAe2 | ||||
|---|---|---|---|---|---|---|---|---|
| F1 # | F2 | K | K2 | S | ||||
| 2011 | 42.05 ± 1.86 | 41.88 ± 1.49 | 43.62 ± 2.75 | 40.60 ± 0.60 | 41.90 ± 2.99 | 42.01 ± 2.33 | −0.769 | 0.231 |
| 2012 | 56.38 ± 2.15 | 59.70 ± 5.91 | 53.65 ± 4.62 | 56.75 ± 4.95 | 54.50 ± 5.42 | 56.20 ± 5.23 | 0.608 | −1.850 |
| 2013 | 55.95 ± 3.50 | 59.42 ± 5.60 | 60.60 ± 6.24 | 57.38 ± 6.62 | 58.58 ± 8.14 | 58.38 ± 6.41 | −1.063 | 0.216 |
| 2014 | 36.83 ± 2.86 | 39.60 ± 3.04 | 41.23 ± 1.60 | 37.40 ± 1.37 | 36.00 ± 1.96 | 38.21 ± 2.97 | −1.304 | −0.238 |
| 2015 | 34.95 ± 1.34 | 35.90 ± 2.23 | 36.98 ± 2.22 | 38.00 ± 2.37 | 36.05 ± 0.55 | 36.38 ± 2.14 | 0.117 | 0.647 |
| 2016 | 30.73 ± 1.15 | 30.60 ± 1.00 | 29.23 ± 4.22 | 35.80 ± 0.67 | 32.07 ± 2.47 | 31.69 ± 3.22 | 1.648 | 0.730 |
| 2017 | 33.38 ± 5.11 | 32.55 ± 2.10 | 31.05 ± 2.55 | 33.58 ± 1.89 | 32.58 ± 2.17 | 32.62 ± 3.14 | 0.723 | −0.099 |
| 2018 | 44.29 ± 2.70 | 42.59 ± 1.56 | 43.81 ± 3.49 | 43.70 ± 2.42 | 43.00 ± 2.53 | 43.48 ± 2.68 | 0.040 | 0.362 |
| Mean | 41.82 ± 9.68 | 42.78 ± 10.98 | 42.52 ± 10.64 | 42.90 ± 9.25 | 41.83 ± 10.10 | 42.37 ± 10.16 | - | - |
| ASV | 1.144 | 1.917 | 4.644 | 3.665 | 1.059 | - | - | - |
| IPCAg1 | 0.470 | −0.372 | −2.012 | 1.592 | 0.322 | - | - | - |
| IPCAg2 | −0.411 | −1.720 | 0.796 | 0.570 | 0.766 | - | - | - |
| Year | Treatment | Mean | IPCAe1 | IPCAe2 | ||||
|---|---|---|---|---|---|---|---|---|
| F1 # | F2 | K | K2 | S | ||||
| 2011 | 2.05 ± 0.52 | 2.00 ± 0.52 | 2.63 ± 0.98 | 1.88 ± 0.30 | 1.75 ± 0.26 | 2.06 ± 0.65 | 0.174 | −0.651 |
| 2012 | 4.03 ± 0.35 | 4.15 ± 0.96 | 3.80 ± 0.52 | 3.60 ± 0.22 | 4.10 ± 0.66 | 3.94 ± 0.64 | −0.097 | 0.237 |
| 2013 | 1.30 ± 0.32 | 1.30 ± 0.16 | 1.40 ± 0.19 | 1.85 ± 0.62 | 1.75 ± 0.15 | 1.52 ± 0.41 | 0.379 | 0.404 |
| 2014 | 2.30 ± 0.19 | 2.80 ± 0.21 | 2.98 ± 0.62 | 2.48 ± 0.25 | 2.58 ± 0.15 | 2.63 ± 0.41 | 0.005 | −0.321 |
| 2015 | 1.28 ± 0.24 | 1.35 ± 0.23 | 1.43 ± 0.08 | 1.35 ± 0.23 | 1.25 ± 0.15 | 1.33 ± 0.21 | 0.145 | −0.030 |
| 2016 | 2.23 ± 0.25 | 4.10 ± 1.82 | 2.17 ± 0.23 | 1.63 ± 0.63 | 2.30 ± 0.12 | 2.49 ± 1.21 | −1.218 | 0.082 |
| 2017 | 2.00 ± 0.37 | 1.95 ± 0.22 | 1.93 ± 0.26 | 2.13 ± 0.20 | 1.85 ± 0.15 | 1.97 ± 0.27 | 0.201 | 0.124 |
| 2018 | 5.50 ± 0.49 | 4.95 ± 0.51 | 5.34 ± 0.48 | 5.35 ± 0.59 | 5.43 ± 0.80 | 5.31 ± 0.62 | 0.411 | 0.155 |
| Mean | 2.59 ± 1.41 | 2.83 ± 1.53 | 2.71 ± 1.34 | 2.53 ± 1.31 | 2.63 ± 1.39 | 2.66 ± 1.40 | - | - |
| ASV | 0.869 | 6.725 | 1.599 | 3.69 | 0.874 | - | - | - |
| IPCAg1 | 0.151 | −1.176 | 0.247 | 0.644 | 0.133 | - | - | - |
| IPCAg2 | 0.072 | 0.023 | −0.745 | 0.222 | 0.428 | - | - | - |
| Year | Treatment | Mean | IPCAe1 | IPCAe2 | ||||
|---|---|---|---|---|---|---|---|---|
| F1 # | F2 | K | K2 | S | ||||
| 2011 | 6.32 ± 1.29 | 6.46 ± 1.67 | 7.88 ± 1.97 | 6.98 ± 1.23 | 7.25 ± 1.71 | 6.97 ± 1.70 | 0.502 | 0.210 |
| 2012 | 24.82 ± 5.48 | 25.45 ± 6.31 | 23.88 ± 6.04 | 23.71 ± 4.96 | 21.71 ± 3.68 | 23.91 ± 5.52 | −0.052 | 0.409 |
| 2013 | 19.66 ± 8.57 | 20.18 ± 4.95 | 17.44 ± 5.82 | 17.84 ± 5.62 | 17.10 ± 5.65 | 18.45 ± 6.37 | −0.162 | 0.793 |
| 2014 | 11.43 ± 3.23 | 18.72 ± 4.47 | 19.71 ± 3.51 | 14.20 ± 3.78 | 14.94 ± 5.10 | 15.80 ± 5.08 | 0.865 | −1.524 |
| 2015 | 27.00 ± 9.61 | 27.25 ± 2.68 | 34.25 ± 7.93 | 30.35 ± 6.67 | 27.88 ± 3.37 | 29.35 ± 7.15 | 1.260 | −1.154 |
| 2016 | 17.77 ± 2.22 | 25.90 ± 8.56 | 15.13 ± 1.10 | 27.38 ± 5.23 | 17.90 ± 0.68 | 20.81 ± 6.73 | −2.866 | −0.804 |
| 2017 | 15.27 ± 3.00 | 13.78 ± 1.69 | 12.80 ± 1.67 | 14.18 ± 1.16 | 13.20 ± 1.56 | 13.84 ± 2.10 | −0.052 | 0.954 |
| 2018 | 26.34 ± 0.85 | 26.55 ± 1.27 | 25.50 ± 2.25 | 24.35 ± 1.48 | 28.80 ± 1.82 | 26.31 ± 2.18 | 0.505 | 1.115 |
| Mean | 18.58 ± 8.71 | 20.54 ± 8.23 | 19.57 ± 8.91 | 19.87 ± 8.54 | 18.60 ± 7.63 | 19.43 ± 8.47 | - | - |
| ASV | 1.996 | 3.212 | 5.598 | 4.223 | 1.745 | - | - | - |
| IPCAg1 | 0.123 | −1.369 | 2.355 | −1.787 | 0.678 | - | - | - |
| IPCAg2 | 1.976 | −0.551 | −1.313 | −0.880 | 0.768 | - | - | - |
| Year | Treatment | Mean | IPCAe1 | IPCAe2 | ||||
|---|---|---|---|---|---|---|---|---|
| F1 # | F2 | K | K2 | S | ||||
| 2011 | 263.9 ± 15 | 251.0 ± 16 | 270.7 ± 47 | 264.1 ± 10 | 232.6 ± 22 | 256.5 ± 29 | 2.001 | −1.915 |
| 2012 | 328.5 ± 84 | 341.3 ± 77 | 324.8 ± 31 | 357.6 ± 69 | 325.2 ± 58 | 335.5 ± 68 | 3.751 | 1.555 |
| 2013 | 244.3 ± 56 | 232.1 ± 14 | 304.2 ± 48 | 234.0 ± 79 | 207.4 ± 30 | 244.4 ± 60 | −0.437 | −6.653 |
| 2014 | 154.4 ± 33 | 191.4 ± 38 | 189.7 ± 35 | 156.4 ± 29 | 169.7 ± 37 | 172.3 ± 38 | 0.057 | 1.255 |
| 2015 | 114.2 ± 28 | 112.5 ± 32 | 124.2 ± 10 | 109.1 ± 15 | 111.9 ± 27 | 114.4 ± 24 | 1.084 | 0.712 |
| 2016 | 282.0 ± 22 | 285.0 ± 27 | 322.9 ± 32 | 117.4 ± 21 | 272.6 ± 39 | 256.0 ± 77 | −11.105 | 1.388 |
| 2017 | 200.2 ± 11 | 200.8 ± 13 | 199.7 ± 21 | 221.0 ± 23 | 217.4 ± 12 | 207.8 ± 19 | 3.024 | 2.658 |
| 2018 | 136.0 ± 21 | 144.8 ± 7 | 148.1 ± 30 | 142.2 ± 62 | 137.9 ± 38 | 141.8 ± 37 | 1.626 | 1.000 |
| Mean | 215.4 ± 82 | 219.9 ± 78 | 235.5 ± 82 | 200.2 ± 92 | 209.3 ± 74 | 216.1 ± 83 | - | - |
| ASV | 13.24 | 13.04 | 31.55 | 66.46 | 10.59 | - | - | - |
| IPCAg1 | −2.157 | −2.091 | −5.073 | 10.840 | −1.518 | - | - | - |
| IPCAg2 | −0.594 | 2.374 | −5.366 | −1.464 | 5.050 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocianowski, J.; Wielkopolan, B.; Jakubowska, M. AMMI Analysis of the Effects of Different Insecticidal Treatments against Agrotis spp. on the Technological Yield from Sugar Beet. Agriculture 2022, 12, 157. https://doi.org/10.3390/agriculture12020157
Bocianowski J, Wielkopolan B, Jakubowska M. AMMI Analysis of the Effects of Different Insecticidal Treatments against Agrotis spp. on the Technological Yield from Sugar Beet. Agriculture. 2022; 12(2):157. https://doi.org/10.3390/agriculture12020157
Chicago/Turabian StyleBocianowski, Jan, Beata Wielkopolan, and Magdalena Jakubowska. 2022. "AMMI Analysis of the Effects of Different Insecticidal Treatments against Agrotis spp. on the Technological Yield from Sugar Beet" Agriculture 12, no. 2: 157. https://doi.org/10.3390/agriculture12020157
APA StyleBocianowski, J., Wielkopolan, B., & Jakubowska, M. (2022). AMMI Analysis of the Effects of Different Insecticidal Treatments against Agrotis spp. on the Technological Yield from Sugar Beet. Agriculture, 12(2), 157. https://doi.org/10.3390/agriculture12020157







