Impact of Zeolite from Coal Fly Ash on Soil Hydrophysical Properties and Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Zeolite Characterization
2.2. Hydrophysical Characterization of the Soil–Zeolite Mixtures
2.3. Plant Growth Experiment
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil and Synthesized Zeolites
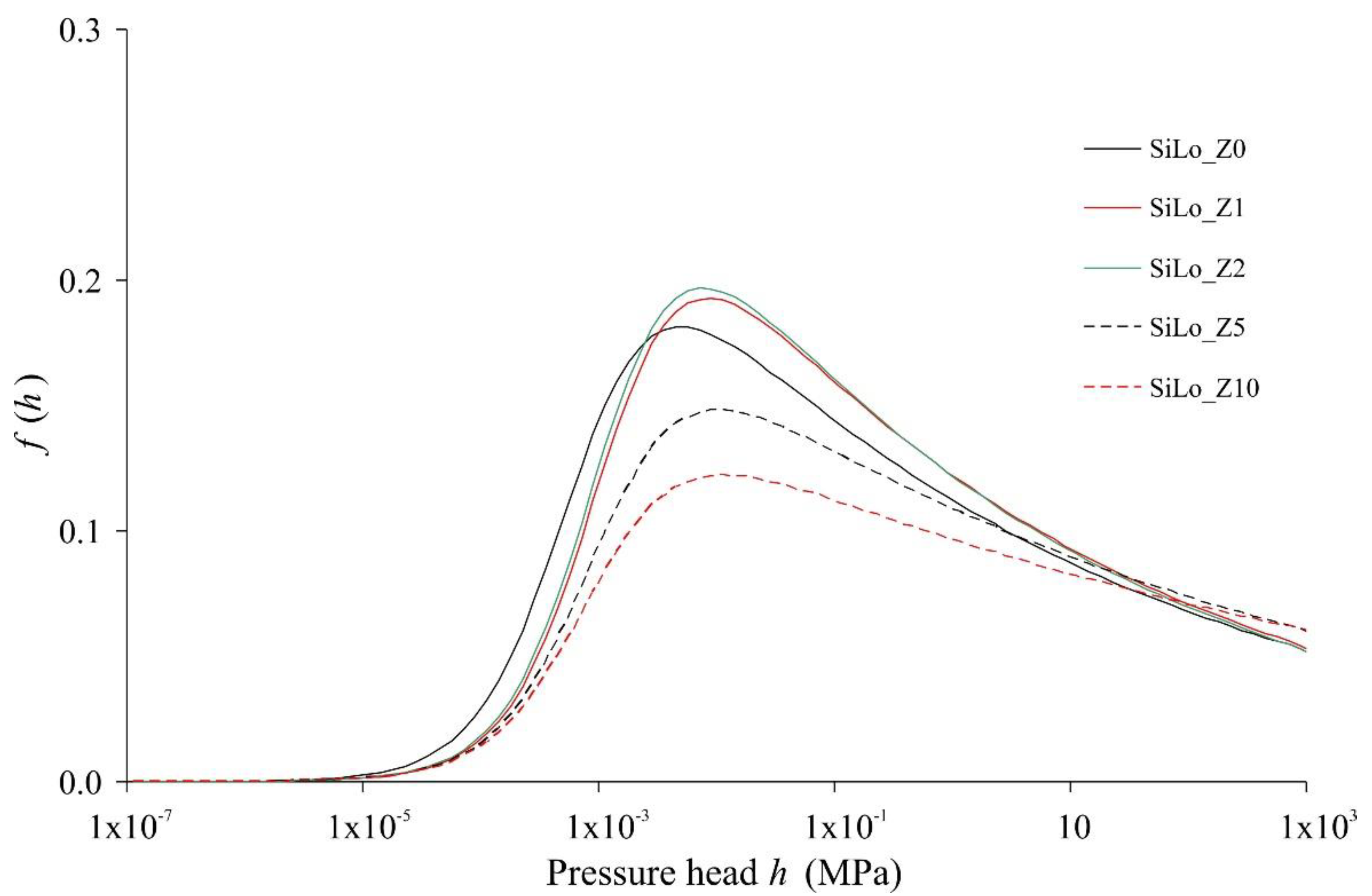
3.2. Effects of Zeolite on Hydrophysical Properties of Soil
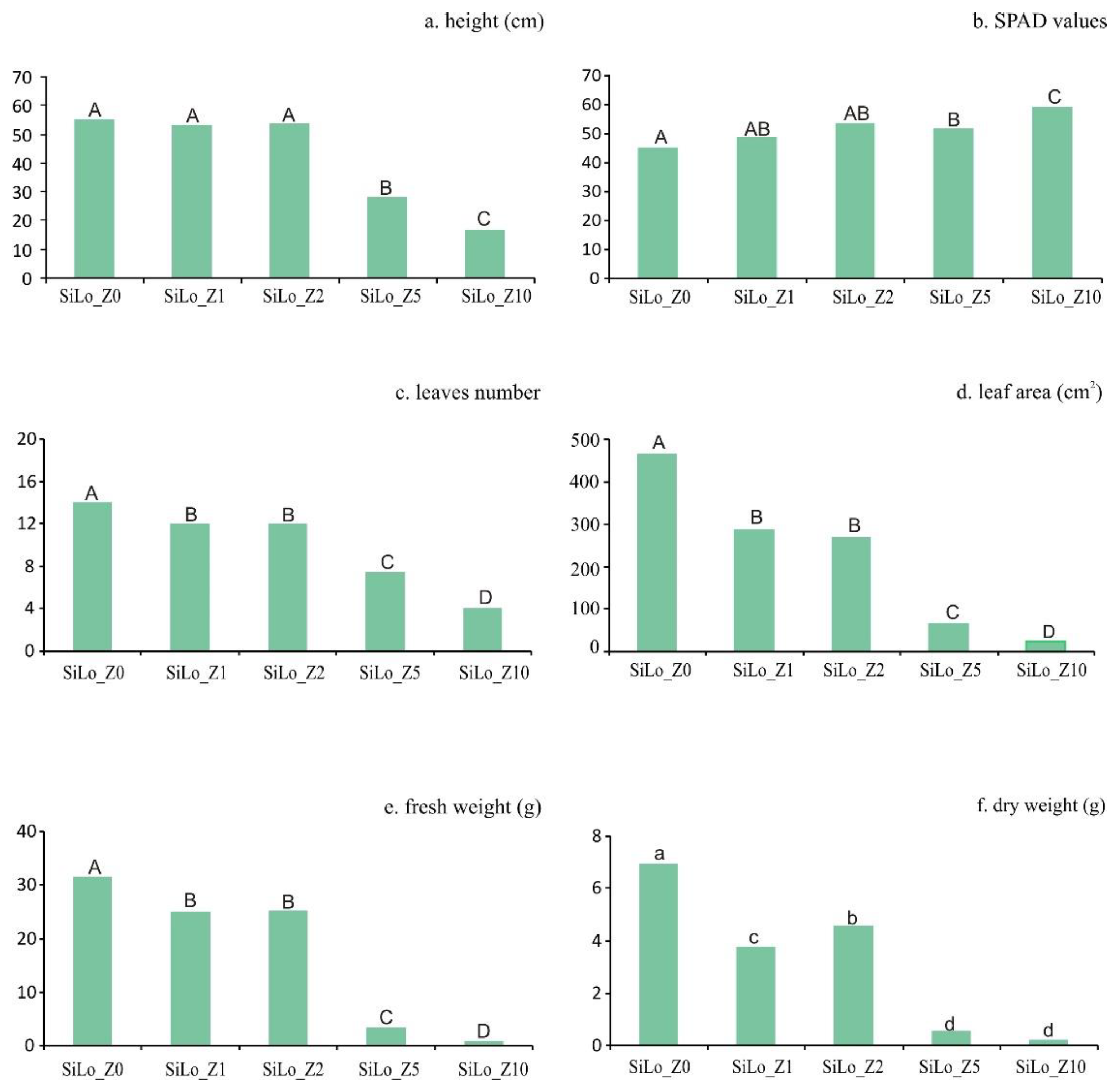
3.3. Effects of Zeolite on Sunflower Growth
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.B.; Zhanbin, H. Zeolite application for enhancing water infiltration and retention in loess soil. Resour. Conserv. Recycl. 2001, 34, 45–52. [Google Scholar]
- Wang, Y.; Li, S.; Quin, S.; Hui, G.; Young, D.; Lam, H.M. How can drip irrigation save water and reduce evapotranspiration compared to border irrigation in arid regions in northwest China. Agr. Water Manag. 2001, 239, 1062560. [Google Scholar] [CrossRef]
- Paknejad, F.; Nasri, M.; Moghadam, H.R.T.; Zahedi, H.; Alahmadi, M.J. Effects of drought stress on chlorophyll fluorescence parameters, chlorophyll content and grain yield of wheat cultivars. J. Biol. Sci 2007, 7, 841–847. [Google Scholar] [CrossRef]
- Juri, W.A.; Vaux, H., Jr. The role of sciencein solving the world’s emerging water problems. Proc. Natl. Acad. Sci. USA 2005, 102, 15715–15720. [Google Scholar] [CrossRef] [Green Version]
- Krumm, M.; Guillaume, J.H.A.; De Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar]
- Gerveni, M.; Avelino, A.F.T.; Dall’erba, S. Drivers of Water Use in the Agricultural Sector of the European Union 27. Environ. Sci. Technol. 2020, 54, 9191–9199. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Mahmood, K.; Ashraf, I.; Siddiqui, M.T.; Knox, J.W. Evaluating socio-economic and environmental factors influencing farm-level water scarcity in Punjab, Pakistan. Irrig. Drain 2020, 70, 797–808. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of cellulose-based superabsorbent hydrogels as water reservoir in agriculture. Int. J. Polym. Sci. 2013, 12, 435073. [Google Scholar] [CrossRef]
- Cannazza, G.; Cataldo, A.; De Benedetto, E.; Demitri, C.; Madaghiele, M.; Sannino, A. Experimental assessment of the use of a novel superabsorbent polymer (SAP) for the optimization of water consumption in agricultural irrigation process. Water 2014, 6, 2056–2069. [Google Scholar] [CrossRef] [Green Version]
- Satriani, A.; Catalano, M.; Scalcione, E. The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: A case-study in Southern Italy. Agric. Water Manag. 2018, 195, 114–119. [Google Scholar] [CrossRef]
- Ai, F.; Yin, X.; Hu, R.; Ma, H.; Liu, W. Research into the super-absorbent polymers on agricultural water. Agric. Water Manag. 2021, 245, 106513. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Alkhasha, A.; Ibrahim, H.M. Effect of biochar particle size on water retention and availability in a sandy loam soil. J. Saudi Chem. Soc. 2020, 24, 1042–1050. [Google Scholar] [CrossRef]
- de Campos Bernardi, A.C.; Peronti Anchao Oliviera, P.; de Melo Monte, M.B.; Souza-Barros, F. Brazilian sedimentary zeolite use in agriculture. Micropor. Mesopor. Mat. 2013, 167, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Ni by synthesising zeolite at low temperatures in a polluted soil. Chemosphere 2010, 78, 1172–1176. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Zn and Pb in polluted soil by in-situ crystallization zeolites from fly ash. Water Air Soil Pollut. 2012, 223, 5357–5364. [Google Scholar] [CrossRef]
- Matthew, D.O.; Soltis, J.A.; Marlon, T.C.; Lee Penn, R.; Rimer, J.D. Nucleation of FAU and LTA zeolites from heterogeneous aluminosilicate precursors. Chem. Mater. 2016, 28, 4906–4916. [Google Scholar]
- Belviso, C. EMT-type zeolite synthesized from obsidian. Micropor. Mesopor. Mat. 2016, 226, 325–330. [Google Scholar] [CrossRef]
- Belviso, C. Ultrasonic vs hydrothermal method: Different approaches to convert fly ash into zeolite. How they affect the stability of synthetic products over time? Ultrason. Sonochem. 2018, 43, 9–14. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Tan, K.-H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice husk. Micropor. Mesopor. Mat. 2015, 204, 2014–2209. [Google Scholar] [CrossRef]
- Belviso, S.; Cavalcante, F.; Lettino, A.; Ragone, P.; Belviso, C. Fly ash as raw material for the synthesis of zeolite-encapsulated porphyrazine and metallo porphyrazine tetrapyrrolic macrocycles. Micropor. Mesopor. Mat. 2016, 236, 228–234. [Google Scholar] [CrossRef]
- Shahsavari, N. Effects of Zeolite and Zinc on Quality of Canola (Brassica napus L.) Under Late Season Drought Stress. Commun. Soil Sci. Plant Anal. 2019, 50, 1117–1122. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, faujasite and A-type zeolite from 2:1dioctahedral and 2:1:1 trioctahedral clay minerals. A singular review of synthesis methods through laboratory trials at a low incubation temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Barzegar, M. Yield, water and nitrogen-use response of rice to zeolite and nitrogen fertilization in a semi-arid environment. Agric. Water Manag. 2010, 98, 38–44. [Google Scholar] [CrossRef]
- Najafinezhad, H.; Tahmasebi-Sarvestani, Z.; Modarres Sanavy, S.A.M.; Naghavi, H. Evaluation of yield and some physiological changes in corn and sorghum under irrigation regimes and application of barley residue, zeolite and superabsorbent polymer. Arch. Agron. Soil Sci. 2014, 61, 891–906. [Google Scholar] [CrossRef]
- Ippolito, A.J.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite soil application method affects inorganic nitrogen, moisture, and corn growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Yamamoto, T.; Tanigawa, T.; Rahman, H.A. Use of zeolite to alleviate water stress on subsurface drip irrigated barley under hot environments. Irrig. Drain. 2011, 60, 473–480. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavya, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Amin, M.S.M.; Anuar, A.R.; Saberioon, M.M. Water stress and natural zeolite impacts on phisiomorphological characteristics of moldavian balm (Dracocephalum moldavica L.). Aust. J. Basic Appl. Sci. 2010, 4, 5184–5190. [Google Scholar]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut 2017, 228, 464–497. [Google Scholar] [CrossRef]
- Szerement, J.; Ambrożewicz-Nita, A.; Kędziora, K.; Piasek, J. Use of zeolite in agriculture and environmental protection. A short review. Вісник Націoнальнoгo Університету 2014, 781, 172–177. [Google Scholar]
- Ramesh, K.; Reddy, D.D. Chapter four—Zeolites and their potential uses in agriculture. Adv. Agron. 2011, 113, 219–241. [Google Scholar]
- Chmielewska, E. Designing clinoptiloliterich tuff columns for adsorptive filtration of water with enhanced ammonium concentration. Fresenius Environ. Bull. 2014, 24, 1277–1283. [Google Scholar]
- Bernardi, A.D.C.; Mendonça, F.; Haim, P.; Werneck, C.; Monte, M.D.M. Water availability and rice yield due to levels of zeolitic concentrate. Irriga 2009, 14, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Demir, A.; Gunay, A.; Debik, E. Ammonium removal from aqueous solution by ion exchange using packed bed natural zeolite. Water SA 2002, 28, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Piñón-Villarreal, A.R.; Bawazir, A.S.; Shukla, M.K.; Hanson, A.T. Retention and transport of nitrate and ammonium in loamy sand amended with clinoptilolite zeolite. J. Irrig. Drain. Eng. 2013, 139, 755–765. [Google Scholar] [CrossRef]
- Zwingmann, N.; Singh, B.; Mackinnon, I.D.; Gilkes, R.J. Zeolite from alkali modified kaolin increases NH 4 retention by sandy soil: Column experiments. Appl. Clay Sci. 2009, 46, 7–12. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, T.; Wu, Q.; Yu, J.; Chen, W.; Che, Y.; Siddique, K.H.M.; Meng, W.; Chi, D.; Xia, G. Effect of zeolite application on phenology, grain yield and grain quality in rice under water stress. Agric. Water Manag. 2018, 206, 241–251. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Naci Onus, A. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Ozbahce, A.; Tari, A.F.; Gonulal, E.; Simsekli, N. Zeolite for Enhancing Yield and Quality of Potatoes Cultivated Under Water-Deficit Conditions. Potato Res. 2018, 61, 247–259. [Google Scholar]
- Bahador, M.; Tadayon, M.R. Investigating of zeolite role in modifying the effect of drought stress in hemp: Antioxidant enzymes and oil content. Ind. Crop. Prod. 2020, 144, 112042. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Progr. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Ayan, S.; Yahyaoglu, Z.; Gercek, V.; Sahin, A. Utilization of zeolite as a substrate for containerised oriental spruce (Picea orientalis L.) seedlings propagation. Int. Symp. Grow. Media 2008, 779, 583–890. [Google Scholar]
- Flores, C.G.; Schneider, H.; Marcilio, N.R.; Ferret, L.; Oliveira, J.C.P. Potassic zeolites from Brazilian coal ash for use as a fertilizer in agriculture. Waste Manag. 2017, 70, 263–271. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Official Gazette of the Italian Republic, General Series n° 248, 1999, Ordinary Supplement n° 12. Available online: www.ecochimicasas.it/web/data/uploads/files/d.m.-13_set_99-metodi-di-analisi-chimica-del-suolo.pdf (accessed on 10 January 2022).
- ASTM D422-63; Standard Test Method for Particle-Size Analysis of Soils. ASTM International: West Conshohocken, PA, USA, 2007.
- Belviso, C.; Cavalcante, F.; Fiore, S. Synthesis of zeolite from Italian coal fly ash. Differences in crystallization temperature using seawater instead of distilled water. Waste Manag. 2010, 30, 839–847. [Google Scholar] [CrossRef]
- Sun, H.; Wu, D.; Guo, X.; Navrotsky, A. Energetic and structural evolution of Na-Ca exchanged zeolite A during heating. Phys. Chem. Chem. Phys 2015, 17, 9241–9247. [Google Scholar] [CrossRef]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity. Laboratory methods. In Methods of Soil Analysis—Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 687–734. [Google Scholar] [CrossRef]
- Stackman, W.P.; Valk, G.A.; van der Harst, G.G. Determination of Soil Moisture Retention Curves: I. Sand Box Apparatus; Range, P.F., Ed.; ICW: Wageningen, The Netherlands, 1969; p. 119. [Google Scholar]
- van Genuchten, M.T.H. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- van Genuchten, M.T.H.; Leij, F.T.; Yates, S.R. The RETC Code for Quantifying the Hydraulic Functions of Unsaturated Soils; Rep. EPA/600/2-91/065; U.S. Environmental Protection Agency: Ada, OK, USA, 1991.
- Comegna, A.; Dragonetti, G.; Kodesova, R.; Coppola, A. Impact of olive mill wastewater (OMW) on the soil hydraulic and solute transport properties. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Durner, W. Hydraulic conductivity estimation for soils with heterogeneous pore structure. Water Resour. Res. 1994, 30, 211–223. [Google Scholar] [CrossRef]
- Jensen, K.L.; Watts, C.W.; Christensen, B.T.; Munkholm, L.J. Soil Water Retention: Uni-Modal Models of Pore-Size Distribution Neglect Impacts of Soil Management. Soil Sci. Soc. Am. J. 2019, 83, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, K. Three-parameter lognormal distribution model for soil water retention. Water Resour. Res. 1994, 30, 891–901. [Google Scholar] [CrossRef]
- Nimmo, J.R. Porosity and Pore Size Distribution. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: London, UK, 2004; pp. 295–303. [Google Scholar]
- Nimmo, J.R. Porosity and Pore Size Distribution. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Yasuda, H.; Takuma, K.; Fukuda, T. Water retention changes of dune sand due to zeolite addition. J. Agric. Meteorol. 1997, 52, 641–644. [Google Scholar] [CrossRef]
- Skopp, J.; Gardner, W.R.; Tyler, E.J. Solute movement in structure soils: Two region model with small interaction. Soil Sci. Soc. Am. J. 1981, 5, 461–472. [Google Scholar]
- Akhter, J.; Mahmood, K.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef] [Green Version]
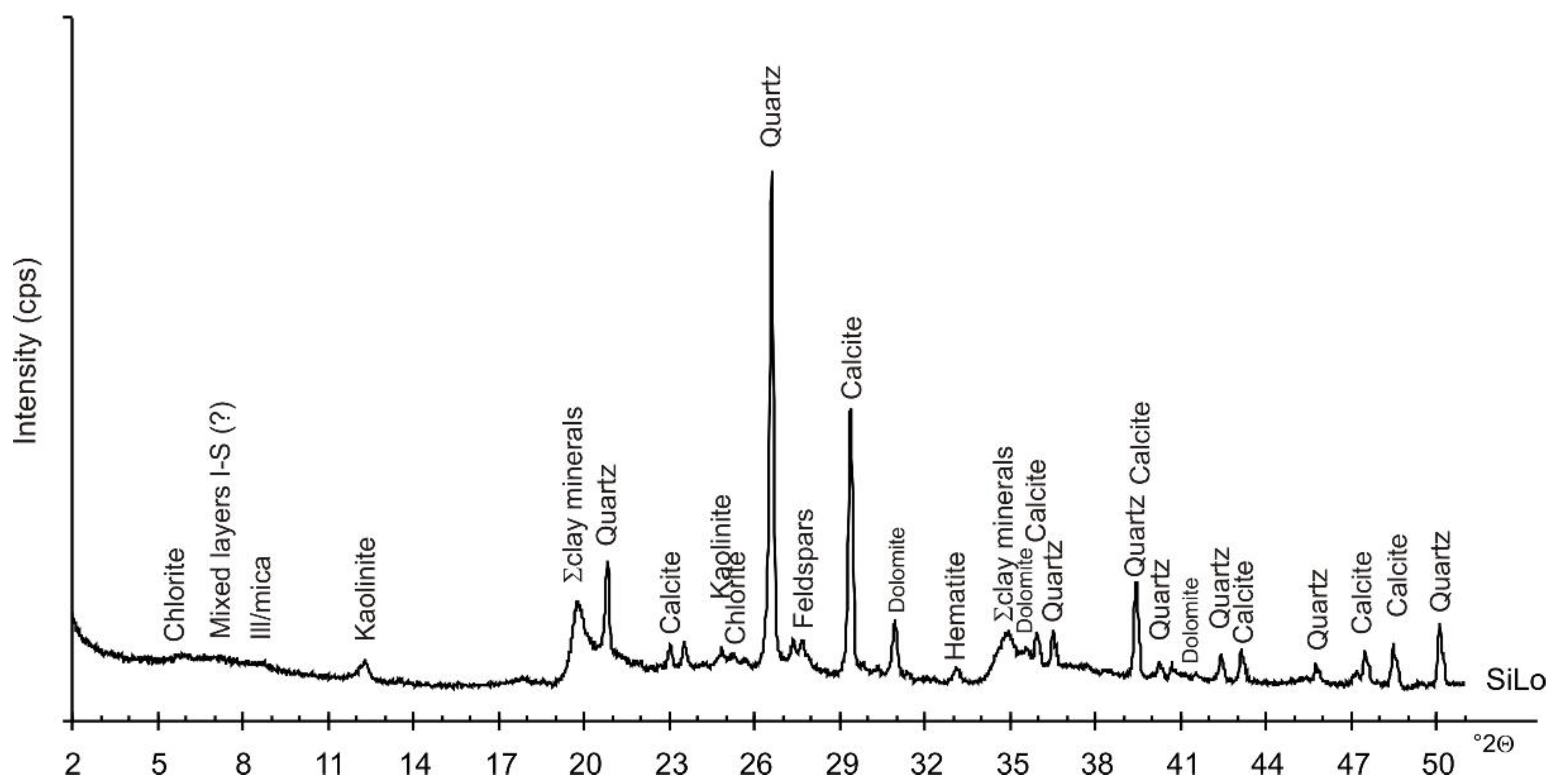
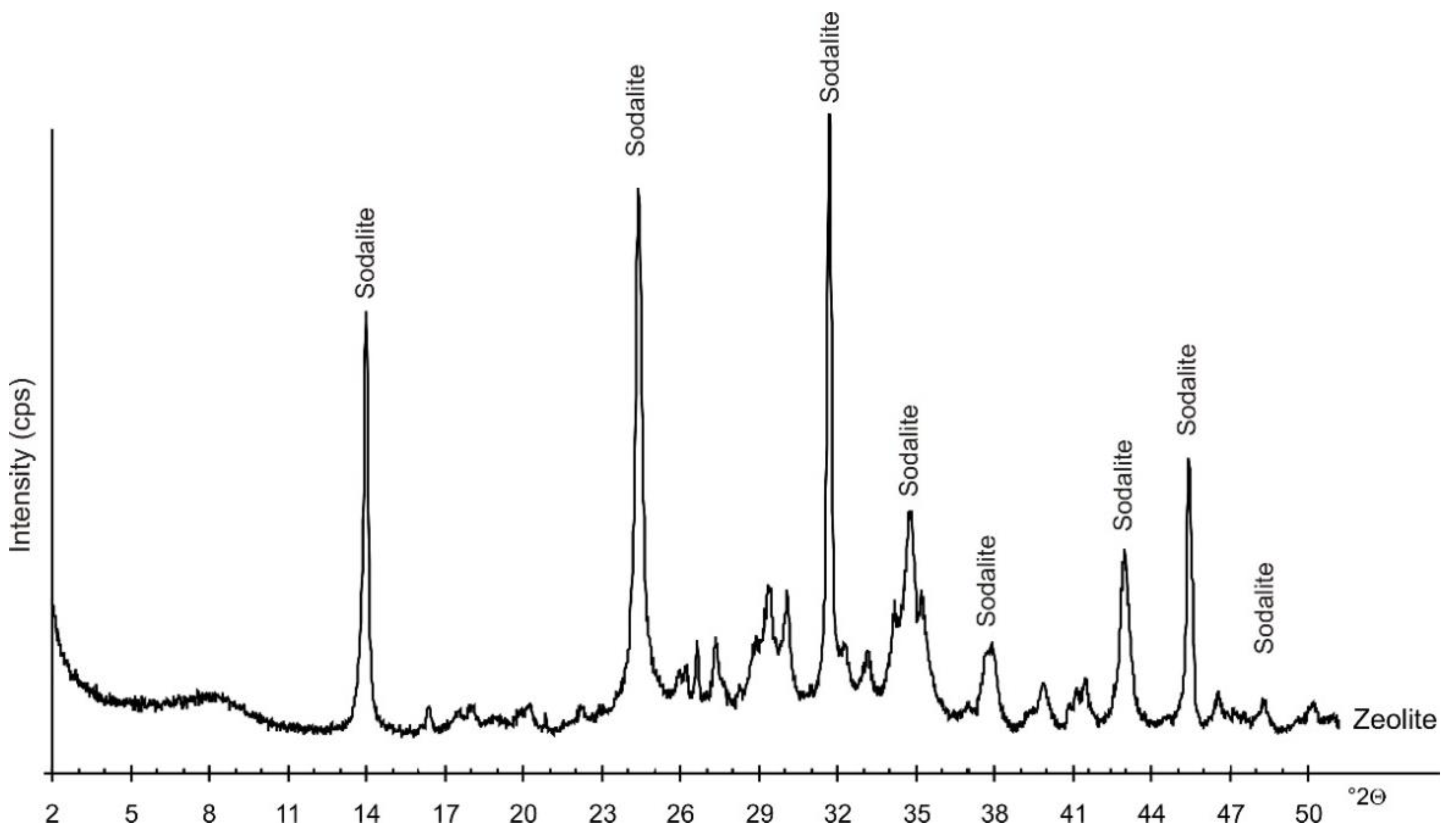

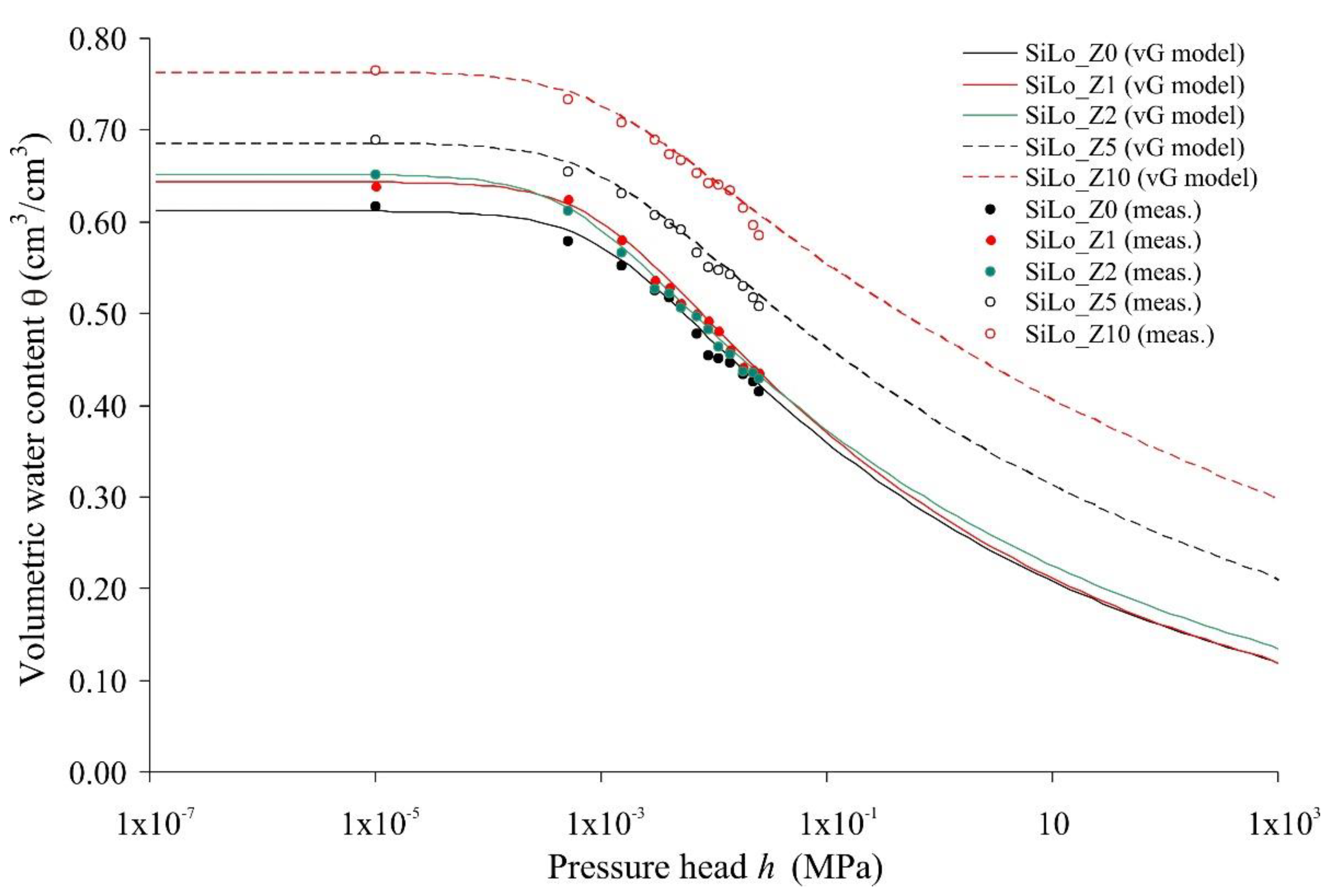
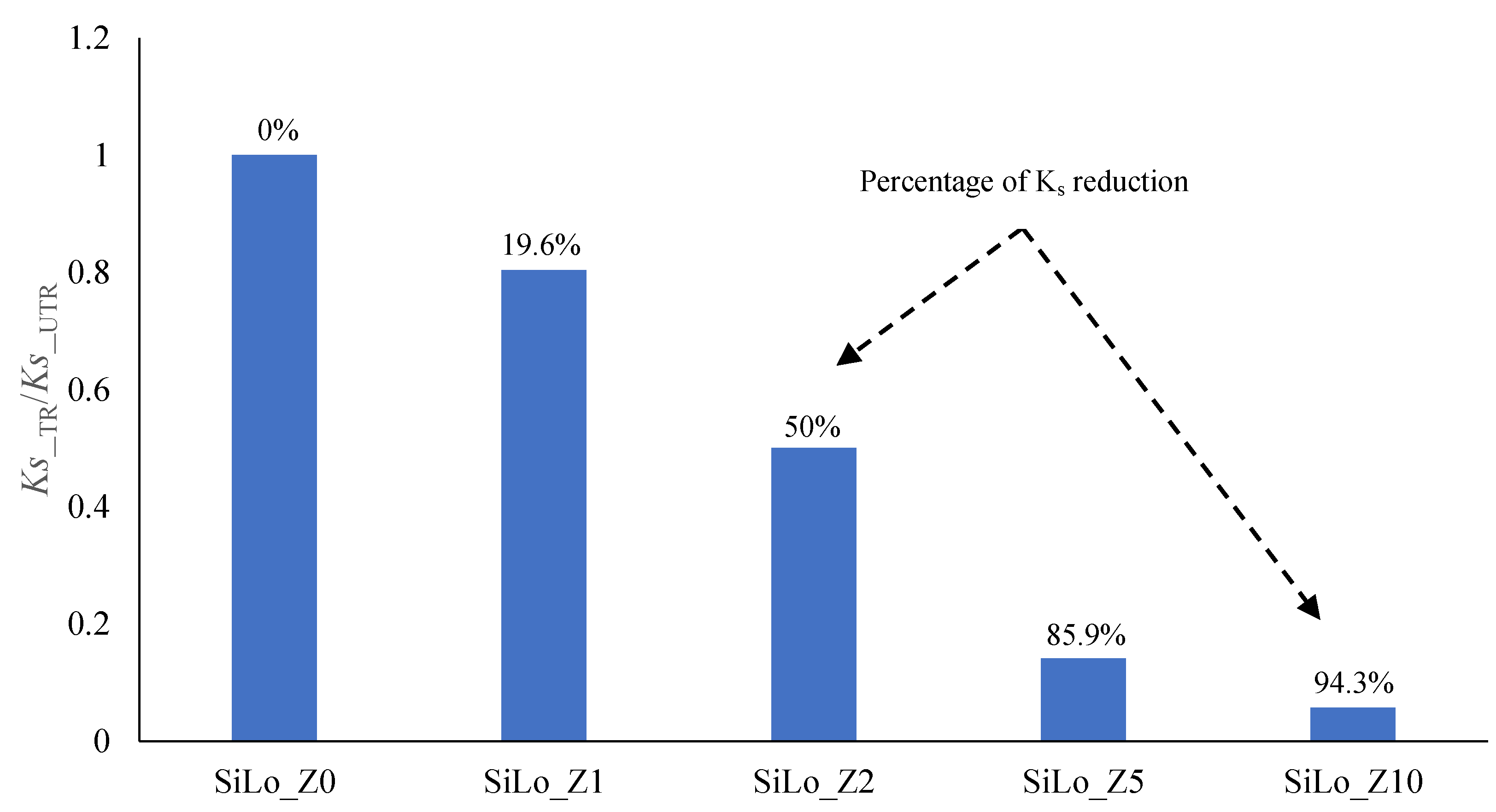
| Property | Soil | Unit | Method |
|---|---|---|---|
| Sand | 9.53 | % | hydrometer method |
| Silt | 66.18 | % | |
| Clay | 24.29 | % | |
| Texture (USDA classification) | Silty loam | - | |
| Soil bulk density (ρb) | 1.369 | g/cm3 | core method |
| Field capacity (FC) | 40.5 | % | retention curve (at h = −0.03 MPa) |
| Wilting point (WP) | 25.5 | % | retention curve (at h = −1.5 MPa) |
| pH (in H2O 1:2.5) | 7.63 | pH meter | |
| Cation exchange capacity | 27.85 | cmol/kg | BaCl2 pH 8.1 |
| Organic matter | 34.90 | g kg−1 | Walkley–Black |
| Exchangeable K+ | 317 | mg kg−1 | BaCl2 pH 8.1 |
| Exchangeable Mg2+ | 277 | mg kg−1 | BaCl2 pH 8.1 |
| Exchangeable Ca2+ | 4852 | mg kg−1 | BaCl2 pH 8.1 |
| Fe | 9.98 | mg kg−1 | Lindsay–Norwell |
| Mn | 5.81 | mg kg−1 | Lindsay–Norwell |
| Zn | 1.45 | mg kg−1 | Lindsay–Norwell |
| Cu | 0.99 | mg kg−1 | Lindsay–Norwell |
| P | 53.02 | mg kg−1 | Olsen |
| Treatments | θs (cm3/cm3) | θFC (cm3/cm3) | θWP (cm3/cm3) | AWC (cm3/cm3) | AC (cm3/cm3) | Ks (cm/min) | α (1/cm) | n (-) |
|---|---|---|---|---|---|---|---|---|
| SiLo_Z0 | 0.612 | 0.405 c | 0.255 C | 0.150 | 0.207 | 0.056 A | 0.123 a | 1.12 |
| SiLo_Z1 | 0.652 | 0.417 c | 0.272 C | 0.145 | 0.235 | 0.045 B | 0.182 b | 1.11 |
| SiLo_Z2 | 0.644 | 0.419 c | 0.260 C | 0.159 | 0.225 | 0.028 C | 0.155 c | 1.12 |
| SiLo_Z5 | 0.685 | 0.504 b | 0.362 B | 0.142 | 0.181 | 0.0079 D | 0.100 d | 1.11 |
| SiLo_Z10 | 0.763 | 0.593 a | 0.456 A | 0.137 | 0.170 | 0.0032 E | 0.099 d | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belviso, C.; Satriani, A.; Lovelli, S.; Comegna, A.; Coppola, A.; Dragonetti, G.; Cavalcante, F.; Rivelli, A.R. Impact of Zeolite from Coal Fly Ash on Soil Hydrophysical Properties and Plant Growth. Agriculture 2022, 12, 356. https://doi.org/10.3390/agriculture12030356
Belviso C, Satriani A, Lovelli S, Comegna A, Coppola A, Dragonetti G, Cavalcante F, Rivelli AR. Impact of Zeolite from Coal Fly Ash on Soil Hydrophysical Properties and Plant Growth. Agriculture. 2022; 12(3):356. https://doi.org/10.3390/agriculture12030356
Chicago/Turabian StyleBelviso, Claudia, Antonio Satriani, Stella Lovelli, Alessandro Comegna, Antonio Coppola, Giovanna Dragonetti, Francesco Cavalcante, and Anna Rita Rivelli. 2022. "Impact of Zeolite from Coal Fly Ash on Soil Hydrophysical Properties and Plant Growth" Agriculture 12, no. 3: 356. https://doi.org/10.3390/agriculture12030356






