Abstract
One of the main reasons for oilseed rape breeding is to improve the quality and composition of seeds by increasing fat and protein content, and to reduce dietary fibre. We attempted to obtain such varieties by crossing two DH lines of winter oilseed rape of different origin, H2-26 and Z-114, which have black and yellow seeds, respectively. The cross was followed by in vitro androgenesis, resulting in a population of 44 DH lines that were studied in a field experiment over two seasons. The following characters of the resulting seeds were analysed: fat, protein, neutral detergent fibre, acid detergent fibre, glucosinolate content and seed colour. The main objective was to check the variability of the DH lines obtained from F1 hybrid black- and yellow-seeded oilseed rape. The range of variability of the DH lines significantly exceeded the range of variability of the parental lines for all traits. These experiments showed that by choosing the appropriate parental genotypes of oilseed rape it is possible to break the negative correlation between protein and fat content. The high level of heritability of traits related to seed quality raises the possibility of improving breeding lines through selection based on phenotype.
1. Introduction
Growing demand for oilseed rape (Brassica napus L. var. oleifera) exerts a permanent pressure on breeders to improve seed yield and quality. The main selection goals in oilseed rape breeding have remained unchanged for several years and include increasing productivity, obtaining oil with a different composition of fatty acids depending on the intended use of oil and increasing the quality of oilseed rape meal.
These goals can, in part, be achieved by improving the chemical composition of oilseed rape seeds, which consist mainly of lipids (37–50%) and proteins (15–26%) as well as carbohydrates, lignins, ash and secondary metabolites (e.g., phenolic compounds) [1,2].
Oilseed rape seeds are a source of high-quality oil, which is used, with modifications, for many food and industrial purposes. Moreover, an important by-product of canola oil extraction is oilseed rape meal—a very rich raw material that contains up to 50% protein on a dry weight basis [3]. It is also rich in calcium, phosphorus, magnesium and zinc. Oilseed rape meal has a similar content of vitamins E, B1, B2, B6 as soybean meal, but it contains much more folic acid, vitamin PP, biotin and choline. The proteins contained in the seeds of oilseed rape are characterized by a favourable composition of amino acids, especially a good content of the exogenous amino acids lysine, threonine and tryptophan. However, the nutritional value of both the expeller and the post-extraction meal of oilseed rape is limited by its high fibre content, which results in the low digestibility of the feed.
In general, dietary fibre is a collection of heterogeneous substances, mainly non-starch polysaccharides and lignins, which derive from plant cell walls [4]. These substances can be divided into four basic groups of compounds: pectin, hemicellulose, cellulose and lignin. Pectins, which are easily dissolved in water, are rapidly fermented and have good digestibility. The remaining three groups of compounds form various fractions of dietary fibre. So-called neutral detergent fibre (NDF) contains all three compounds, while acid detergent fibre (ADF) contains only cellulose and lignin. The third fraction–ADL–is made solely of lignin [5].
Much oilseed rape breeding research has focused on reducing levels of fibre and anti-nutritional substances such as glucosinolates (GSL) and phenolic compounds and increasing protein and fat content. The breeding of yellow-seeded oilseed rape is very promising, because the seeds of such varieties have a low fibre content due to their transparent coat [6,7,8]. Reducing the fibre content of oilseed rape cake or post-extraction meal increases the availability of protein and improves the overall digestibility of the feed.
Modern breeding programmes make extensive use of biotechnology [9,10]. In winter oilseed rape breeding, doubled haploid (DH) technology, which uses an in vitro androgenesis process, has already become a routine way of obtaining completely homozygous and fully genetically stable lines in one generation. The benefits of DH lines are primarily acceleration of the breeding process by reducing the time taken to obtain homozygous lines compared to inbreeding and an increase in the efficiency of selection in early generations [11]. In addition, for polygenic traits, DH technology requires significantly fewer genotypes compared to classical breeding [12,13].
The main objective of the study was to evaluate the DH line population of winter oilseed rape derived from F1 hybrids obtained by crossing black- and yellow-seeded DH lines for fat, protein, fibre and GSL content and to select the best genotypes.
2. Materials and Methods
2.1. Plant Material
This study used the population of DH lines of winter oilseed rape derived from F1 hybrids obtained by crossing the yellow-seeded DH line Z-114 and the black-seeded DH line H2-26. The cross was followed by in vitro androgenesis in isolated microspore culture [14,15]. The plant material thus comprised a set of 44 DH lines together with the 2 parental lines. The parental yellow-seeded line Z-114 was selected from the population of DH lines developed from yellow-seeded breeding materials obtained at the Plant Breeding and Acclimatization Institute–National Research Institute (PBAI-NRI) in Poznań, Poland, from a natural mutant with brighter seeds and the Canadian spring line [16]. The seeds of DH Z-114 line were characterized by excellent yellow colour and low fibre content. In contrast, the black-seeded parental line DH H2-26 obtained at PBAI-NRI by in vitro androgenesis from breeding line 10199 had relatively high protein content, good plant habit and high resistance to frost.
2.2. Experimental Conditions
The DH line population along with the parental lines were used in a field experiment conducted over 2 seasons, 2016/2017 and 2017/2018. The field experiment was carried out in a randomized design with 4 replications in the sandy loam soils at the Łagiewniki Experimental Station of the Smolice Plant Breeding company. Each genotype was grown in a plot 1.2 m × 5.0 m in size. Oilseed rape genotypes were sown on 28 August in 2016 and 31 August in 2017 and harvested 23 July 2017 and 20 July 2018, respectively, at the seed maturity stage using a plot harvester. In both seasons, the level of mineral fertilization was the same and was as follows: 205 N, 40 P2O5, 60 K2O, and 30 S (kg·ha−1). Full chemical protection of plants was applied, and other agronomic practices were similar to those used for commercial planting in the area. The weather conditions varied during 2 analysed growing seasons, especially during spring and early summer. The sums of precipitation were 365.1 mm from March to July 2017 and 303.8 mm from March to July 2018. Mean temperatures for these periods at the experimental site were 12.8 °C and 13.2 °C for the first and second year, respectively. The monthly temperatures and precipitations in each growing season, from the beginning of March till the end of July (harvest), are presented in Table 1.

Table 1.
Meteorological data for seasons of experiments (mean value).
Just before flowering, several plants in each plot were covered with bags to obtain self-pollinated seeds that were then analysed for GSL content, protein and two fractions of dietary fibre, NDF and ADF. In contrast, the fat content was measured in samples of seeds collected from open-pollinated plants because the instrument for fat content analysis in place required a sample of 60–80 g per analysis. Seed samples for chemical analyses were taken from each plot in every replication of the trial.
GSL content was analysed by gas chromatography [17]. To determine the content of protein and both fibre fractions, a near-infrared reflectance spectroscopy (NIRS) method was used. The fat content in dry seeds (about 5% moisture) was determined by pulsed nuclear magnetic resonance (NMR; MQA7005, Oxford Instruments, Abingdon, UK). Seed coat colour was determined with a Color Flex spectrophotometer on a scale from 0 to 5 (black to yellow; Figure 1).

Figure 1.
Seed colour on a scale from 0 (black) to 5 (yellow).
2.3. Methods of Statistical Analysis
Experimental data were analysed using uni- and multivariate statistical methods [18,19]. Two-factor analysis of variance was performed for seed quality traits and seed colour. This enabled the determination of statistical characteristics for all genotypes for each individual year. Heritability in the broad sense was calculated using the following formula [20,21]:
where:
H2 = σg2/σp2
- σg2—genotypic variance
- σp2—phenotypic variance.
The phenotypic variance was calculated from the formula:
where:
σp2 = σg2 + σge2/m + σe2/rm
- σge2—variance of genotype-environmental interaction
- σe2—error variance
- m—number of environments
- r—number of replications
The assessment of the relationships between analysed traits was made on the basis of correlation coefficients for the appropriate pairs of variables (quantitative traits), determined on the basis of the mean values of DH lines for both years jointly. The results are presented in the form of a correlation matrix.
To identify transgressive lines with advantageous and significant effects for the studied traits, each DH line was compared with parent lines using the F-statistic. A given line was considered as transgressive if its value was significantly higher than the value of the better-scoring parent or significantly lower than the value of the lower-scoring parent. Positive transgressive effects were sought for fat and protein content, and negative transgressive effects were investigated for NDF, ADF, and GSL content. Moreover, each DH line with low fibre content was compared and tested by F-statistic with the Z-114 line to identify DH lines with significantly better effects than the yellow-seeded parental line in terms of other studied traits.
The studied genotypes were divided into homogeneous groups according to seed colour using the procedure of Gabriel [22]. The affiliation of genotypes to particular colour groups was described by Szała et al. [23].
To classify the studied DH lines in terms of six traits treated simultaneously, hierarchical clustering was performed using Ward’s method based on Mahanalobis distance, which was treated as a measure of multitrait phenotypic distance between genotypes.
3. Results
3.1. Statistical Characteristics of Studied Traits
A total of 44 DH lines and their parental lines H2-26 and Z-114 were studied in a field experiment over two seasons. The following characters were analysed: fat, protein, NDF, ADF, GSL content and seed colour. The results of two-factor analyses of variance for genotypes (DH lines and parental lines) and environments (years), carried out for all the investigated traits, are presented in Table 2. They indicate that both sources of variation tested in the trial, environments and genotypes, were significant (p < 0.01) for all monitored traits, apart from NDF content. With regard to NDF content, only genotype effects were significantly expressed. Furthermore, a significant genotype by environment interaction was found for fat, protein, ADF and GSL content as well as for seed colour.

Table 2.
Two-factor analysis of variance of genotypes (DH lines and parents) and environments (years) for six traits of interest.
Coefficients of variation for all traits were higher for the DH line population than for the parental lines (Table 3). Among the seed quality traits, the highest variation in the DH line population was observed for GSL content (CV = 17.3% and CV = 22.1%, in the first and second year of the experiment, respectively), and for ADF content (CV = 20.5% and CV = 18.1%, in the first and second year of the experiment, respectively). The lowest variability among the DH lines was obtained for fat content (CV = 3.3% and CV = 4.8%, in the first and second year, respectively). This latter trait also turned out to be the most constant, as its coefficients of variation for both parental lines and in both years ranged from 0.7% to 2.8%.

Table 3.
Characteristics of trait variability in the DH population and parental lines.
The broad-sense heritability coefficients were high for all six traits and ranged from 0.69 for GSL content to 0.97 for ADF content (Table 3).
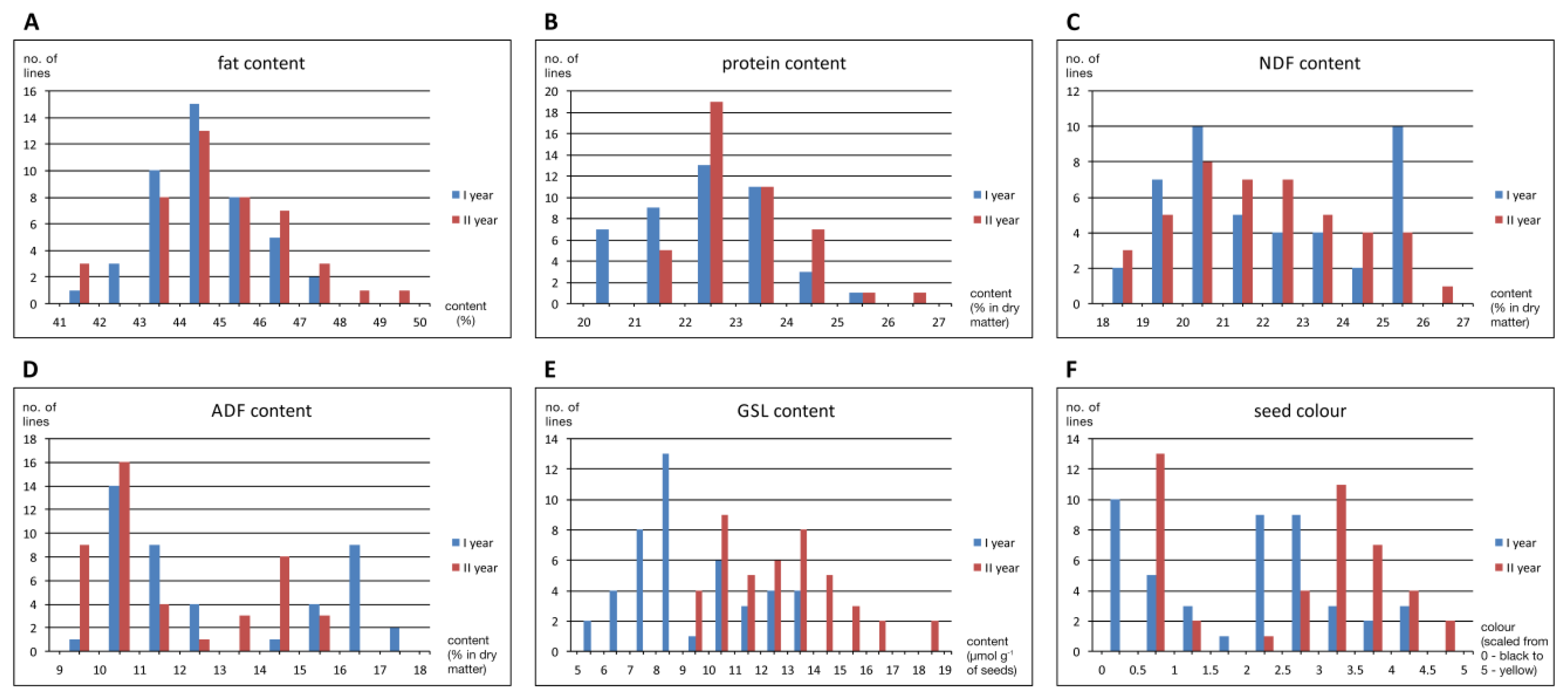
Frequency distribution of seed biochemical traits and seed colour for two years were presented as histograms in Figure 2. The studied DH population showed typical quantitative variation for fat and protein contents. The distribution of NDF and GSL contents was close to quantitative, whereas ADF content and seed colour were qualitative. The GSL content was significantly lower in the first year of the study. The protein content tended to be lower in the first year as well, but the difference was very small (less than 1 percentage point on average). In addition, in the first year of the study, seeds were slightly darker than in the second year.
Figure 2.
Frequency distribution of oilseed rape seed biochemical traits and seed colour for two years in the population of DH lines (no parental lines included). The (A) fat, (B) protein, (C), neutral detergent fibre—NDF (D) acid detergent fibre—ADF, (E) glucosinolates—GSL content, and (F) scaled seed colour values were shown.
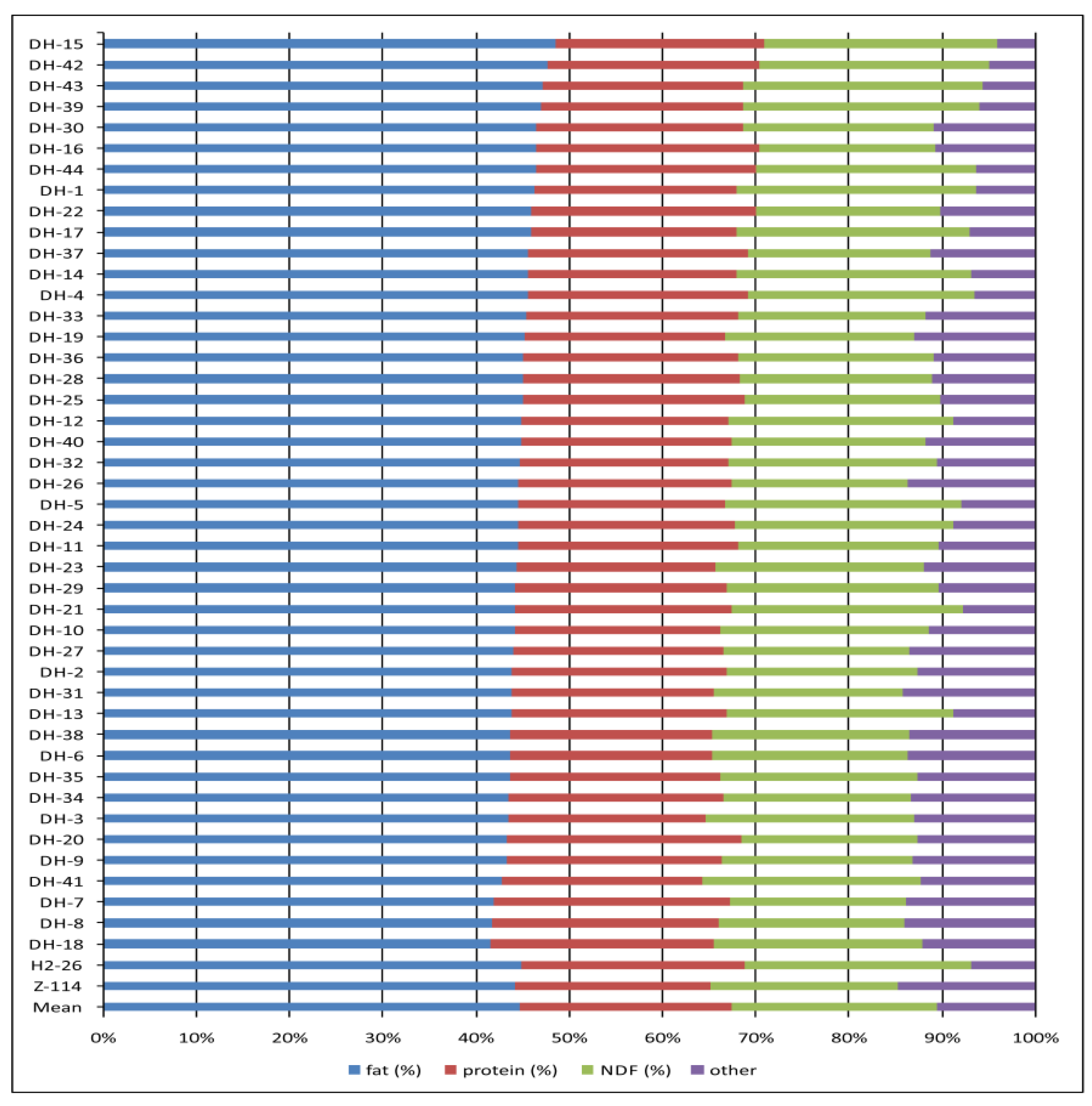
The profiles of main seed compounds in each individual DH line, based on the two-year means, were shown in Figure 3. Mean values for all monitored traits for individual genotypes and both years were presented in Supplementary Table S1.
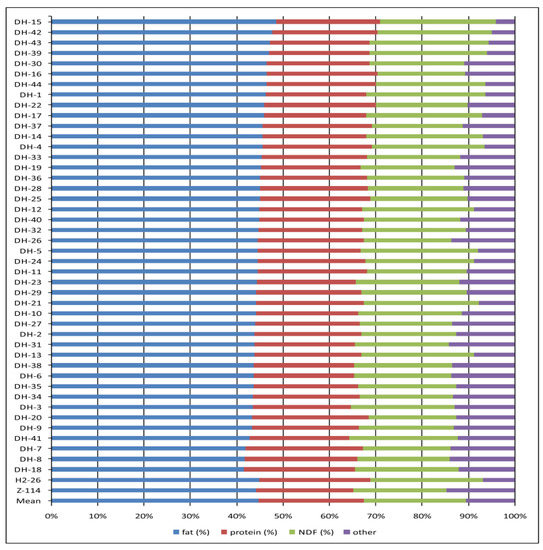
Figure 3.
The profiles of main seed compounds in each individual DH line and two parental oilseed rape lines included in the study. The population mean was also shown.
3.2. Correlation between Fat, Protein, NDF, ADF and GSL Content and Seed Colour
The results of our two-year investigation revealed a weak but statistically significant negative correlation between seed colour and content of fat (r = −0.34 **), and a strong negative correlation between seed colour and NDF (r = −0.85 **), as well as between seed colour and ADF (r = −0.91 **), which means that seeds of a lighter colour have both a lower fat content and a lower fibre content (Table 4). A weak, but significant positive correlation was noticed between fat and NDF content (r = 0.35 **), and fat and ADF content (r = 0.32 **), while protein content was negatively correlated with NDF (r = −0.33 **) and with ADF (r = −0.27 **) content. However, a significant correlation between protein and fat content was not observed (r = −0.12). This last result suggests that it should be possible to develop genotypes with simultaneously increased protein and fat content in further breeding work.

Table 4.
Correlation coefficients for the six traits in winter oilseed rape DH lines.
3.3. Transgressive Effects
DH lines were also examined for positive transgressive effects for fat and protein content and negative transgressive effects for NDF, ADF, and GSL content (Table 5). Among 44 analysed genotypes, 10 DH lines revealed positive transgressive effects for fat content, and 2 DH lines showed such effects for protein content. We found three DH lines with negative transgression for NDF content and one DH line for ADF content. However, for GSL content, no transgressive lines were detected. Among the transgressive lines in terms of fat content, six lines showed black seed colour, one line was yellow-brown and three lines were yellow seeded. Transgressive effects for protein content occurred only in yellow-seeded lines. In the DH population, three lines with yellow seeds were particularly distinctive: line DH-20, which showed favourable transgressive effects for protein, NDF and ADF content; line DH-7, with favourable transgressive effects for protein and NDF content; and line DH-16, with significantly increased fat content and significantly reduced NDF content.

Table 5.
DH lines with transgressive effects.
3.4. Selection of the Best Yellow-Seeded DH Lines
Each DH line with low fibre content, i.e., not significantly higher than the fibre content in seeds of parental line Z-114, was compared with this parental line for the studied traits (Table 6). Among 17 selected DH lines, five lines were notable: DH-16, DH-22, DH-30, DH-33 and DH-37. These lines were characterized by significantly higher fat content and protein content (p < 0.01) than the yellow-seeded parental line. Two lines, DH-16 and DH-22, were among five lines with the highest sum of fat and protein contents (70%) in the studied population (Figure 3). Moreover, line DH-16 had significantly reduced NDF content (p < 0.05) compared to parental line Z-114. These five lines also had a significantly higher GSL content than the Z-114 line, but the GSL level in seeds was still within the standard range. The lines DH-7 and DH-20 are also worth mentioning, as they had the highest protein content and significantly reduced fibre content.

Table 6.
Estimates and testing results of contrasts between chosen DH lines with low fibre content and yellow-seeded parental DH line Z-114.
3.5. Hierarchical Cluster Analysis
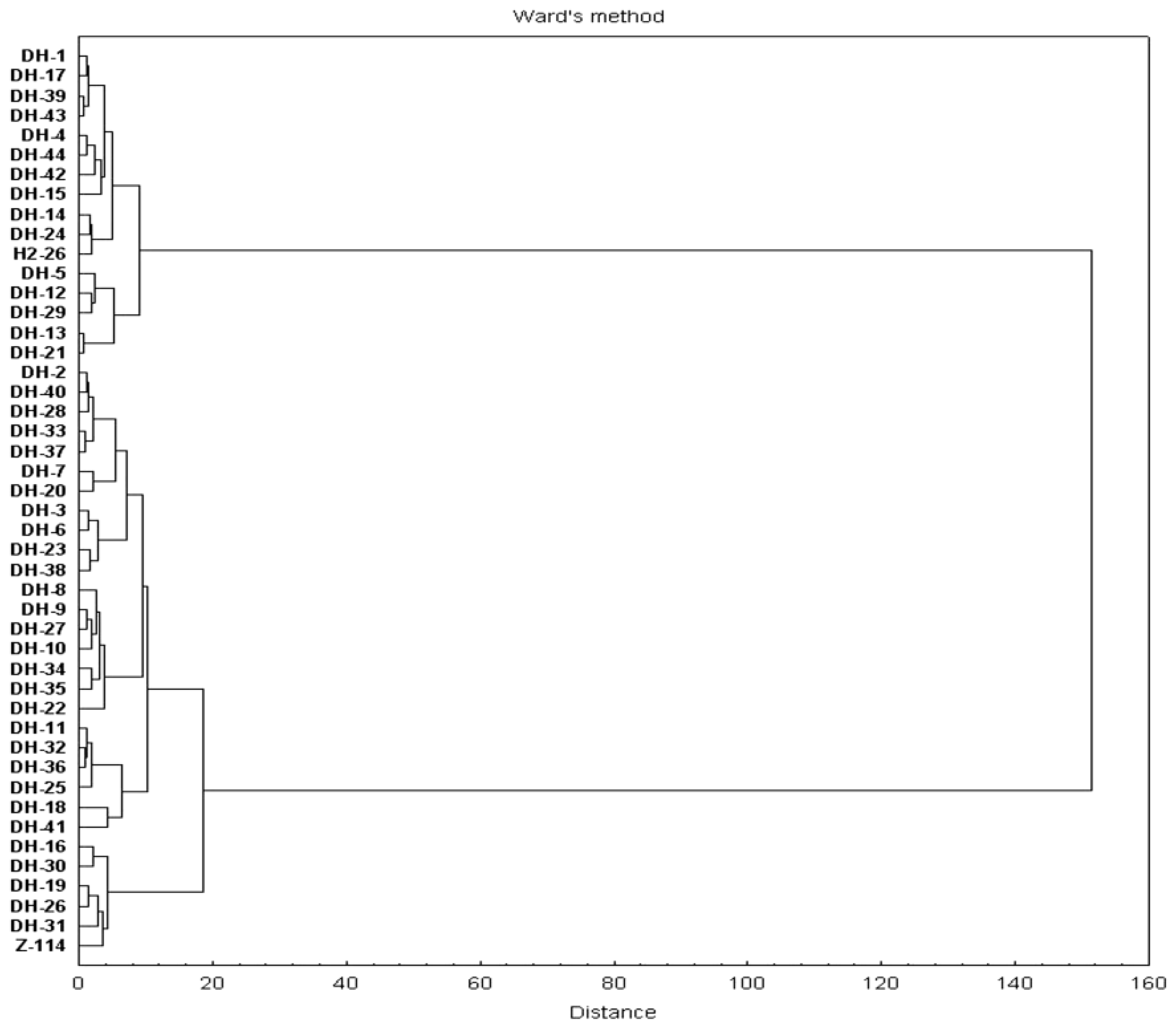
Based on the Mahalanobis distance of the DH lines, a dendrogram was created of similarities between DH lines and parental lines with respect to the quality traits and seed colour (Figure 4). The cluster analysis showed that the genotypes could be divided into two main groups: a group comprising 16 black-seeded lines and a group of 30 lines with yellow, yellow-brown and brown seed colour.
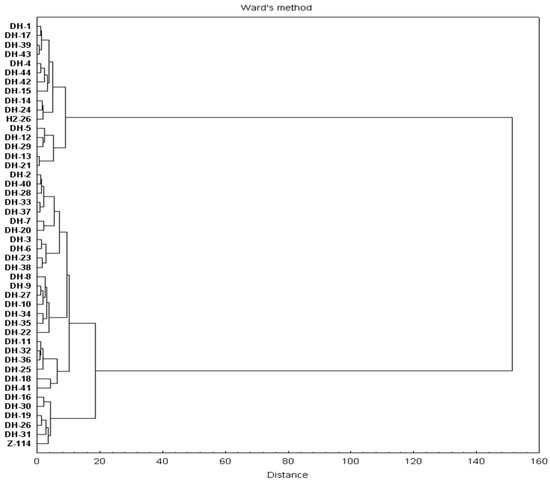
Figure 4.
Clustering dendrogram obtained on the basis of six studied traits.
4. Discussion
The development of yellow-seeded cultivars of Brassica napus is especially desirable because of their improved seed meal quality [24]. Yellow-seeded cultivars have a thinner seed coat, higher protein and lower fibre content than black-seeded cultivars, resulting in a higher fat content in the seed [7,25]. Breeding of yellow-seeded oilseed rape is one way to reduce the fibre level in oilseed rape meal, and this helps to increase its digestibility and to improve energy intake from the feed [26,27,28].
The cross between the black-seeded H2-26 line and the yellow-seeded Z-114 line aimed to produce high-yielding genotypes [23] with low fibre content, the cake of which would be a valuable animal feed. Additionally, analyses of the protein, NDF, ADF and GSL contents, as well as the assessment of seed colour, allowed the variability in these traits and the relationships between them to be determined. Seeds of all genotypes collected in the first year tended to be darker than the seeds coming from the second year, with the exception of DH-1 and DH-7 lines. It was a reaction to heavy rainfall during the last stage of seed maturation, with lower sunlight and temperature. Seed colour is not a selective trait itself, but the light seed colour that results from a very thin, transparent seed coat may be a determinant and phenotypic marker of low fibre content. Relatively high values of coefficients of variation for the content of both fractions of dietary fibre, especially ADF, show high genetic variability for these traits among DH lines. In contrast, low coefficients of variation for parental lines and high coefficients of heritability for all studied genotypes indicate a high level of genetic variation for both these traits. The substantial efforts of breeders to reduce the fibre content in oilseed rape seeds aim to increase the digestibility of feeds obtained from oilseed cake or meal. According to many authors, high fibre content in oilseed rape meal can reduce energy and protein availability in monogastric animals [7,29,30,31]. In the DH population studied here, three lines were found with a negative transgression for NDF content. One of these lines also contained significantly less ADF and significantly more protein than the best parent. It is interesting that the low-fibre DH lines identified in this study tended to have a significantly increased protein content, with no corresponding effect on fat content. The same tendency was noticed by Wittkop [32] and Liu [33], who observed the relationship between ADL (acid detergent lignine) and protein contents. According to these authors, the tendency for a higher level of protein in lines with low ADL, without a corresponding increase in fat content suggests a direct biochemical relationship between the seed coat and the biosynthesis of storage proteins in the embryo. The consequence of the low fibre content is a reduction in the content of fibre-related tannins and polyphenols, as well as the yellow colour of seeds, or rather the colour of the cotyledons visible under the transparent seed coat. The correlation coefficients between the colour of seeds and the content of NDF and ADF confirm a very strong relationship between these features, which is explained by some authors to be due to their genetic control being shared in part [34].
The protein content in seeds was strongly influenced by the environment and genotype-environment interaction, but a genetic factor also had a significant influence on this trait. The variability among DH lines was low and slightly exceeded the variability of the parental lines in both years of the experiment, and the heritability rate was relatively high. It is worth noting that the yellow-seeded Z-114 parent line has a significantly lower protein content than the black-seeded H2-26 parental line, with similar fat content in both lines. However, transgressive effects for protein content occurred only in the yellow-seeded DH lines. Similar results were obtained by Wolko et al. [35], indicating that selection for a lighter colour could lead to higher protein content in seeds.
Cultivars with different protein content can be found among black-seeded oilseed rape, although the genetic variability within this trait is small. Among the 15 cultivars of winter oilseed rape assessed by Kowalska et al. [36], the protein content in seeds ranged from 17.7 to 20.1% in the first year, 18.8 to 23.1% in the second year, and 21.2 to 23.3% in the third year, respectively. Similar results were obtained by Marjanović-Jeromela et al. [37], who assessed 30 genotypes of different geographic origins. Against this background, the variability we observed within the DH lines seems high (18.1–26.4% in the first year and 20.0–26.6% in the second year of the study). Higher variability for seed protein content was observed by Chao et al. [38] and Wolko et al. [35] in mapping populations.
The lowest coefficient of variation was found for the fat content. In the studied population of DH lines, this was mainly due to genetic variation, which was confirmed by the high value of the heritability coefficient. A high degree of heritability in two very numerous populations of DH lines was also observed by Delourme et al. [39], who conducted research on the genetic determinants of fat content in black-seeded oilseed rape. However, the generated variability in the analysed population turned out to be sufficient to find 10 segregants with positive transgressive effects based on two years of experience. Furthermore, although fat content was positively correlated with dark seed colour, three transgressive lines had a light seed colour. The positive significant correlation between dark seed colour and seed fat content did not confirm many reports of increased fat content in yellow-seeded oilseed rapes [7,25,40]. Many reports also indicate the additive effect of genes as the main factor controlling the fat content in seeds [39,41,42,43,44]. According to Zhao et al. [45], crossing appropriately selected varieties and the derivation of DH lines allows the breeding of genotypes with a higher fat content than the parents by combining the positive alleles of genes derived from different gene pools. Nevertheless, Wang et al. [46] believe that the inheritance of seed fat content is explained by a more complex model, where additive and dominant effects play major roles, but there is also a small epistatic effect.
The correlation between protein and fat content in our sample turned out to be negative but insignificant, which contrasts with many reports of a very strong negative correlation between these features [38,41,47,48]. This indicates the possibility of finding desirable “correlation breakers” for these traits. Progress in breeding is largely dependent on breaking unfavorable linkage between genes by recombination through appropriate selection of parental components.
GSL content turned out to be the most variable of the biochemical traits. The coefficients of variation for genetically stable parental forms were high, and there were significant differences in the content of these compounds in both years of the study. Of all the quality traits, GSL content was characterized by the lowest heritability. Apart from the genetic factors, the content of GSL is determined by the availability of sulfur in soil and moisture conditions. Under conditions of rainfall deficiency, sulfur content increases significantly [49]. Jensen et al. [50] indicated that a higher increase of GSL content is due to the drought conditions in the period of vegetative development than during the pod development. Low rainfall in April in the second year of the study might result in a higher GSL content in the seeds. The critical importance of rainfall in April was pointed out by Wójtowicz [51], who found that the content of GSL increased under low rainfall conditions before and during the initial flowering period. For GSL content, no line with negative transgressive effects was found. This is not favorable, because alkene GSLs, especially progoitrin, are anti-nutritional substances and their levels in seeds need to be reduced to further improve the biological value of the meal. On the other hand, breeding for reduced seed GSL content could have a negative impact on seed yield and resistance to pests and diseases [52].
The study of this population was previously described in terms of yield and its components [23]. Four yellow-seeded lines gave significantly higher yields than the parental line Z-114, including the DH-16 and DH-30 lines, which were characterized by low fibre content and increased fat and protein content.
Breeding programmes for many crop species involve simultaneous improvement of a number of traits, usually taking into account both productivity and yield quality [24,53,54]. The plant materials are evaluated for multiple traits from the initial stages of breeding. Cluster analysis on the basis of a multidimensional evaluation of genotypes enables them to be comprehensively assessed for practical use in plant breeding. However, a multi-trait evaluation of our DH lines for seed quality and colour simultaneously did not identify the best genotypes. Seed colour had the most discriminating power to distinguish cluster groups, which means that the other traits had much less variation.
5. Conclusions
Crossing two DH lines of different origin, line H2-26 and line Z-114, followed by in vitro androgenesis, resulted in a population of DH lines characterized by a significant range of variability. Although the described population was relatively small, the range of variability of the obtained DH lines exceeded the range of variability of the parental forms. The calculated coefficients of variation for the traits studied in the DH population took into account the overall variability, while the coefficients of variation of the parental lines were indicative of non-heritable variability. Our data show that by choosing appropriate parental lines it is possible to break the negative correlation between protein content and fat content in seeds.
The high level of heritability of traits relating to seed quality suggests the possibility of improving breeding materials through effective selection based on phenotype. It seems that the selected DH-16 and DH-30 lines can be the starting material for breeding cultivars with reduced fibre content and increased fat and protein content.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12030340/s1. Table S1: Mean values of monitored traits of 44 DH lines and parental lines for individual years and both years together.
Author Contributions
Conceptualization, L.S.; methodology, Z.K.; software, Z.K.; validation, L.S. and T.C.-T.; formal analysis, Z.K.; investigation, L.S.; resources, T.C.-T.; data curation, L.S. and M.M.; writing—original draft preparation, L.S.; writing—review and editing, L.S., Z.K., M.M. and T.C.-T.; visualization, M.M.; supervision, T.C.-T.; project administration, T.C.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yonsel, S. Extraction of Protein Mixture from Rapeseed for Food Applications. Msc Thesis, Biobased Chemistry and Technology. Wageningen University & Research, Wageningen, The Netherlands, 2018. Available online: https://edepot.wur.nl/444991 (accessed on 15 March 2018).
- Beszterda, M.; Nogala-Kałucka, M. Current research developments on the processing and improvement of the nutritional quality of rapeseed (Brassica napus L.). Eur. J. Lipid Sci. Technol. 2019, 121, 1800045. [Google Scholar] [CrossRef]
- Aider, M.; Barbana, C. Canola proteins: Composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity—A practical and critical review. Trends Food Sci. Technol. 2011, 22, 21–39. [Google Scholar] [CrossRef]
- Prosky, L. What is fibre? Current controversies. Trends Food Sci. Technol. 1999, 10, 271–275. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and Biochemistry of Seed Flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Rahman, M.; Mc Vetty, P.B.E. A review of Brassica seed color. Can. J. Plant Sci. 2011, 91, 437–446. [Google Scholar] [CrossRef]
- Lin, A.; Ma, J.; Xu, F.; Xu, W.; Jiang, H.; Zhang, H.; Qu, C.; Wei, L.; Li, J. Differences in Alternative Splicing between Yellow and Black-Seeded Rapeseed. Plants 2020, 9, 977. [Google Scholar] [CrossRef]
- Forster, B.P.; Thomas, W.T.B. Doubled haploids in genetics and plant breeding. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 57–88. [Google Scholar]
- Germana, M.A. Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Rep. 2011, 30, 839–857. [Google Scholar] [CrossRef]
- Cegielska-Taras, T.; Szała, L.; Matuszczak, M.; Babula-Skowrońska, D.; Mikołajczyk, K.; Popławska, W.; Sosnowska, K.; Hernacki, B.; Olejnik, A.; Bartkowiak-Broda, I. Doubled haploid as a material for biotechnological manipulation and a modern tool for breeding of oilseed rape (Brassica napus L.). BioTechnologia 2015, 96, 171–177. [Google Scholar] [CrossRef]
- Möllers, C.; Iqbal, M.C.M. Doubled haploids in breeding of winter oilseed rape. In Advances in Haploid Production in Higher Plants; Touraev, A., Foster, B.P., Jain, S.M., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2009; pp. 161–169. [Google Scholar]
- Ferrie, A.M.R.; Caswell, K.L. Application of doubled haploidy for improving industrial oilseed. In Industrial Oil Crops; McKeon, T.A., Hayes, D.G., Hildebrand, D.F., Weselake, R.J., Eds.; Elsevier Inc.: London, UK, 2016; pp. 359–378. [Google Scholar]
- Cegielska-Taras, T.; Tykarska, T.; Szała, L.; Kuraś, L.; Krzymański, J. Direct plant development from microspore-derived embryos of winter oilseed rape Brassica napus L. ssp. oleifera (DC.) Metzger. Euphytica 2002, 124, 341–347. [Google Scholar] [CrossRef]
- Szała, L.; Sosnowska, K.; Cegielska-Taras, T. Induced chromosome doubling in microspores and regenerated haploid plants of Brassica napus. Acta Biol. Cracov. Bot. 2020, 62, 23–31. [Google Scholar] [CrossRef]
- Bartkowiak-Broda, I.; Piotrowska, A.; Hernacki, B.; Cegielska-Taras, T.; Michalski, K. Development of yellow seeded winter oilseed rape (Brassica napus L. var. oleifera). In Proceedings of the Technical Meeting, Manesar, India, 2–4 February 2009; GCIRC Bulletin 2011. Volume 26. [Google Scholar]
- Michalski, K.; Kołodziej, K.; Krzymański, J. Quantative analysis of glucosinolates in seeds of oilseed rape—Effect of sample preparation on analytical results. In Proceedings of the 9th International Rapeseed Congress, Cambridge, UK, 4–7 July 1995; Volume 3, pp. 911–913. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Morrison, D.F. Multivariate Statistical Methods; McGraw-Hill Kogakusha Ltd.: Tokyo, Japan, 1976. [Google Scholar]
- Nyquist, W.E. Estimation of heritability and prediction of selection response in plant populations. Crit. Rev. Plant Sci. 1991, 10, 235–322. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. An Introduction to Quantitative Genetics, 4th ed.; Longmans Green: Harlow, UK, 1996. [Google Scholar]
- Gabriel, K.R. Procedure for testing the homogeneity of all sets of means in analysis of variance. Biometrics 1964, 20, 459–477. [Google Scholar] [CrossRef]
- Szała, L.; Kaczmarek, Z.; Wójtowicz, M.; Sosnowska, K.; Cegielska-Taras, T. Genetic variability in oilseed rape DH line population developed from F1 hybrids obtained by crossing black- and yellow-seeded DH lines. I. Yield and yield components. Euphytica 2021, 217, 99. [Google Scholar] [CrossRef]
- Rahman, M.H.; Joersbo, M.; Poulsen, M.H. Development of yellow-seeded Brassica napus of double low quality. Plant Breed. 2001, 120, 473–478. [Google Scholar] [CrossRef]
- Kaczmarek, P.; Korniewicz, D.; Lipiński, K.; Mazur, M. Chemical composition of rapeseed products and their use in pig nutrition. Pol. J. Nat. Sci. 2016, 31, 545–562. [Google Scholar]
- Simbaya, J.; Slominski, B.A.; Rakow, G.; Campbell, L.D.; Downey, R.K.; Bell, J.M. Quality characteristics of yellow-seeded Brassica seed meals: Protein, carbohydrates, and dietary fiber components. J. Agric. Food Chem. 1995, 43, 2062–2066. [Google Scholar] [CrossRef]
- Slominski, B.A. Developments in the breeding of low fibre rapeseed/canola. J. Anim. Feed Sci. 1997, 6, 303–317. [Google Scholar] [CrossRef][Green Version]
- Slominski, B.A.; Simbaya, J.; Campbell, L.D.; Rakow, G.; Guenter, W. Nutritive value for broilers of meals derived from newly developed varieties of yellow-seeded canola. Anim. Feed Sci. Technol. 1999, 78, 249–262. [Google Scholar] [CrossRef]
- Bell, J.M. Factors affecting the nutritional value of canola meal: A review. Can. J. Anim. Sci. 1993, 73, 679–697. [Google Scholar] [CrossRef]
- Khajali, F.; Slominski, B.A. Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Mejicanos, G.; Sanjayan, N.; Kim, I.H.; Nyachoti, C.M. Recent advances in canola meal utilization in swine nutrition. J. Anim. Sci. Technol. 2016, 58, 7. [Google Scholar] [CrossRef]
- Wittkop, B.; Snowdon, R.J.; Friedt, W. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica 2009, 170, 131. [Google Scholar] [CrossRef]
- Liu, L.; Stein, A.; Wittkop, B.; Sarvari, P.; Li, J.; Yan, X.; Dreyer, F.; Frauen, M.; Friedt, W.; Snowdon, R.J. A knockout mutation in the lignin biosynthesis gene CCR1 explains a major QTL for acid detergent lignin content in Brassica napus seeds. Theor. Appl. Genet. 2012, 124, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Badani, A.G.; Snowdon, R.J.; Wittkop, B.; Lipsa, F.D.; Baetzel, R.; Horn, R.; De Haro, A.; Font, R.; Lühs, W.; Friedt, W. Colocalization a partially dominant B. napus gene for seed colour with a major QTL influencing acid detergent fibre (ADF) content in different crosses of oilseed rape (Brassica napus). Genome 2006, 49, 1499–1509. [Google Scholar] [CrossRef]
- Wolko, J.; Dobrzycka, A.; Bocianowski, J.; Szala, L.; Cegielska-Taras, T.; Bartkowiak-Broda, I.; Gacek, K. Genetic variation of traits affecting meal quality in black × yellow seeded doubled haploid population of winter oilseed rape. Agron. Res. 2020, 18, 2259–2270. [Google Scholar] [CrossRef]
- Kowalska, G.; Kowalski, R.; Hawlena, J.; Rowiński, R. Seed of oilseed rape as aa alternative source of protein and minerals. J. Elem. 2020, 25, 515–522. [Google Scholar] [CrossRef]
- Marjanović-Jeromela, A.; Kondić-Spika, A.; Saftić-Panković, D.; Marinković, R.; Hristov, N. Phenotypic and molecular evaluation of genetic diversity of rapeseed (Brassica napus L.) genotypes. Afr. J. Biotechnol. 2009, 8, 4835–4844. [Google Scholar]
- Chao, H.; Wang, H.; Wang, X.; Guo, L.; Gu, J.; Zhao, W.; Li, B.; Chen, D.; Raboanatahiry, N.; Li, M. Genetic dissection of seed oil and protein content and identification of networks associated with oil content in Brassica napus. Sci. Rep. 2017, 7, 46295. [Google Scholar] [CrossRef]
- Delourme, R.; Falentin, C.; Huteau, V.; Clouet, V.; Horvais, R.; Gandon, B.; Specel, S.; Hanneton, L.; Dheu, J.E.; Deschaps, M.; et al. Genetic control of oil content in oilseed rape (Brassica napus L.). Theor. Appl. Genet. 2006, 113, 1331–1345. [Google Scholar] [CrossRef]
- Rakow, G.; Relf-Eckstein, J.A.; Olson, T. Review and update on the development of yellow seed Brassica napus canola. In Proceedings of the 13th International Rapeseed Congress, Prague, Czech Republic, 5–9 June 2011; p. 55. [Google Scholar]
- Grami, B.; Stefansson, B.R. Gene action for protein and oil content in summer rape. Can. J. Plant Sci. 1977, 57, 625–631. [Google Scholar] [CrossRef]
- Engqvist, G.M.; Becker, H.C. Relative importance of genetic parameters for selecting between oilseed rape crosses. Hereditas 1991, 115, 25–30. [Google Scholar] [CrossRef]
- Nesi, N.; Delourme, R.; Brégeon, M.; Falentin, C.; Renard, M. Genetic and molecular approaches to improve nutritional value of Brassica napus L. seed. Comptes Rendus Biol. 2008, 331, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xia, S.; Zeng, X.; Gu, J.; Yang, Y.; Xu, J.; Liu, C.; Liu, K.; Wu, J. Identification of quantitative trait loci associated with oil content and development of near isogenic lines for stable qOC-A10 in Brassica napus L. Can. J. Plant Sci. 2016, 96, 423–432. [Google Scholar] [CrossRef]
- Zhao, J.; Becker, H.C.; Zhang, D.; Zhang, Y.; Ecke, W. Oil content in a European × Chinese rapeseed population: QTL with additive and epistatic effects and their genotype-environment interactions. Crop Sci. 2005, 45, 51–59. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Yang, Q.; Hua, W.; Liu, J.; Wang, H. Genetic analysis on oil content in rapeseed (Brassica napus L). Euphytica 2010, 173, 17–24. [Google Scholar] [CrossRef]
- Zhao, J.; Becker, H.C.; Zhang, D.; Zhang, Y.; Ecke, W. Conditional QTL mapping of oil content in rapeseed with respect to protein content and traits related to plant development and grain yield. Theor. Appl. Genet. 2006, 113, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, Y.; Yokoi, S.; Sato, T.; Daimon, H.; Nishida, I.; Takahata, Y. Genetic variation of storage compounds and seed weight in rapeseed (Brassica napus L.) germplasms. Breed. Sci. 2011, 61, 311–315. [Google Scholar] [CrossRef]
- Bouchereau, A.; Clossais-Besnard, N.; Bensaoud, A.; Leport, L.; Renard, M. Water stress effects on rapeseed quality. Eur. J. Agron. 1996, 5, 19–30. [Google Scholar] [CrossRef]
- Jensen, C.R.; Mogensen, V.O.; Mortensen, G.; Fieldsend, J.K.; Milford GF, J.; Andersen, M.N.; Thage, J.H. Seed glucosinonate, oil and protein contents of field-grown rape (Brassica napus L.) affected by soildrying and evaporative demand. Field Crop Res. 1996, 47, 93–105. [Google Scholar] [CrossRef]
- Wójtowicz, M. Effect of Environmental and Agronomical Factors on Quantity and Quality of Yield of Winter Oilseed Rape (Brassica napus L.); Plant Breeding and Acclimatization Institute—National Research Institute, Monographs and Dissertations: Radzików, Poland, 2013. No 45. (In Polish) [Google Scholar]
- Velasco, P.; Rodríguez, V.M.; Francisco, M.; Cartea, M.E.; Soengas, P. Genetics and Breeding of Brassica Crops. In Glucosinolates; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Szała, L.; Cegielska-Taras, T.; Adamska, E.; Kaczmarek, Z. Assessment of genetic effects on important breeding traits in reciprocal DH populations of winter oilseed rape (Brassica napus L.). J. Integr. Agric. 2018, 17, 76–85. [Google Scholar] [CrossRef]
- Bocianowski, J.; Nowosad, K.; Bujak, H.; Luczkiewicz, T.; Piesik, D. Evaluation of the breeding value of the spring oilseed rape (Brassica napus L.) inbred lines based on a multi-trait analysis. Indian J. Genet. Plant Breed. 2016, 76, 284–289. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).