1. Introduction
Biochar (BC) is a carbonaceous porous material produced by biomass pyrolysis in oxygen-starved conditions [
1]. A complete definition of such material has been given by the European Biochar Foundation (EBF), which stated that [
2]:
“Biochar is a porous, carbonaceous material that is produced by pyrolysis of plant biomasses and is applied in such a way that the contained carbon remains stored as a long-term C sink or replaces fossil carbon in industrial manufacturing. It is not made to be burnt for energy generation.”
According to EBF’s definition, the sole biomass eligible for BC production is that obtained by fast–growing plants, plant residues from certified forestry management, agricultural residues, and organic wastes from urban areas [
3]. Moreover, the EBF definition also accounts for many different BC uses, such as applications in paper and cellulose manufacturing, advanced building materials, electronics, 3D printing, water and sewage sludge decontamination, mining, air filtration, textile industry, and animal farming [
4].
In soils, biochar is widely applied as an amendment in order to improve soil quality and fertility. As an example, BC proved its efficiency in ameliorating soil structure [
5], increasing crop productivity [
6], improving rill flow resistance [
7], affecting pH, enhancing ionic exchange capacity, porosity, surface area, bulk density, water-holding capacity (WHC), nutrient use efficiency, as well as increasing phosphorus and nitrogen availability to plant nutrition [
8,
9]. From an environmental point of view, biochar can also be used for air, water and soil decontamination [
10,
11,
12,
13,
14] as well as for carbon sequestration in soils [
1], thereby allowing negative atmospheric carbon balance [
15] and mitigating greenhouse effects [
16]. For the sake of completeness, it must be outlined that some studies also highlighted the negative effects of biochars after application to soils [
17,
18,
19]. In some cases, a reduction on crop yield was observed, whereas, in other ones, crop production appeared magnified [
17]. It is not clear the reason why these contrasting effects are observed. Either biochar may interfere with phosphorous supply to plants [
18] or favor nitrogen leaching towards deep soil horizons [
19]. However, the significant limits of the studies where adverse biochar effects are described are related to the lack of a complete biochar characterization. As an example, Xu et al. [
18] only reported that:
“The feedstock was pyrolyzed under oxygen-limited atmosphere in muffle furnace at 300 °C, 400 °C, 500 °C, and 600 °C for 4 h, respectively”
without any indication about physicochemical biochar characteristics. Conversely, Gonzaga et al. [
19] reported proximate (ash content, volatile matter, and fixed carbon) and ultimate (elemental C, N, H, and S content) analyses, but no indication on biochar surface areas, porosity, etc. As indicated in a recent paper by Conte [
20], in order to understand how biochar may affect nutrient dynamics in soils and, therefore, crop yield, ultimate and proximate investigations are not sufficient. Detailed information about biochar spatial conformation and surface physical–chemistry must be achieved [
1,
5,
20,
21].
One of the paradigms about biochar uses in soils is that this material shows great stability, thereby making it unchanged for centuries up to millennia [
1,
21]. Although this can be true for biochar persistence in soils, in the sense that it remains in soils for long periods, changes are reported for what concerns its physical aspect and chemical properties as a consequence of its effects on soil properties [
21]. In particular, in their seminal paper, Hammes and Schmidt [
21] reported that biochar is subjected to particle fragmentation once in soil because of freeze–thaw cycles, rain and wind impact, plant roots and fungal hyphae penetration, and bioturbation (i.e., fragmentation due to the activity of micro- and mesofauna). From a chemical point of view, right after biochar incorporation into soils, its outer surfaces are subjected to oxidation, thereby making it more hydrophilic [
21].
During the last decade, fast field cycling (FFC) NMR relaxometry has emerged as a valuable tool to investigate biochar and its interactions with organic and inorganic systems [
22,
23,
24,
25,
26,
27]. Indeed, differentiation among different biochars having the same chemical composition was assessed [
22], biochar efficacy in removing heavy metals from water was evaluated [
24], the effect of biochar on hydraulic properties of a sandy-clay soil was investigated [
5], biochar hydrophobic character was studied [
25], and so on. The FFC NMR relaxometry technique is based on the rapid change in the intensity of the magnetic field where a sample is placed in order to monitor the overall molecular dynamics of complex systems providing no preliminary separation and/or purification procedure, as needed in the case of classical NMR spectroscopy [
28]. Relevant details of the technique have been reported in Conte and Lo Meo [
29].
Understanding the changes of biochar surfaces after addition to soils is a mandatory requirement in order to evaluate its role in ameliorating soil quality and to address the best agricultural practices for soil management, as well. To this purpose, with the present study, we intend to investigate the transformations of two different biochars obtained from pyrolysis of fir-wood pellets when they are applied to a soil. In particular, the two biochars were produced by quenching the pyrolysis in two different ways, i.e., (i) by letting the pyrolyzed biomass cool down at room temperature; (ii) by using water. Both biochars were added to a soil placed into a lysimeter. Then, seeds of watercress were sown, and the soil irrigated to let seeds germinate, and the plants grow. After 7 weeks of treatment, the biochars were collected for the analyses. Results revealed not only changes in porosity but also in the hydrophilic character of the biochar surface, thereby confirming the relatively fast oxidation following its application to soil, as described in the literature [
21]. Nevertheless, an additional aim of the present study consists in the testing of the mathematical models pointed out in previous papers from Conte’s group [
30,
31,
32,
33,
34] for the evaluation of the experimental data from fast field cycling NMR relaxometry experiments carried out on both hard and soft materials.
2. Materials and Methods
2.1. Biochar Production
Fir-wood pellets were placed into an Elsa Research pyrolytic stove (BLUECOMB™) that works with natural ventilation and does not require any power supply. The production process initially started as combustion. The top of the feedstock was lighted with a combustible for charcoal, and then it was allowed to burn for about one minute. The reaction continued as gasification once the chimney was placed on the top of the kiln. Then, two different quenching procedures were applied in order to produce two different biochar samples. The first one was obtained by letting the pyrolyzed biomass cool spontaneously (dry quenching), whereas the second biochar sample was obtained by quenching with water (wet quenching). The dry quenching procedure was carried out by sealing the kiln until the biochar cooling was complete. Conversely, the wet quenching was performed by pouring room temperature water on the pyrolyzed biomass directly into the kiln once the conversion of the feedstock into biochar was complete.
The two biochars were analyzed for the BET-specific surfaces by using a Beckman Coulter SA 3100 apparatus (Indianapolis, IN, USA). The dry−quenched biochar and the wet−quenched biochar revealed BET-specific surfaces of 378 m2 g−1 and 352 m2 g−1, respectively. Biochar pH was measured by using a biochar-to-water ratio of 1:20. The dry−quenched biochar revealed a pH of 9.44 ± 0.02, while the wet−quenched biochar showed 9.00 ± 0.04. The water content of the two biochars were: 1.13 ± 0.04% for the dry−quenched sample and 32.5 ± 0.4% for the wet−quenched biochar. Finally, elemental analysis (CHN) of the biochars was performed after a suitable drying procedure in order to eliminate all the water present in the samples. C and N content were respectively 76.0 ± 0.5% and 0.29 ± 0.03% for the biochar from dry quenching and 58 ± 3% and 0.24 ± 0.02% for the biochar from the wet quenching procedure.
2.2. Biochar Application to Soil
Soil was sampled from a residential area located in the near proximity of the University of Udine, Italy (46°04′52″ N, 13°12′33″ E; top 20 cm). After air-drying at room temperature and 2 mm sieving, the soil was characterized for its texture, pH, cation exchange capacity (CEC), and organic matter (OM) content. Results showed that the soil could be classified as clay soil (sand 26%, silt 6.4%, and clay 67.6%) with pH 7.40 ± 0.01, CEC 13.9 ± 0.3 cmol kg−1 of dry matter, and organic matter content of 4.4 ± 0.6%.
The sieved soil was mixed with cerium dioxide nanoparticles (CeO2-to-soil ratio of 1:1000 w w−1). Then, the soil–CeO2 mixture was split into two aliquots, each added with one of the two biochars produced as described above. The amount of each biochar added to the aforementioned mixture was 5% w w−1. The bulk densities of the three mixtures were 387 ± 2 g dm−3, 366 ± 2 g dm−3, and 366 ± 2 g dm−3 for the control, the mixture with dry−quenched biochar and that with the water-quenched biochar, respectively. The biochar was added to the soil without any alteration after its production (neither sieving nor milling was done). The biochar particles resembled the wood pellets of the feedstock, which had a cylindrical shape with a diameter of 4.5 mm by 10 mm length. The soil–CeO2-biochar mixtures were placed into column lysimeters (height: 55 cm; diameter: 15 cm). Seeds of watercress were sown in the soil/biochar mixtures placed into the lysimeters, which were irrigated to let the seeds germinate and the plants grow. After 7 weeks, the biochars were manually collected. Such a procedure was performed with tweezers and a magnifier on air-dried soil samples carefully collected from each column. The manual sampling was facilitated by the contrast between the light color of the soil and the black one of the biochar particles. Four types of biochar samples were then retrieved and analyzed by the experimental procedures reported below. Namely, BCDB and BCDA represent the biochar (BC) obtained by the dry−quenched (D) procedure, before (B) and after (A) application to soil. BCWB and BCWA are the biochars (BC) obtained by the wet−quenched (W) procedure, before (B) and after (A) soil application, respectively.
2.3. FFC NMR Experiments
As reported in the Introduction, the theory of fast field cycling NMR is discussed in detail elsewhere [
28,
29]. FFC NMR experiments were performed at the constant temperature of 25 °C by using a Stelar SmarTracer relaxometer (Stelar s.r.l., Mede, PV, Italy). The samples for the FFC NMR relaxometric analyses were prepared by suspending 1 g of each solid material in 3 mL of MilliQ grade water (electrical resistivity 18.2 MΩ cm at 25 °C). Data were acquired according to the procedure reported in Conte and Ferro [
31], to which the readers are addressed for details.
2.4. General Considerations on FFC NMR Experiments
By means of the FFC NMR technique, valuable information can be achieved on the molecular dynamics of material systems in general, provided that suitable data analysis is performed [
20]. In the particular case of porous systems, such as biochars, soils, nano sponges (NS), etc., which have been equilibrated with an aqueous medium, most of the
1H magnetization (induced in the sample after its introduction into a magnetic field) is due to the water molecules. Hence, the observed relaxometric behavior will specifically provide information related to the dynamics of water, which, in turn, mirrors the texture properties of the porous structure. In particular, the more constrained the water molecules are due to inclusion into small-sized pores, the shorter the
value and the faster the
value (
) they will show. Conversely, as water moves in larger-sized pores, the
lengthens and
slows down. A complete description of the relationship between pore systems and parameters from FFC NMR relaxometry is given in references [
20,
28,
29,
35].
2.5. FFC NMR Relaxometry Elaboration to Obtain NMRD Profiles
The decay/recovery curves (see
Appendix A for illustrative examples) obtained by changing the applied magnetic field in the proton Larmor frequency range 0.01–10 MHz have been fitted by using Equations (1) and (2) for the pre-polarized (PP) and non-polarized experiments, respectively [
28]:
The aforementioned equations are also indicated as exponential stretch functions. Their use is advantageous because they enable describing a large variety of behaviors within a single model. For this reason, assumptions about the number of exponentials to be applied in modeling relaxometric data are no longer needed. In Equations (1) and (2), is the offset and is the magnetization intensity at the Boltzmann equilibrium; is a heterogeneity parameter that is related to the stretching of the decay/recovery processes.
The resulting values are reported versus the proton Larmor frequency values, thus obtaining the nuclear magnetic resonance dispersion (NMRD) profiles.
2.6. Time Domain Distributions
Equations (1) and (2) cannot correctly represent the continuous distribution of
values when multi-phase systems are investigated, and the different components of the molecular dynamics are described by longitudinal relaxation times with values very close to each other. In this case, a better description of the
distribution is obtained by applying an inverse Laplace transformation, which is in the form of Equation (3) when pre-polarized experiments are performed and in the form of Equation (4) when non-polarized experiments are carried out.
In Equations (3) and (4),
and
are the longitudinal relaxation time limits;
is the distribution function that must be determined by solving either Equation (3) or Equation (4);
is an unknown noise component. The latter term makes it impossible to find the exact distribution of relaxation times, thereby allowing infinite possible solutions for Equations (3) and (4). However, the most probable distribution of relaxation times can be obtained when some constraints, such as smoothness of the solution and variance of the experimental data, are accounted for. For the present study, the algorithm used to solve Equations (3) and (4) was the uniform penalty regularization, also referred to as UPEN. Details about this algorithm can be found in transform. The result of the UPEN transformation is referred to as relaxogram and reports
-vs.-
. Examples of relaxograms are reported in
Appendix B.
2.7. The FFC NMR Models to Interpret NMRD Profiles and Relaxograms
In the sub-sessions below, the mathematical description of different models applied to interpret FFC NMR relaxometry data collected on porous systems having different chemical–physical natures are described. In particular, the
SCI-
FCI model was introduced by Conte and Ferro [
31,
32,
33] to provide a quantitative measurement of the hydrological connectivity inside the soil (
HCS), which is applied to evaluate the effects of the heterogeneities in complex environmental systems. In particular,
HCS refers to both the spatial patterns inside the soil (i.e., the structural connectivity,
SC) and the physical–chemical processes at a molecular level (i.e., the functional connectivity,
FC). The
SCI-
FCI model has been elaborated to provide the quantitative evaluation of the structural and functional connectivities via the elaboration of a Structural Connectivity Index (
SCI) and a Functional Connectivity Index (
FCI).
The
PCI model (Pore Connectivity Index) was elaborated by Lo Meo et al. [
35], on the basis of the
SCI-
FCI model, to solve the intrinsic difficulties involved in the quantitative assessment of the textural properties of soft porous matter, such as NS. Indeed, due to their softness, the evaluation of NS textural characteristics via the typical analyses, such as the Brunauer–Emmett–Teller (BET) for surface area evaluation, and Barrett–Joyner–Halenda (BJH) for pore size and volume investigation, fail, thereby providing artifacts [
35]. The NMR-based
PCI model appeared very suitable for quantitatively describing how the pores in nano sponges are connected to each other [
35].
The heuristic algorithm was recently introduced by Lo Meo and co-workers [
34] to simplify the plethora of different mathematical models which have been developed in the past for the investigation of organic and inorganic systems having different nature and provenience. A list of all the possible models is reported in references [
20,
28]. The algorithm, whose details are reported below, appeared to be suitable for the evaluation of the chemical–physical characteristics of
β-cyclodextrins, nano sponges, cheese, and cellulose [
34].
2.7.1. The SCI-FCI Model
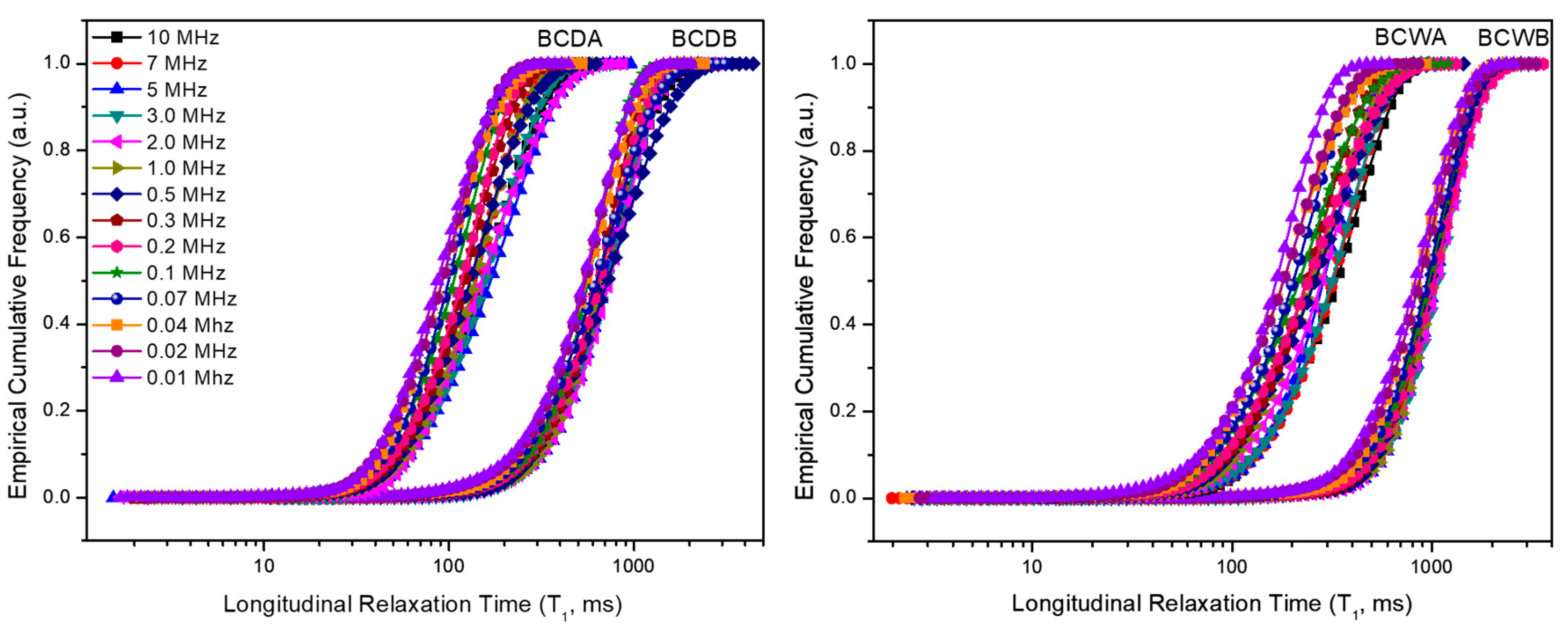
As previously mentioned, the inverse Laplace transform relaxograms describe distributions of longitudinal relaxation times that, in turn, are directly related to the pore size distribution of a porous material (assuming that the surface relaxivity is the same for all the surfaces). It is worth recalling here that the shorter the value, the more constrained the water molecules are as a consequence of their entrapment into micro-pores. As the value lengthens, water molecules move into pores that are progressively larger (e.g., meso- and macropores). The integration of the NMR relaxogram provides an S-shaped curve representing a non-exceeding empirical cumulative frequency , i.e., the longitudinal relaxation time assumes a value that is less than or equal to a given value.
Two different parts can be recognized in the
function. The first one is defined by the integral given in (5), where
is the total area of the relaxogram:
This describes the zone corresponding to the shortest values within a time range referred to as . Equation (5) means that only 1% of the measured relaxation times are less or equal to (fast relaxing component).
The second
part is given in the integral reported in (6), where
has the same meaning as before:
It describes the zone associated with the longest longitudinal relaxation times, included in a range indicated as . Equation (6) means that only 1% of the measured values are larger than or equal to (slow relaxing component).
According to Conte and Ferro [
31,
32,
33], the solution of the integrals in (5) and (6) provides the parameters (i.e.,
, and
) which can be applied to define two “connectivity indexes”. They are the Structural Connectivity Index (
SCI) and the Functional Connectivity Index (
FCI) calculated according to the following expressions:
in which
is the coefficient of variation of the relaxation time
values occurring in the range 0.01 <
< 0.99
The SCI represents the overall spatial organization of the soil particles leading to the formation of pores and channels where water molecules move. The FCI represents the ability of water exchange among different pores or particle aggregates mediated by the chemical–physical interactions with the pore boundaries.
2.7.2. The PCI Model
According to general theory, the longitudinal relaxation rate
R1 of a wet porous material depends on the different behavior of the water molecules either flowing in the pore lumen or interacting with the pore surface:
where
is the relaxation rate of bulk water,
and
are the mole fraction and the intrinsic relaxation rate for surface water, respectively. The assumption is that water molecules are continuously exchanged between the pore surface and the bulk. Thus:
In turn,
can be related to the texture properties of the material through the relationships:
, and
, where
is the thickness of the hydration shell of the pore walls,
is a geometry parameter (its value depends on the pore shape, i.e.,
m = 4 for cylindrical pores, 6 for spherical pores),
and
are the specific pore surface and volume, respectively,
is the average pore diameter. The combination of the latter relationships leads to the following expressions, which can be found in literature:
where
is the so-called “surface relaxivity”.
By examining the actual relaxation kinetics of the sample, different conditions can be observed. Indeed, relaxation of different nuclei actually occurs at different rates, according to a frequency distribution,
, given by the inverse Laplace transform of the kinetic data. Therefore, the relaxation kinetics should be actually expressed in an integral form as follows:
In the simplest case, this reduces to a relatively narrow unimodal Lognormal distribution, and, hence, the relaxation kinetics can be reasonably fitted as an ordinary first-order mono-exponential expression. In general, this is not the case, and the inverse Laplace transform instead consists of an asymmetric distribution, which frequently appears as the convolution of two (or more) partly superimposed contributions. Under these circumstances, in the case of wet systems, Conte and Ferro [
31,
32,
33] proposed (see above) to define two suitable “connectivity indexes”, namely
SCI and
FCI, which are intended to quantify the functional transport properties of a solid porous system, such as soil.
A further interesting behavior can be observed for the wet nano sponges (i.e., hyper-reticulated cyclodextrin or calixarene polymers) which can be considered soft, porous materials. In fact, in this case, the distribution function
obtained from the inverse Laplace transform features a bimodal distribution with two distinct asymmetric Lognormal components, which can be attributed to the relaxometric behavior of (i) the free water molecules flowing within the pore network (“slow” relaxation component
) and (ii) cumulatively to the surface water molecules and the skeletal hydrogen atoms of the organic framework (“fast” relaxation component
). Under these circumstances, the relaxation kinetics follows a trend that can be suitably fitted as the sum of two independent exponential terms, from which two distinct relaxation rates
and
can be obtained. Hence, Lo Meo et al. [
35] introduced
PCI defined as follows:
where the integration limits are defined as follows:
The same treatment can be applied, in principle, even in the case that the Φ(T1) frequency distribution function can be reasonably subjected to deconvolution analysis into two distinct (though superimposed) distributions.
2.7.3. The Heuristic Algorithm
In whatever way the relaxation kinetics has to be treated, and the relevant relaxation rates
as a function of the magnetic field are obtained, the problem arises of how the relevant NMRD dispersion curves have to be further analyzed in order to obtain the final information about the correlation times
(i.e., the time needed for a molecule to rotate one radian or to move for a distance as long as its gyration diameter). By the way, in the case of the soft NS, for which two distinct rates (“slow” and “fast”) are obtained, two distinct NMRD curves have to be built as well. According to the well-known Bloembergen–Purcell–Pound (BPP) theory [
36], for an ideal “simple” molecular system, subjected to a single dynamic regime, relaxation rates should depend on
according to the relationship:
where
is the internuclear distance,
is the proton Larmor angular frequency (rad s
−1),
is the gyromagnetic ratio that is a constant for each NMR observable nucleus,
is the Dirac constant, and
is the proton magnetic permeability. For complex systems, possibly made of different components subjected to different dynamics, one should derive, in principle, the correct expression based on the spectral density functions, which, in turn, derive from the autocorrelation function for the relaxing nuclei. Unfortunately, such a rigorous approach needs a robust mathematical elaboration. Alternatively, a “model-free” approach may provide a viable solution. The main conceptual issues involved in the model-free analysis have been particularly addressed in the seminal papers by Halle et al. [
37], who proposed to adapt a generic NMRD curve as a sum of an adjustable number of Lorentzian functions:
Hence, the requested apparent correlation time
can be calculated as follows:
Subsequently, Kruk et al. [
38] proposed to treat the NMRD curves of proteins as the sum of three terms:
where
is an offset term accounting for very fast dynamic components (
),
describes the homonuclear relaxation as a sum of three BPP-like terms, and
is a complex ad hoc term to take into account the effect (i.e., the “quadrupolar peaks” in the NMRD profiles) of the quadrupolar interactions with nitrogen nuclei. In order to overcome the conceptual difficulties arising from the degree of discretionarily in the choice of Lorentzian or BPP components typical of model-free approaches, very recently Lo Meo et al. [
34] proposed a heuristic analysis method, based on the assumption that, in principle, a complex system may be viewed as a continuum of virtual dynamic domains, each describable in terms of a BPP-like function with a proper
value. Hence, the second term of Equation (18) can be transformed into an integral form:
where a suitable distribution function
is present, providing a complete description of the system dynamics, which constitutes the Inverse Integral Transform of the experimental NMRD curve, with the BPP function as the kernel. By applying this type of analysis to diverse systems (including NS, cellulose, and cheese), it unexpectedly turned out that the
function features a limited number of sharp peaks, each pointing out at an individual dynamic domain, which in turn can be related to a particular physical–chemical characteristic of the system, without introducing any element of discretionarily in the analysis.
5. Conclusions
The present study was aimed at understanding the physicochemical alterations of biochar when it was applied to the soil. In particular, fir wood pellets were used to produce dry–quenched and wet–quenched biochar. The two types of biochar produced by the two different procedures were subjected to different mechanisms of alteration when placed into the same soil. In fact, while the dry–quenched biochar was mainly chemically modified, that obtained by the wet–quenching procedure was both physically and chemically altered. In other words, the dry−quenching produced a material that mainly underwent surficial oxidization, while its porosity was almost unchanged. Conversely, the porosity degree and surficial chemistry of the biochar produced by the wet–quenching procedure were modified when it was incorporated into the soil. Due to these diverse transformations, the dry– and wet–quenched biochars revealed different attitudes to retaining water. From a practical point of view, the results of the present study suggest that the biochar production procedure can be changed according to the use of the biochar one intends to implement to solve specific soil problems. As an example, we can expect that the application of the dry–quenched biochar may result in a better crop yield as compared to the soil treated with the wet–quenched material. In fact, due to its higher hydrophilic character following aging in soil, the dry−quenched biochar may promote higher water retention, hence, reduced nutrient leaching and higher crop yield. Conversely, the wet–quenched biochar became more hydrophobic because of the aging in soil. Therefore, it may allow water and nutrient leaching towards the deeper horizons of the soil profile and a reduction in plant growth. Inferences can, therefore, be drawn from these observations when biochar is applied for soil remediation purposes. In fact, it can be expected that the higher hydrophilicity of BCDA may allow better contaminant retention on the soil surface, thus preventing leaching towards groundwaters.
In the present study, a number of different mathematical models to interpret relaxometric data were used. In particular, a model (referred to as SCI-FCI) used for the analyses of soils (i.e., hard matter) was compared to that (referred to as PCI) developed for the investigation of nano sponges (i.e., soft matter). With a different grade of sensitivity, both mathematical models provided the same answers, thereby revealing that they can also be applied to biochar that can be considered a material with physical properties intermediate between hard and soft systems. Moreover, the results reported in the present study suggest also that the SCI-FCI and the PCI models can be joined together for the elaboration of a unified model. The latter could have more general applicability than the two single ones being usable on any kind of material. However, this is the aim of an ongoing study from our research group. The present study also revealed the suitability of the heuristic algorithm for the evaluation of the water behavior in biochar. In particular, it was possible to differentiate between water molecules in hydration spheres and those directly bound to the biochar surface.