Agroenvironmental Performances of Biochar Application in the Mineral and Organic Fertilization Strategies of a Maize–Ryegrass Forage System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments
2.3. Experiment Design and Data Recording
2.4. Soil Parameters
2.5. Biochar Characterization
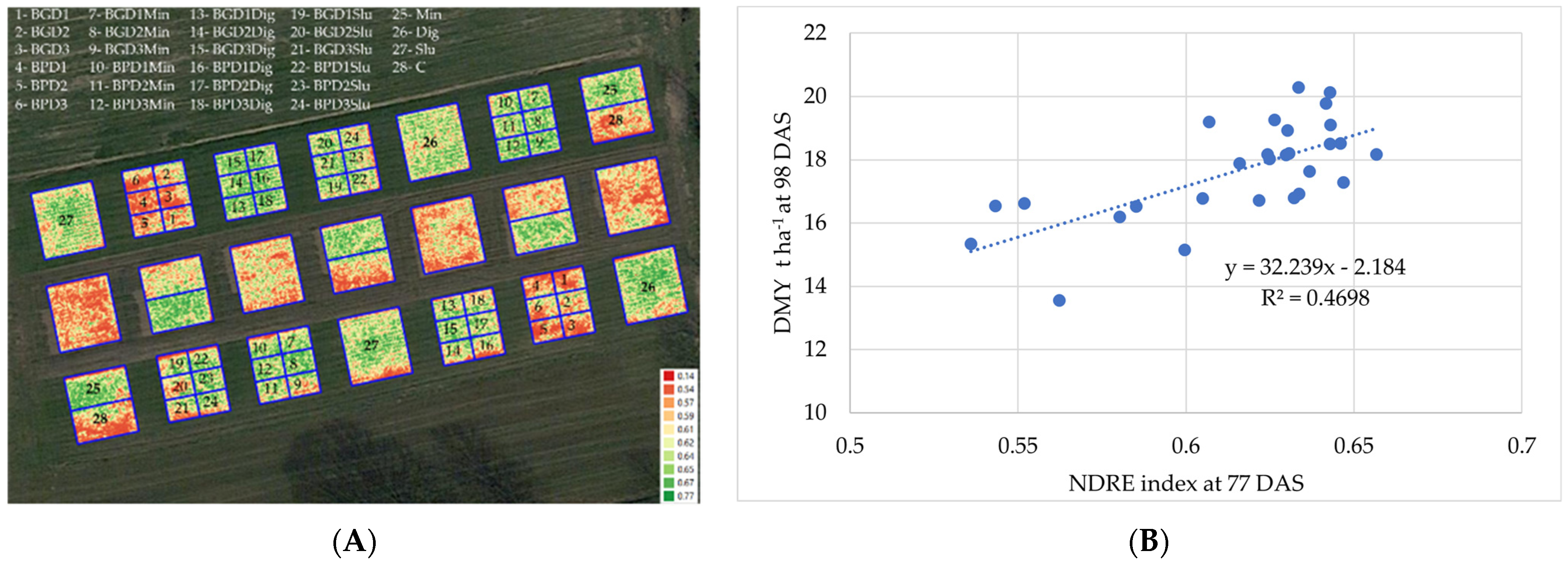
2.6. Multispectral Imaging
2.7. Nitrous Oxide Emissions
2.8. Statistical Analyses
3. Results
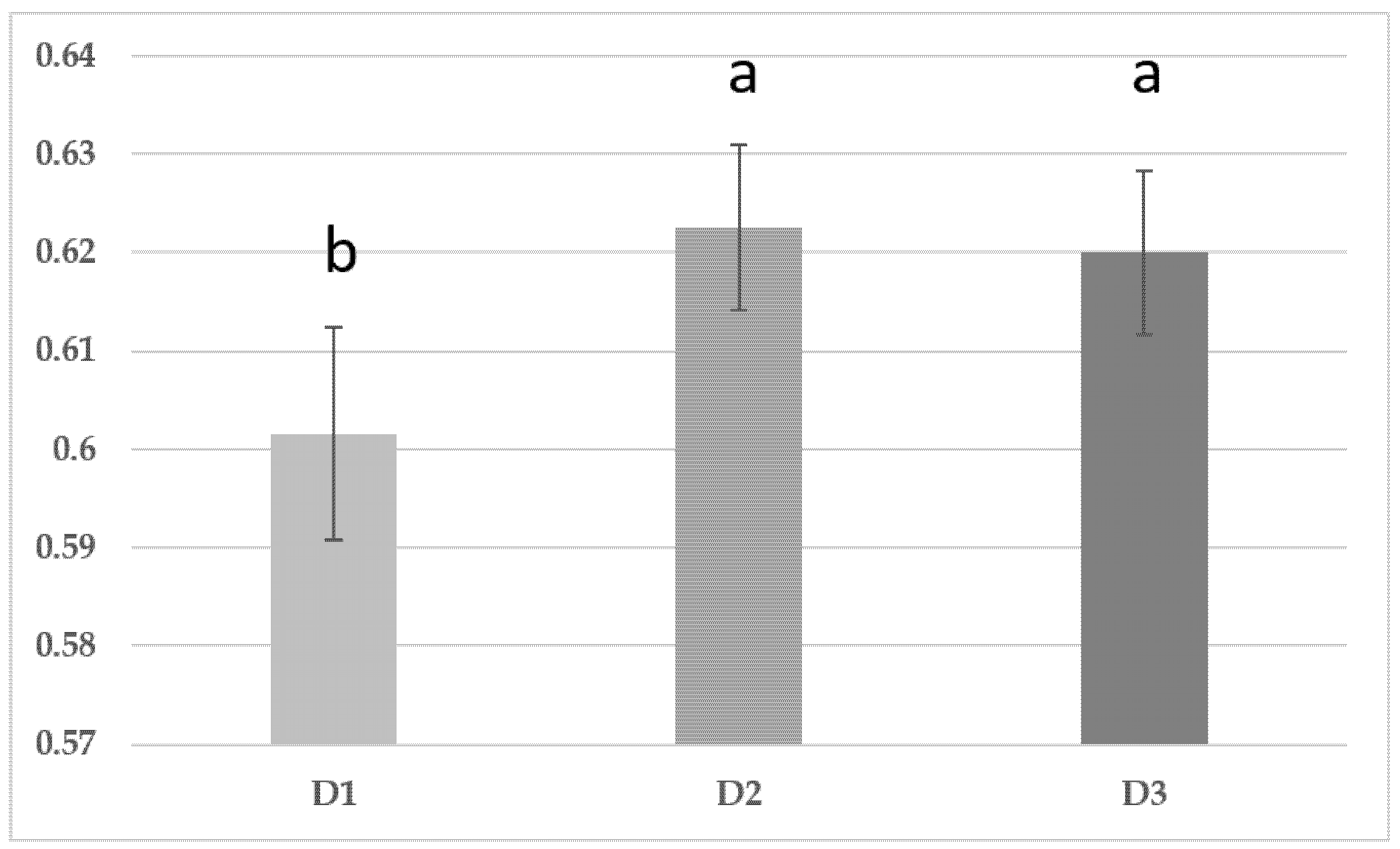
3.1. Maize Yield and Growth Dynamics
3.2. Italian Ryegrass (Lolium multiflorum) Yield
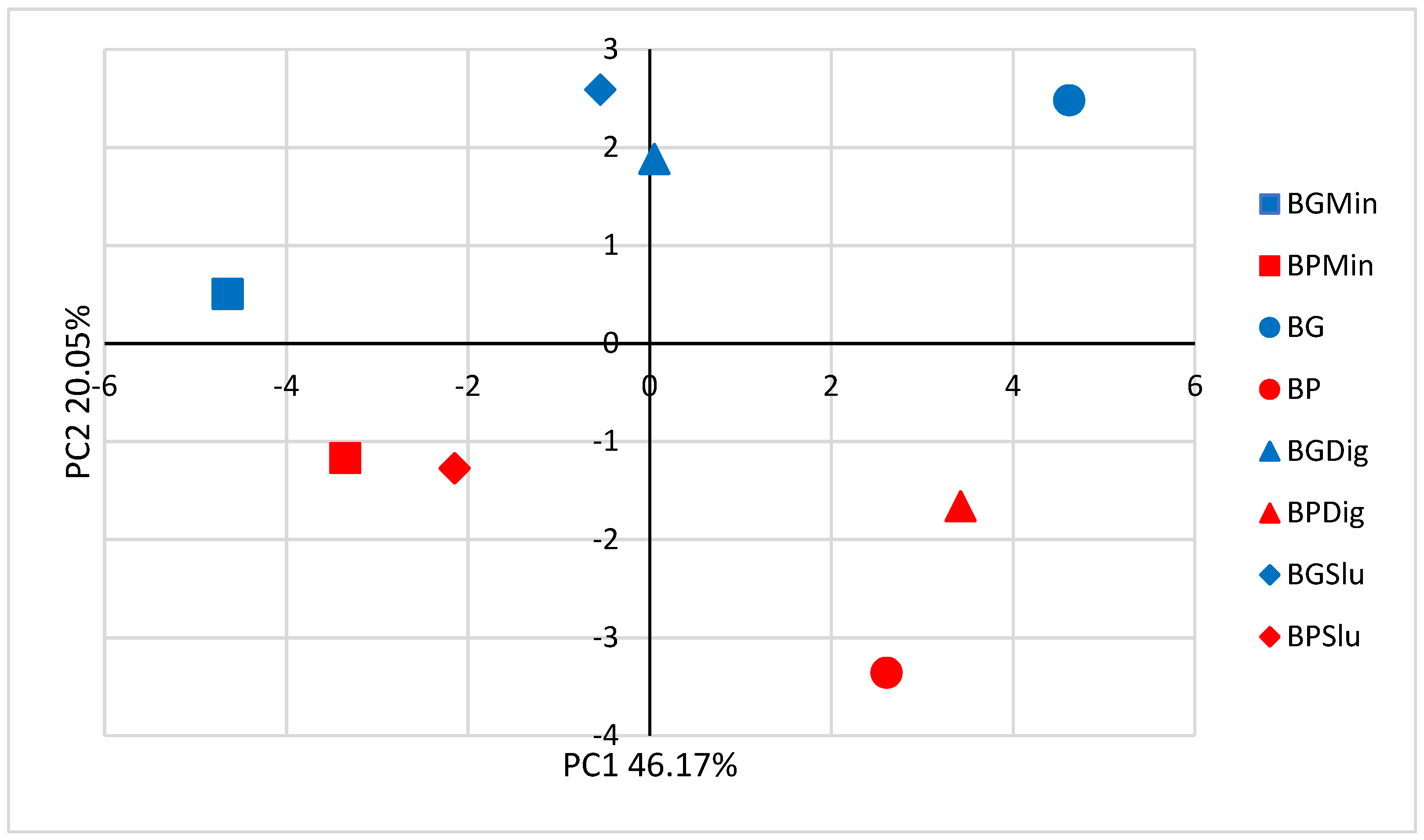
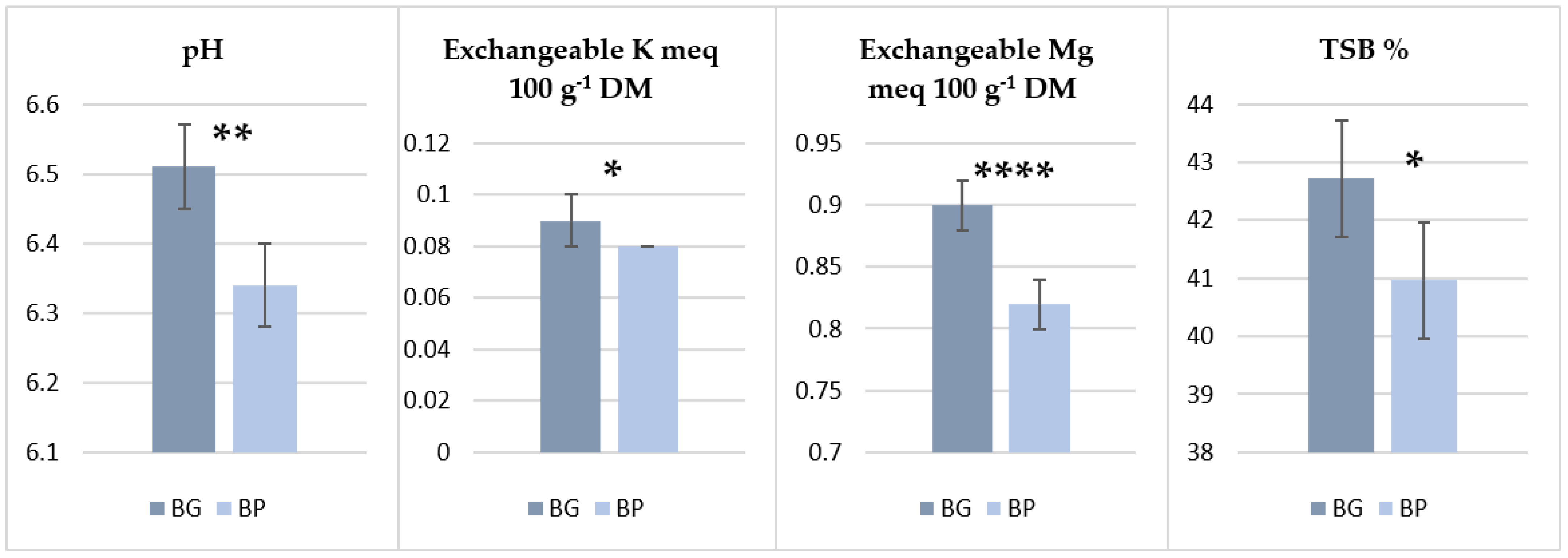
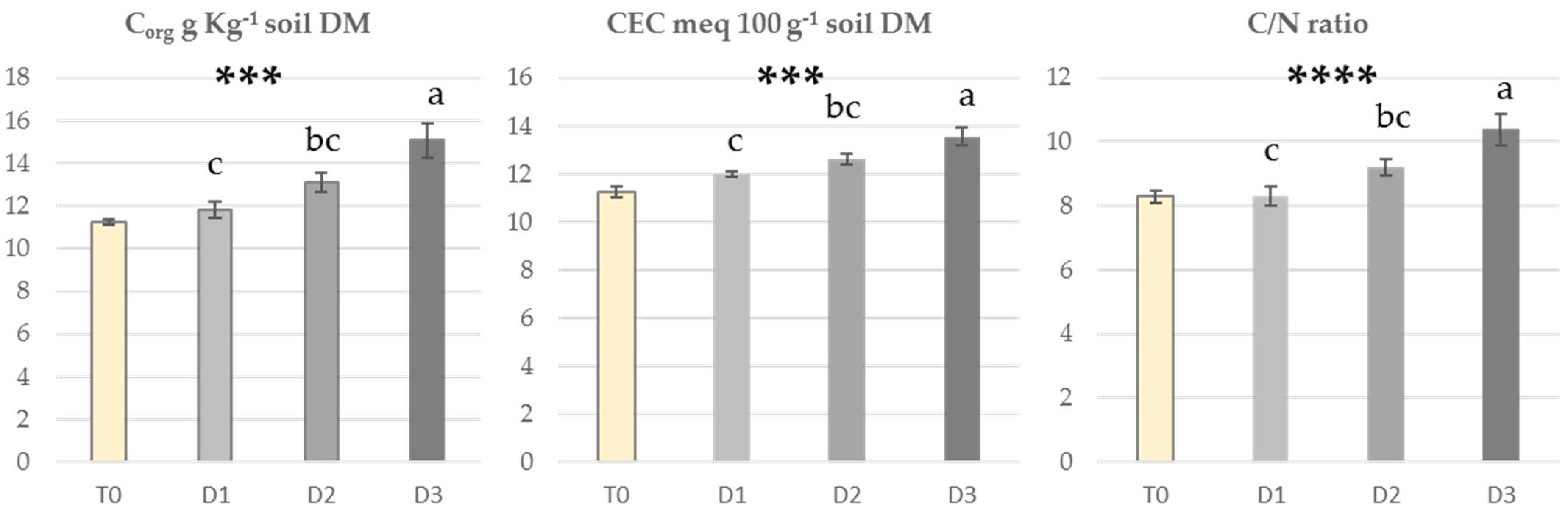
3.3. Soil Fertility
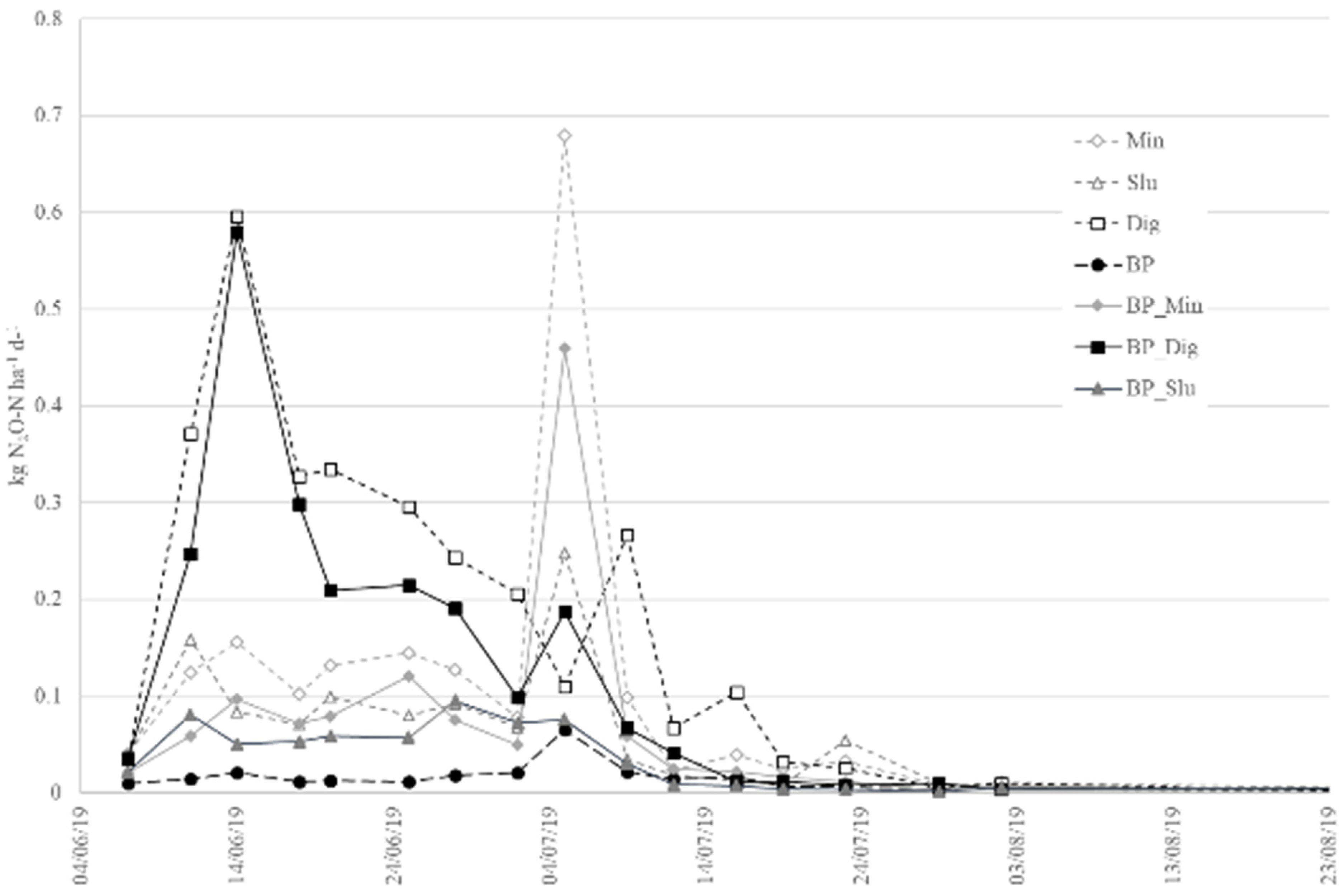
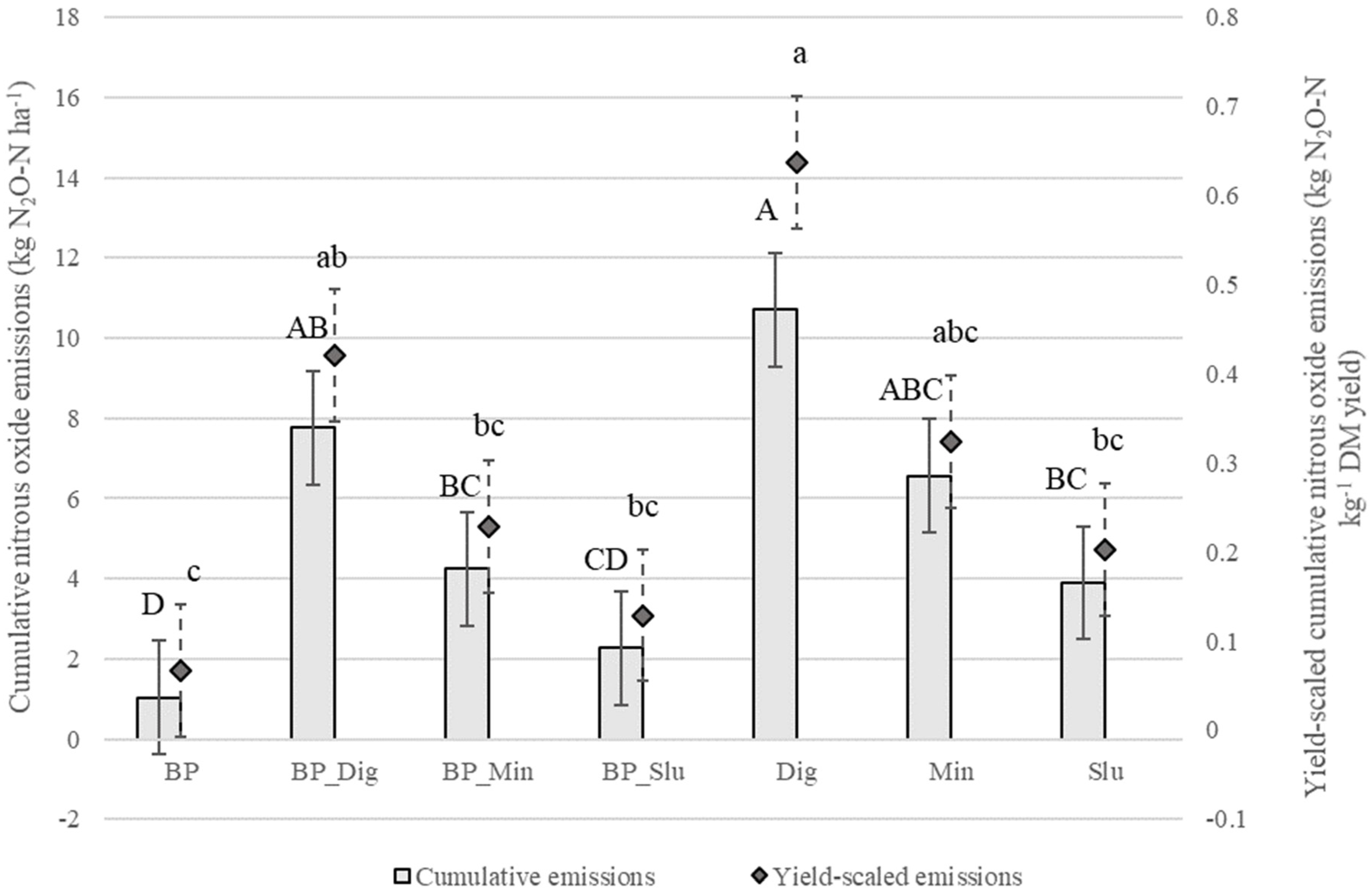
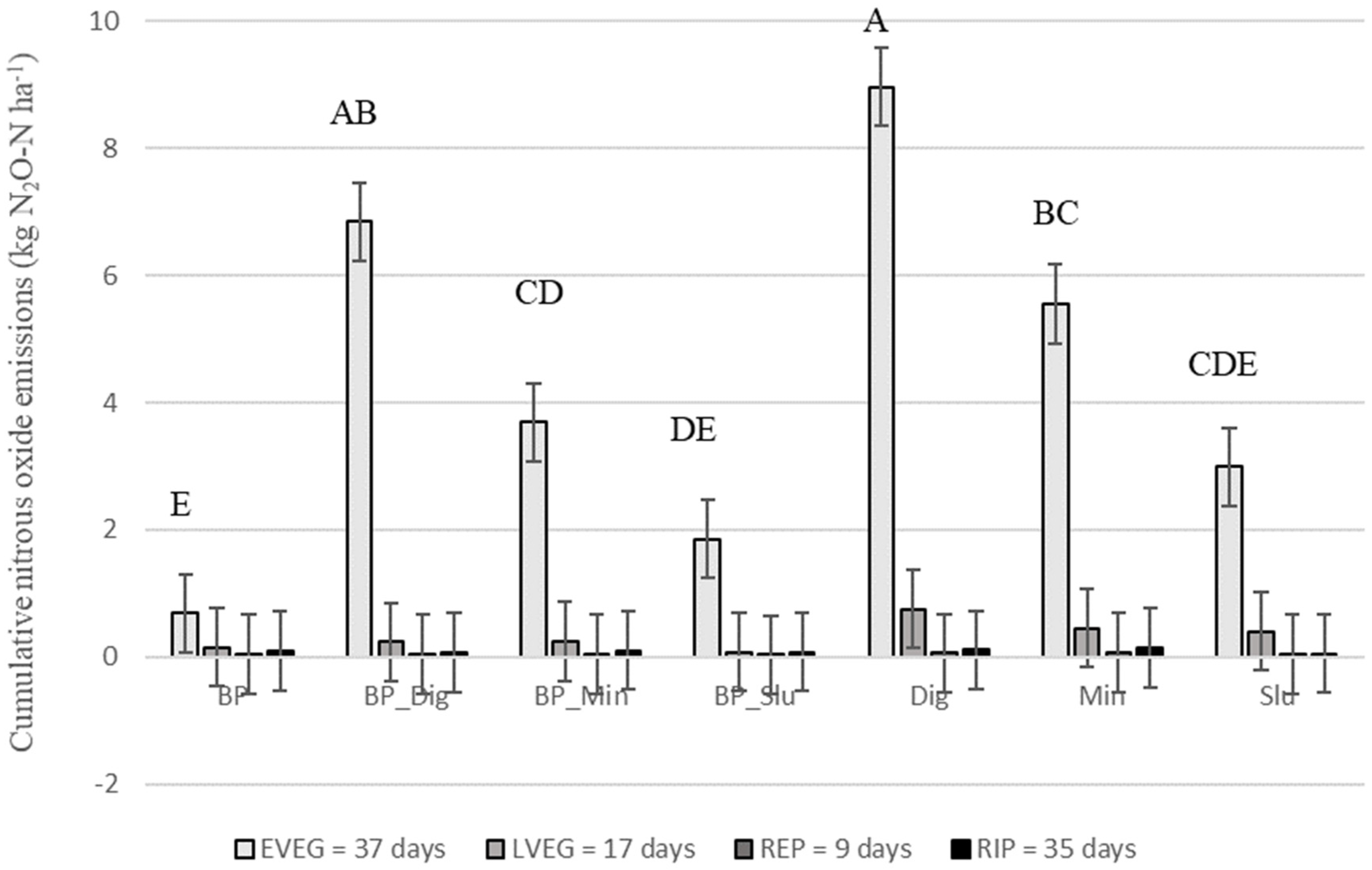
3.4. Nitrous Oxide Emissions
4. Discussion
4.1. Effect of Biochar Introduction on Growth Dynamics and Yield of Maize and Italian Ryegrass
4.2. Effect of Biochar Incorporation on Soil Fertility Parameters
4.3. Effect of Biochar Introduction on N2O Emissions from Maize
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavalli, D.; Cabassi, G.; Borrelli, L.; Geromel, G.; Bechini, L.; Degano, L.; Marino Gallina, P. Nitrogen fertilizer replacement of undigested liquid cattle manure and digestates. Eur. J. Agron. 2016, 73, 34–41. [Google Scholar] [CrossRef]
- Tabacco, E.; Comino, L.; Borreani, G. Production efficiency, costs and environmental impacts of conventional and dynamic forage systems for dairy farms in Italy. Eur. J. Agron. 2018, 99, 1–12. [Google Scholar] [CrossRef]
- Bertora, C.; Zavattaro, L.; Sacco, D.; Monaco, S.; Grignani, C. Soil organic matter dynamics and losses in manure maized-based forage systems. Eur. J. Agron. 2009, 30, 77–186. [Google Scholar] [CrossRef]
- Tomasoni, C.; Borrelli, L.; Ceotto, E. Effect of integrated forage rotation and manure management on yield, nutrient balance and soil organic matter. Ital. J. Agron. 2011, 6, e10. [Google Scholar] [CrossRef]
- Herrmann, A.; Kage, H.; Taube, F.; Sieling, K. Effect of biogas digestate, animal manure and mineral fertilizer application on nitrogen flows in biogas feedstock production. Eur. J. Agron. 2017, 91, 36–73. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Michels, E.; Meers, E. The potential of digestate and the liquid fraction of digestate as chemical fertilizer substitutes under the RENURE criteria. Agronomy 2021, 11, 1374. [Google Scholar] [CrossRef]
- Lehman, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Pete Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Martinsen, V.; Mulder, J.; Shitumbanuma, V.; Sparrevik, M.; Børresen, T.; Cornelissen, G. Farmer-led maize biochar trials: Effect on crop yield and soil nutrients under conservation farming. J. Plant Nutr. Soil Sci. 2014, 177, 681–695. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Martinsen, V.; Schimdt, H.P.; Cornelissen, G. Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 2018, 625, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, S.D.; Alavifazel, M.; Nurmohammadi, G.; Ardakani, M.R.; Sarajughi, M. Soil respiration, root traits and dry matter yield of sorghum (Sorghum bicolor L.) as affected by biochar application under different cropping patterns and irrigation method. Int. Agrophys. 2020, 34, 495–502. [Google Scholar] [CrossRef]
- Haider, F.U.; Virk, A.L.; Rehmani, M.I.A.; Skalicky, M.; Ata-ul-Karim, S.T.; Ahmad, N.; Soufan, W.; Brestic, M.; Sabagh, A.E.L.; Liqun, C. Integrated Application of Thiourea and Biochar Improves Maize Growth, Antioxidant Activity and Reduces Cadmium Bioavailability in Cadmium-Contaminated Soil. Front. Plant Sci. 2022, 12, 809322. [Google Scholar] [CrossRef] [PubMed]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237–238, 105–116. [Google Scholar] [CrossRef]
- De Sousa Lima, J.R.; de Moraes Silva, W.; de Medeiros, E.V.; Duda, G.P.; Corrêa, M.M.; Martins Filho, A.P.; Clermont-Dauphin, C.; Antonino, A.C.; Hammecker, C. Effect of biochar on physicochemical properties of a sandy soil and maize growth in a greenhouse experiment. Geoderma 2018, 319, 14–23. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of Nutrient-Enriched Biochar as a Soil Amendment during Maize Growth: Exploring Practical Alternatives to Recycle Agricultural Residuals and to Reduce Chemical Fertilizer Demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, I.; Kaiser, M.; Gunina, A.; Ledesma, P.; Polifka, S.; Wiedner, K.; Mueller, C.W.; Glaser, B.; Ludwig, B. Substitution of mineral fertilizers with biogas digestate plus biochar increases physically stabilized soil carbon but not crop biomass in a field trial. Sci. Total Environ. 2019, 680, 181–189. [Google Scholar] [CrossRef]
- Videgain-Marco, M.; Marco-Montori, P.; Martí-Dalmau, C.; Jaizme-Vega, M.C.; Manyà-Cervelló, J.J.; García-Ramos, F.J. Effects of Biochar Application in a Sorghum Crop under Greenhouse Conditions: Growth Parameters and Physicochemical Fertility. Agronomy 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Velthof, G.L.; Rietra, R.P.J.J. Nitrous Oxide Emission from Agricultural Soils; Report 2921; Wageningen Environmental Research: Wageningen, The Netherlands, 2018; ISSN 1566-7197. [Google Scholar]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (NO2) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [Green Version]
- FAO. Food Energy-Methods of Analysis and Conversion Factors; FAO Food and Nutrition Paper 77; FAO: Rome, Italy, 2003; pp. 7–17. ISSN 0254-4725. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–426. [Google Scholar]
- Isermeyer, H. Eine einfache Methode sur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Pflanzanernah. Bodenk. 1952, 56, 6–38. [Google Scholar]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000; ASA-CSSA-SSSA: Madison, WI, USA, 2000. [Google Scholar]
- Boiarskii, B.; Hasegawa, H. Comparison of NDVI and NDRE Indices to Detect Differences in Vegetation and Chlorophyll Content. J. Mech. Cont. Math. Sci. 2019, 4, 20–29. [Google Scholar] [CrossRef]
- Moretti, B.; Bertora, C.; Grignani, C.; Lerda, C.; Celi, L.; Sacco, D. Conversion from Mineral Fertilisation to MSW Compost Use: Nitrogen Fertiliser Value in Continuous Maize and Test on Crop Rotation. J. Plant Nutr. Soil Sci. 2020, 705, 135308. [Google Scholar] [CrossRef] [PubMed]
- Bertora, C.; Peyron, M.; Pelissetti, S.; Grignani, C.; Sacco, D. Assessment of methane and nitrous oxide fluxes from paddy field by means of static closed chambers maintaining plants within headspace. J. Vis. Exp. 2018, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Dai, H. Modeling maize above-ground biomass based on machine learning approaches using UAV remote-sensing data. Plant Methods 2019, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Gilliot, J.M.; Michelin, J.; Hadjard, D.; Houot, S. An accurate method for predicting special variability of maize yield from UAV-BASED plant height estimation: A tool for monitoring agronomic field experiments. Precis. Agric. 2021, 22, 897–921. [Google Scholar] [CrossRef]
- Borrelli, L.; Pecetti, L. Wheat yield as a measure of the residual fertility after 20 years of forage cropping systems with different manure management in Northern Italy. Ital. J. Agron. 2019, 14, 1359. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parik, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Nasielski, J.; Earl, H.; Deen, B. Luxury vegetative nitrogen uptake in maize buffers grain yield under post-silking water and nitrogen stress: A mechanistic understanding. Front. Plant Sci. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms ? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Nyambo, P.; Taeni, T.; Chiduza, C.; Araya, T. Effects of Maize Residue Biochar Amendments on Soil Properties and Soil Loss on Acidic Hutton Soil. Agronomy 2018, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Z.; Lou, Y.; He, X. A one-year short-term biochar application improved carbon accumulation in large macroaggregate farctions. Catena 2015, 127, 26–31. [Google Scholar] [CrossRef]
- Paetsch, L.; Mueller, C.W.; Rumpel, C.; Angst, Š.; Wiesheu, A.C.; Girardin, C.; Ivleva, N.P.; Niessner, R.; Kögel-Knabner, I. A multi-technique approach to assess the fate of biochar in soil and to quantify its effect on soil organic matter composition Organic. Geochemistry 2017, 112, 177–186. [Google Scholar] [CrossRef]
- Subedi, R.; Taupe, N.; Pelissetti, S.; Petruzzelli, L.; Bertora, C.; Leahy, J.; Grignani, C. Greenhouse gas emissions and soil properties following amendment with manure-derived biochars: Influence of pyrolysis temperature and feedstock type. J. Environ. Manag. 2016, 16, 73–83. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Xue, J.; Zhang, L. Quantifying the effects of biochar application on greenhouse gas emissions from agricultural soils: A global meta-analysis. Sustainability 2020, 12, 3436. [Google Scholar] [CrossRef] [Green Version]
- Yanai, J.; Sawamoto, T.; Oe, T.; Kusa, K.; Yamakawa, K.; Sakamoto, K.; Naganawa, T.; Inubushi, K.; Hatano, R.; Kosaki, T. Spatial variability of nitrous oxide emissions and their soil-related determining factors in an agricultural field. J. Environ. Qual. 2003, 32, 1965–1977. [Google Scholar] [CrossRef]
- Bertora, C.; Alluvione, F.; Zavattaro, L.; van Groenigen, J.W.; Velthof, G.; Grignani, C. Pig slurry treatment modifies slurry composition, N2O, and CO2 emissions after soil incorporation. Soil Biol. Biochem. 2008, 40, 1999–2006. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the Nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Horák, J.; Kotuš, T.; Toková, L.; Aydın, E.; Igaz, D.; Šimanský, V. A Sustainable Approach for Improving Soil Properties and Reducing N2O Emissions Is Possible through Initial and Repeated Biochar Application. Agronomy 2021, 11, 582. [Google Scholar] [CrossRef]
- Verhoeven, E.; Pereira, E.; Decock, C.; Suddick, E.; Angst, T.; Six, J. Toward a better assessment of biochar–nitrous oxide mitigation potential at the field scale. J. Environ. Qual. 2017, 46, 237–246. [Google Scholar] [CrossRef]
| Soil Parameter | Unit | |
|---|---|---|
| Total sand | % | 53.90 |
| Silt | % | 34.40 |
| Clay | % | 11.70 |
| Water content at field capacity | % | 13.10 |
| Water content at wilting point | % | 7.10 |
| pH in H2O | 6.35 | |
| Corg | g kg−1 | 11.25 |
| Total N | g kg−1 | 1.35 |
| C/N | 8.30 | |
| CEC | meq 100 g−1 | 11.25 |
| Exchangeable Ca | meq 100 g−1 | 4.24 |
| Exchangeable Mg | meq 100 g−1 | 0.97 |
| Exchangeable K | meq 100 g−1 | 0.09 |
| Exchangeable Na | meq 100 g−1 | 0.09 |
| Assimilable P2O5 | mg kg−1 | 18.00 |
| Biochar Parameter | Unit | BG | BP |
|---|---|---|---|
| Moisture | % | 67.6 | 68.3 |
| pH | 9.9 | 9.2 | |
| Electrical conductivity | 10−1 S m−1 | 73 | 6 |
| Total C | % DM | 77.9 | 77.9 |
| Total organic C | % DM | 76.8 | 77.6 |
| H: Corg Molar ratio | <0.1 | 0.1 | |
| Stable C | % Corg | 87.1 | 91.9 |
| Ashes at 550 °C | % DM | 17.06 | 6.26 |
| Total N | % DM | 0.16 | 0.20 |
| Total P2O5 | % DM | 0.26 | 0.05 |
| Total K | % DM | 1.03 | 0.27 |
| Maximum water retention | % | 80.2 | 77.01 |
| Particle size fraction > 5 mm | % | >20 | >48 |
| Particle size fraction > 2 mm | % | >53 | >77 |
| PAHs a | mg kg−1 | <1 | <1 |
| Heavy metals b | <permitted limits | <permitted limits | |
| SOLUBLE ELEMENTS (per liter of wet biochar) | |||
| NH4–N | mg L−1 | 17.24 | 11.69 |
| NO3–N | mg L−1 | <5 | <5 |
| N | mg L−1 | 17.24 | 11.69 |
| P | mg L−1 | 8.43 | 0.95 |
| Ca | mg L−1 | 18.77 | 16.5 |
| Mg | mg L−1 | 8.89 | 1.79 |
| K | mg L−1 | 1362.86 | 18.97 |
| Na | mg L−1 | 46.45 | 6.69 |
| Treatment | Yield t ha−1 DM | Plant-To-Ear Ratio | Protein Content % DM | N Uptake kg ha−1 | |||
|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2019 | 2019 | ||
| B | 17.21 ± 0.41 | 15.57 ± 0.32 b | 2.41 ± 0.08 | 1.96 ± 0.05 b | 6.93 ± 0.18 c | 173.5 ± 5.9 b | |
| BMin | 18.33 ± 0.33 | 18.64 ± 0.34 ab | 2.26 ± 0.09 | 1.85 ± 0.06 b | 8.58 ± 0.18 ab | 256.8 ± 7.3 ab | |
| BDig | 18.47 ± 0.33 | 18.44 ± 0.29 ab | 2.45 ± 0.09 | 2.24 ± 0.06 ab | 8.62 ± 0.17 ab | 254.5 ± 5.6 ab | |
| BSlu | 18.59 ± 0.45 | 17.59 ± 0.34 ab | 2.29 ± 0.07 | 2.13 ± 0.06 ab | 7.82 ± 0.20 b | 220.5 ± 6.3 ab | |
| Min | 20.05 ± 1.34 | 20.29 ± 0.79 a | 2.62 ± 0.41 | 1.87 ± 0.15 b | 9.42 ± 0.61 a | 306.9 ± 23.2 a | |
| Dig | 15.77 ± 0.22 | 16.80 ± 0.47 ab | 2.73 ± 0.23 | 2.48 ± 0.05 a | 8.34 ± 0.13 ab | 224.3 ± 7.9 ab | |
| Slu | 16.35 ± 0.90 | 19.25 ± 1.21 ab | 2.22 ± 0.25 | 2.17 ± 0.50 ab | 8.40 ± 0.37 ab | 259.8 ± 21.8 ab | |
| C | nd | 16.53 ± 0.72 ab | nd | 1.98 ± 0.10 b | 6.68 ± 0.31 c | 176.8 ± 10.7 b | |
| Block | 1.44 ns | 0.18 ns | 0.05 ns | 0.04 ns | 1.63 ns | 1.91 ns | |
| Treatment | 1.73 ns | 6.04 * | 1.81 ns | 7.20 ** | 18.8 **** | 10.6 *** | |
| Biochar effect | BMin vs. Min 1 | 1.35 ns | 2.09 ns | 1.24 ns | 0.03 ns | 5.1 ǂ | 4.3 ns |
| BDig vs. Dig 1 | 3.29 ns | 2.07 ns | 0.80 ns | 3.20 ns | 0.6 ns | 1.6 ns | |
| BSlu vs. Slu 1 | 2.29 ns | 2.11 ns | 0.04 ns | 0.09 ns | 2.5 ns | 2.6 ns | |
| B vs. C 1 | nd | 0.71 ns | nd | 0.01 ns | 0.5 ns | 0.0 ns | |
| Fertilization effect | BMin vs. B 1 | 1.97 ns | 29.39 *** | 0.74 ns | 2.33 ns | 69.8 **** | 41.6 **** |
| BDig vs. B 1 | 2.50 ns | 22.22 *** | 0.06 ns | 15.38 ** | 72.2 **** | 39.3 **** | |
| BSlu vs. B 1 | 3.03 ns | 11.05 * | 0.49 ns | 3.45 ns | 20.1 *** | 13.2 ** | |
| Treatment*bl. | 2.36 * | 1.87 ns | 2.53 * | 0.90 ns | 0.62 ns | 2.36 * | |
| Treatment | Vegetative Stages | Tasseling | ||||
|---|---|---|---|---|---|---|
| H1 (22–23 DAS) | H2 (33–34 DAS) | H3 (43–44 DAS) | H4 (54–55 DAS) | H5 (62–63 DAS) | ||
| B | 12.99 ± 0.61 d | 34.38 ± 1.62 d | 81.86 ± 3.12 c | 182.27 ± 5.89 b | 249.82 ± 2.29 a | |
| BMin | 14.37 ± 0.58 c | 41.58 ± 2.27 bc | 101.60 ± 3.38 b | 206.61 ± 5.30 a | 258.69 ± 8.88 a | |
| BDig | 17.36 ± 0.62 ab | 52.15 ± 1.76 a | 118.51 ± 2.14 a | 220.15 ± 4.13 a | 263.80 ± 2.36 a | |
| BSlu | 16.85 ± 0.61 ab | 49.74 ± 2.16 a | 112.46 ± 2.44 ab | 214.09 ± 4.89 a | 258.63 ± 3.23 a | |
| Min | 16.21 ± 0.84 b | 45.28 ± 3.09 ab | 107.63 ± 2.71 ab | 217.33 ± 3.00 a | 261.17 ± 1.13 a | |
| Dig | 17.98 ± 0.75 a | 52.31 ± 2.81 a | 115.23 ± 3.19 a | 218.94 ± 5.41 a | 261.96 ± 1.94 a | |
| Slu | 17.16 ± 0.89 ab | 49.14 ± 3.00 a | 110.03 ± 4.91 ab | 210.16 ± 11.30 a | 255.06 ± 6.77 a | |
| C | 13.63 ± 0.84 cd | 37.64 ± 3.09 cd | 89.16 ± 4.59 c | 193.28 ± 7.67 b | 259.59 ± 2.85 a | |
| Block | 137.0 **** | 117.2 **** | 30.3 **** | 20.3 **** | 1.6 ns | |
| Treatment | 61.1 **** | 36.4 **** | 36.9 **** | 22.3 **** | 3.0 ns | |
| Biochar effect | BMin vs. Min 1 | 19.7 *** | 3.5 ns | 2.5 ns | 4.4 ns | 0.3 ns |
| BDig vs. Dig 1 | 2.9 ns | 0.01 ns | 1.0 ns | 0.1 ns | 0.2 ns | |
| BSlu vs. Slu 1 | 0.7 ns | 0.1 ns | 0.5 ns | 1.0 ns | 0.8 ns | |
| B vs. C 1 | 2.4 ns | 2.7 ns | 3.7 ns | 4.7 ns | 4.3 ns | |
| Fertilization effect | BMin vs. B 1 | 22.2 *** | 26.2 *** | 53.8 **** | 45.8 **** | 7.1 * |
| BDig vs. B 1 | 222.9 **** | 160.2 **** | 185.4 **** | 110.8 **** | 17.6 *** | |
| BSlu vs. B 1 | 173.4 **** | 119.6 **** | 129.2 **** | 78.2 **** | 7.0 * | |
| Treatment*bl | 0.49 ns | 0.90 ns | 0.78 ns | 0.33 ns | 0.76 ns | |
| Treatment | Yield t ha−1 DM | ||
|---|---|---|---|
| 2019 | 2020 | ||
| B | 7.52 ± 0.51 | 3.35 ± 0.22 | |
| BMin | 8.04 ± 0.46 | 4.98 ± 0.26 | |
| BDig | 7.83 ± 0.34 | 4.89 ± 0.25 | |
| BSlu | 7.79 ± 0.41 | 5.51 ± 0.22 | |
| Min | 7.57 ± 0.99 | 3.03 ± 0.50 | |
| Dig | 5.61 ± 0.49 | 3.70 ± 0.98 | |
| Slu | 7.25 ± 0.05 | 4.08 ± 0.01 | |
| C | 5.73 ± 0.51 | 2.89 ± 0.45 | |
| Treatment | 1.25 ns | 2.24 ns | |
| Biochar effect | BMin vs. Min 1 | 0.25 ns | 2.33 ns |
| BDig vs. Dig 1 | 5.60 ǂ | 0.86 ns | |
| BSlu vs. Slu 1 | 0.33 ns | 1.25 ns | |
| B vs. C 1 | nd | 0.13 ns | |
| Fertilization eff. | BMin vs. B 1 | 1.08 ns | 5.72 * |
| BDig vs. B 1 | 0.37 ns | 5.08 ǂ | |
| BSlu vs. B 1 | 0.29 ns | 10.02 * | |
| Treatment | Corg g kg−1 DM | C/N Ratio | CEC meq 100 g−1 DM | |
|---|---|---|---|---|
| B | 12.18 ± 0.36 abc | 8.72 ± 0.29 a | 12.18 ± 0.17 ab | |
| BMin | 14.13 ± 0.88 a | 9.57 ± 0.52 a | 13.09 ± 0.41 a | |
| BDig | 14.30 ± 1.08 a | 10.08 ± 0.60 a | 13.15 ± 0.47 a | |
| BSlu | 12.73 ± 0.54 ab | 8.89 ± 0.37 a | 12.43 ± 0.27 ab | |
| Min | 10.05 ± 0.25 c | 7.45 ± 0.45 a | 11.25 ± 0.15 b | |
| Dig | 10.85 ± 0.35 bc | 7.50 ± 0.0 a | 11.50 ± 0.10 b | |
| Slu | 10.45 ± 0.35 bc | 7.45 ± 0.25 a | 11.35 ± 0.25 b | |
| C | 9.85 ± 0.45 c | 7.30 ± 0.10 a | 11.35 ± 0.05 b | |
| Block | 0.21 ns | 0.13 ns | 0.31 ns | |
| Treatment | 16.31 **** | 3.89 * | 18.05 **** | |
| Biochar effect | BMin vs. Min 1 | 31.42 **** | 5.56 * | 35.09 **** |
| BDig vs. Dig 1 | 22.52 *** | 7.96 * | 28.17 *** | |
| BSlu vs. Slu 1 | 9.79 * | 2.59 ns | 11.96 * | |
| B vs. C 1 | 10.23 * | 2.49 ns | 7.19 * | |
| Fert. effect | BMin vs. B 1 | 25.18 *** | 3.14 ns | 29.88 **** |
| BDig vs. B 1 | 29.90 **** | 7.53 * | 33.84 **** | |
| BSlu vs. B 1 | 2.00 ns | 0.13 ns | 2.11 ns | |
| Treatment*bl. | 0.13 ns | 0.56 ns | 0.11 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti, C.; Bertora, C.; Valagussa, M.; Borrelli, L.; Cabassi, G.; Tosca, A. Agroenvironmental Performances of Biochar Application in the Mineral and Organic Fertilization Strategies of a Maize–Ryegrass Forage System. Agriculture 2022, 12, 925. https://doi.org/10.3390/agriculture12070925
Scotti C, Bertora C, Valagussa M, Borrelli L, Cabassi G, Tosca A. Agroenvironmental Performances of Biochar Application in the Mineral and Organic Fertilization Strategies of a Maize–Ryegrass Forage System. Agriculture. 2022; 12(7):925. https://doi.org/10.3390/agriculture12070925
Chicago/Turabian StyleScotti, Carla, Chiara Bertora, Massimo Valagussa, Lamberto Borrelli, Giovanni Cabassi, and Alberto Tosca. 2022. "Agroenvironmental Performances of Biochar Application in the Mineral and Organic Fertilization Strategies of a Maize–Ryegrass Forage System" Agriculture 12, no. 7: 925. https://doi.org/10.3390/agriculture12070925
APA StyleScotti, C., Bertora, C., Valagussa, M., Borrelli, L., Cabassi, G., & Tosca, A. (2022). Agroenvironmental Performances of Biochar Application in the Mineral and Organic Fertilization Strategies of a Maize–Ryegrass Forage System. Agriculture, 12(7), 925. https://doi.org/10.3390/agriculture12070925







