Abstract
The validity to promote seed vigor of sand priming had been reported; however, its feasibility in pine seeds remained unknown. In this study, Masson pine (Pinus massoniana Lamb.) seeds of three varieties were used to investigate the effect of sand priming on seed germination, respiratory metabolism and antioxidant capacity. Seeds treated with hydrated sand, about 5% moisture over 2 day, were employed as the priming group with non-primed seeds as the control. The germination test and field test showed that the germination rate, germination potential, vigor index and field emergence rate of sand-primed seeds were significantly enhanced by 8.3~12.3%, 3.9~11.5%, 40.4~72.3% and 5.8~8.9% compared to non-primed seeds. The oxygen-sensing test indicated that sand priming accelerated seed respiration. Besides these, antioxidant enzyme activities and endogenous GA1 and IAA are also enhanced after sand-priming treatment. Furthermore, the variation tendency of gene expression of GID1, POD and SDH was in line with the physiological parameters. Based on the results, the regulatory mechanism of germination promotion of the Masson pine by sand priming was clarified. Sand priming might promote respiratory metabolism, endogenous hormones and antioxidant capacity through influencing the related gene expression, which would have a conjoint promotion on the seed germination of Pinus massoniana.
1. Introduction
Masson pine (Pinus massoniana Lamb.) is an economical and eco-admirable evergreen conifer in China. The eighth national forest inventory revealed that the area of the Masson pine was 10.01 million hm2, accounting for 6.08% of the total arboreal forest area [1]. Owing to the characteristics involving fast growth, high yield, strong adaptability, as well as comprehensive utilization, the Masson pine played crucial roles in forest ecosystem, afforestation and re-vegetation projects [2]. Seeds are the basis of seedling cultivation of the Masson pine; however, seeds become aged and deteriorated, accompanied by damage of the cell membrane function and some enzymatic activities, during storage, which severely restricts the afforestation program of Pinus massoniana. In this regard, how to ameliorate seed performance of Pinus massoniana becomes a difficult problem in production practice. Seed priming is a high-efficiency and easily operated method for enhancing the seed vigor, seedling uniformity and stress resistance [3,4]. It is a seed technology carried out by moisture absorption then re-drying without radicle emergence. Multiple seed priming techniques have been developed. PEG (polyethylene glycol) priming promotes the germination characteristics of barley seeds under drought conditions [5], while KNO3 priming enhances canola seed performance when subjecting salt stress [6]. Sodium nitroprusside as chemical priming agents had a well-recorded property for increasing the capacity of stress tolerance of Ocimum basilicum L. [7]. Bio-priming with Trichoderma harzianum reduced the damage of salt stress to bread wheat [8].
However, the above methods were not suitable to apply in treating a large quantity of seeds because huge cost or special equipment was required. The use of the above methods is complicated, making it difficult to popularize the technology for ordinary farmers. Solid matrix priming combines the seeds, water and organic/inorganic solid material with a proper ratio at a certain temperature [9,10]. Sand as a solid matrix has the definite capacities to hold water and release water to seeds slowly, and it is cheap, easily separated and can be repeatedly utilized. In practice, sand priming was not only employed for improving the germination rate under stress conditions of Oryza sativa L. [11] but also Medicago sativa L. [12]. As mentioned above, priming promotion usually occurred in the germination stage and young seedling establishment, which was in line with the research [13].
Over-accumulation of ROS (reactive oxygen species) has a negative effect on proteins, lipids and carbohydrates, as well as DNA, and might further give rise to the oxidative damage of the organisms or plant cells [14]. Proline was accumulated extensively in plants under stress conditions to stabilize the structure of biomacromolecules and improve the stress tolerance [15]. MDA (malondialdehyde) was commonly employed for reflecting the lipid peroxidation levels and the antioxidant status in plant cells [16]. For self-preservation, plant cells have evolved defensive system to minimize the damage of ROS over-accumulation, such as SOD (superoxide dismutase), CAT (catalase), POD (peroxidase), APX (ascorbate peroxidase), GPX (glutathione peroxidase) and so on [17,18]. Sand priming enhanced the activities of CAT, SOD, APX and POD and inhibited MDA gathering during the germination stage [19]. The afforestation program needs both economic efficiency and practical feasibility. Previous research had proved that sand priming was a valid pretreatment to promote seed germination in some field crops. Therefore, a hypothesis was proposed about whether sand priming could be applied to promote seed germination of Pinus massoniana. In order to evaluate the effects of sand priming Pinus massoniana, seed respiration capacity (by oxygen-sensing technique and SDH activity measurement), main antioxidant enzymes (POD, CAT and SOD) and endogenous phytohormones (GA1, IAA and ABA) between sand-primed seeds and non-primed seeds were analyzed. To further clarify the mechanism at the gene level, several related genes were determined to verify the possible regulatory mechanism of the germination promotion of Pinus massoniana by sand priming. The objective of this study will provide a new and efficient pretreatment of Pinus massoniana seeds before germination.
2. Materials and Methods
2.1. Plant Materials
The seeds of Masson pine varieties “Dai 1.0”, “Dai 1.5” and “Dai 2.0” were obtained in 2017 from Laoshan forestry center in Hangzhou, China, located at 29°50′35″ N, 119°04′01″ E. Laoshan forestry center preserves the most important germplasm resources of Pinus massoniana Lamb. in China. The above three varieties of seed orchard of Masson pine have gradually improved, and they have been widely used in genetic improvement and afforestation. The landform of this region is mainly low mountains and hilly, and it has a subtropical monsoon climate with 1063 mm of average annual precipitation and 17~18 °C of mean temperature. To break dormancy, seeds were put into zippered plastic bags and subjected to moist chilling at 4 °C at 35% moisture content for 60 d in the dark. The initial germination rate ranged from about 10–40%, which was increased to approximately 20–65% after moist chilling. Finally, seeds were stored at 4 °C for 3 months before experiment.
2.2. Sand-Priming Treatment
The objective of this experiment was to address whether and how sand priming promotes seed germination of Pinus massoniana. The priming treatment followed [12] with slight modifications. Sand was screened by sieve (d = 2 mm) to remove the large sand particles and sterilized by oven at 130 °C for 2 h. Each gram of seeds was mixed with 100 g of sand, about 5% moisture and then sealed by preservative film in plastic boxes at 20 °C for 48 h. It was conducted in dark conditions to minimize the influence of light. Post priming, seeds were laid on the dry filter paper to dehydrate until initial moisture level. Except for priming procedure, all conditions between sand-primed seeds and non-primed seeds were absolutely identical. Before germination test, a portion of seeds were instantaneously frozen then stored at −80 °C for oxygen-sensing test, antioxidant capacity and endogenous hormone determination.
2.3. Laboratory Germination Experiment
Three independent replicates with 100 seeds per replicate were conducted in germination test. Seeds were evenly floored on fully hydrated filtration paper at 25 °C/dark. The quantity of germinated seeds was recorded until there was no further germination (12 d). Germination rate (GR), germination index (GI), vigor index (VI) [20] and the mean germination time (MGT) [11] were calculated according to the following formulas:
here, Gt is corresponding number of seeds germinated in the t day; Dt is time corresponding to Gt in days.
here, GI refers to germination index. S is the average length of 10 seedlings.
here, Ni refers to the number of the new germination seeds in day Ti.
here, n1 represented the number of germinated seeds; n2 represented the number of totally tested seeds. The seedlings of 3, 6, 9 and 12 d were collected and stored at −80 °C for RNA extraction.
GR = the number of germinated seeds within the 12 days/the total number of seeds × 100%.
GI = ∑ (Gt/Dt)
VI = GI × S
MGT = ∑TiNi / ∑Ni
Germination potential = n1 within 5 days/n2 × 100%
2.4. Field Emergence Rate Test
The field emergence rate test was conducted at the Guantang Farm of Zhejiang Agriculture and Forestry University (ZAFU) located in lin’an, Hangzhou, Zhejiang, China for 21 days in May 2018. In the 10 cm soil layer, the average temperature was 16.8 °C, and the average relative water content was 67.9%. The field emergence rate test was conducted using a complete random design on three replicates of 100 seeds per sample. The plant and row spacing was 20 cm × 30 cm. Seeds were sown in 2 cm deep soil covered with 1~2 cm deep plant ash. The number of seedlings was recorded every two days after emergence until 21 d after sowing.
2.5. Oxygen-Sensing Measurement
Oxygen-sensing technology was found that was an effective and rapid method in seed vigor assessment of Pinus massoniana [21]. In this study, we wanted to clarify whether sand priming could enhance the seed vigor by strengthening the seed respiration of Pinus massoniana. There were three replicates, and each replicate contained 50 seeds in oxygen-sensing test. The seeds were airproofed with photosensitive material and oxygen content was measured at hourly intervals by Q2 instrument (ASTEC Global, Maarssen, The Netherlands). After 12 days of monitoring, four oxygen-sensing parameters (1-IMT: increased metabolism time; 2-OMR: oxygen metabolism rate; 3-COP: critical oxygen pressure; 4-RGT: relative germination time) were calculated by oxygen-sensing software (ASTEC Global, Maarssen, The Netherlands) [21].
2.6. Antioxidant Capacity Assessment
Antioxidant capacity assessment was established in triplicate and on 1 g seeds for each sample. Superoxide dismutase (SOD) activity was measured by determining the inhibition to photochemical reduction of Nitroblue tetrazolium as monitored at 560 nm, catalase (CAT) activity was measured by the absorbance decrease at 240 nm based on the H2O2 decomposition at 25 °C and peroxidase (POD) activity was measured by monitoring the increase in absorbance at 470 nm due to guaiacol oxidation at 25 °C [22]. Succinate dehydrogenase (SDH) activity was determined by mg of SDH per g protein an hour using the extinction coefficient 1.03 × 103/M/cm at 420 nm [23]. The content of Malondialdehyde (MDA) was calculated by the formula “μmol MDA g−1 FW = 6.45 (OD532 − OD600) − 0.56 × OD450”; proline content was assayed by the spectrophotometer at 520 nm [24].
2.7. Endogenous Hormones Determination
For endogenous hormone determination, three biological replicates were conducted, and 0.5 g seeds were used for each replicate. The cryopreserved seed embryos of Pinus massoniana were ground to powder. Based on the 1:10 ratio (M/V), 0.5 g of powder was added into 5 mL of 80% methanol extract in the centrifuge tube. During this period, vortex once every half an hour and repeat it 5 times. After standing for 12 h at 4 °C in the dark, centrifugation at 12,000× g at 4 °C for 20 min was conducted to separate of precipitate. A volume of 500 μL of 30% methanol solution was used to dissolve the precipitate. Centrifuge at 12,000× g for 15 min at 4 °C to collect the precipitate and store it in the sample vial. Endogenous gibberellin A1 (GA1), abscisic acid (ABA) and indole-3-acetic acid (IAA) were measured by LC-ESI-MS/MS [25]. MS system: ion spray voltage −4500 V, temperature 550 °C, curtain gas 40 psi, gas 1:50/gas 2:50. Chromatographic system: HSS T3 liquid chromatography column (100 × 2.1 mm, 1.8 µm), mobile phase A (0.1% formic acid-aqueous solution), mobile phase B (0.1% formic acid-acetonitrile) and sample volume = 5 μL.
2.8. Quantitative Real-Time PCR Assay
Total RNA was extracted using Spin Column Plant Total RNA Purification Kit (Sangon Biotech, Shanghai, China). cDNA was synthesized from 1000 ng of total RNA according to reverse transcription kit (Takara, Beijing, China). The sequences of 3 candidate genes were acquired from the GenBank. Gene annotation and primer sequence were summarized in Table 1. qRT-PCR was performed in three biology replications and two technique replications by TB Green Premix Ex Taq (Takara, Beijing, China), and the result was evaluated by 2−∆∆Ct method.

Table 1.
The primer sequence and annotation information of candidate genes used in this study.
2.9. Data Analysis
Two-way analysis of variance was carried out with SPSS 19.0 (IBM SPSS Statistics, Chicago, IL, USA). The effect of treatment and variety are recognized as the first and second factors. Duncan’s multiple range test was employed to determine if there were significant differences among different treatments at p < 0.05.
3. Results
3.1. Effect of Sand Priming on Seed Germination Traits and Field Emergence of Pinus massoniana Lamb.
The major purpose of this experiment was an attempt to clarify the effect of sand priming on the seed germination and field emergence of Pinus massoniana. From Table 2, it can be observed that the vigor of “Dai 2.0” appeared to be very high; the germination rate was over 73.6% without priming, while “Dai 1.5” and “Dai 1.0” were only 48.5% and 22.4%, respectively. The results showed that sand priming at 25 °C for 48 h significantly improved seed germination and field emergence among all of three Masson pine varieties (Table 2). For “Dai 2.0” seeds, the germination rate, germination potential, germination index and vigor index and field emergence rate were 1.14, 1.57, 1.29, 1.40 and 1.14 times the non-primed seeds, respectively. For “Dai 1.5” seeds, the above indicators were 1.17, 1.61, 1.31, 1.47 and 1.14 times. For “Dai 1.0” seeds, the above indicators were 1.55, 1.85, 2.27, 1.72 and 1.46 times. Meanwhile, the mean germination time was shortened about 0.6–1.2 day. Interestingly, by analyzing the data, we found that the most significant promotion occurred on low-vigor seeds (Dai 1.0), and the least significance occurred on high-vigor seeds (Dai 2.0). Therefore, we speculated there was a negative correlation between the degree of promotion of sand priming and the seed vigor itself.

Table 2.
Effect of sand priming on seed germination of Pinus massoniana Lamb.
3.2. Effect of Sand Priming on Seed Respiration of Pinus massoniana
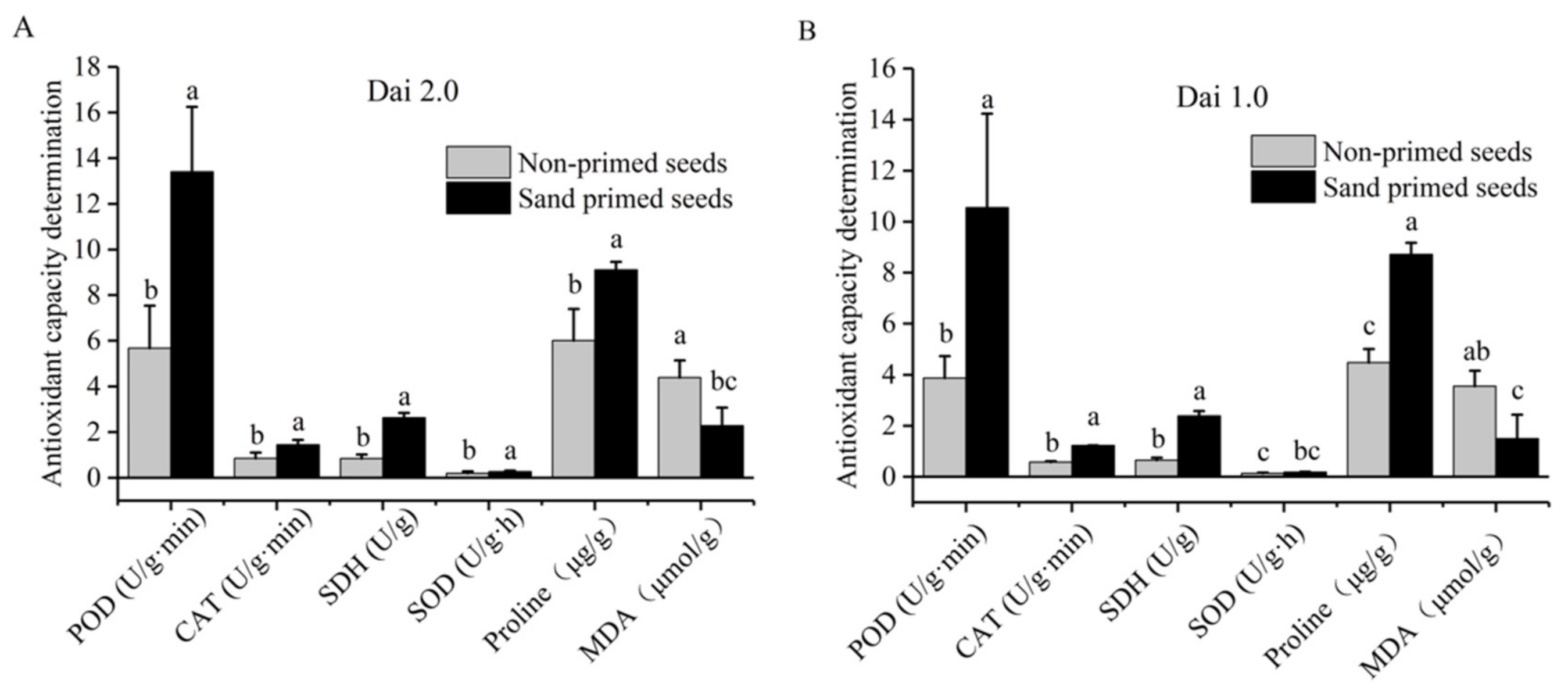
An oxygen-sensing test was conducted to reveal the dynamic changes of respiration metabolism at the initial stage of seed germination. The research [21] indicated that IMT, COP and RGT negatively reflected the germination rate, whereas OMR was in accord with the laboratory germination performance. In this paper, the OMR value of sand-primed group was significantly increased by 5.0~20.0%, whereas IMT, COP and RGT values were lower than those of non-primed seeds (Table 3). Interestingly, SDH participated in step six of the TCA cycle as well as the respiratory chain, which might reflect respiratory capacity to some extent. SDH activity was promoted by 211.6~265.0% (Figure 1) in subsequent experiments. Therefore, the opinion that sand priming improves seed respiration of Pinus massoniana could be verified.

Table 3.
Effect of sand priming on seed respiration of Pinus massoniana.
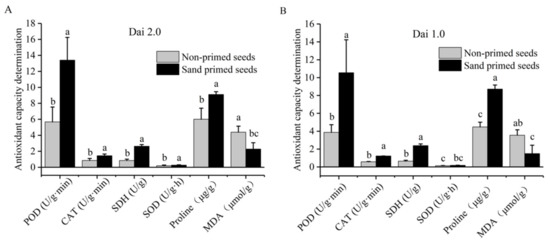
Figure 1.
Effects of sand priming on enzymatic activities and the content of proline and MDA of Pinus massoniana. (A) The result of Masson pine variety “Dai 2.0”. (B) The result of Masson pine variety “Dai 1.0”. The same lowercase letters are not significantly different at p < 0.05, according to Duncan’s multiple range test. MDA: malondialdehyde; CAT: catalase; SOD: superoxide dismutase; POD: peroxidase, SDH: succinate dehydrogenase.
3.3. Effect of Sand Priming on Antioxidant Capacity of Pinus massoniana
Sand priming at 25 °C for 48 h improved the antioxidant capacity of both high-vigor seeds and low-vigor seeds (Figure 1). For “Dai 2.0”, the activities of POD, CAT and SOD after sand priming were increased by 135.9%, 70.8% and 39.4% compared with the non-primed controls (Figure 1A). For “Dai 1.0”, the enhancement was more obvious; POD and CAT activities were enhanced by 172.3% and 112.8%, respectively (Figure 1B). For the SOD activity, there was a little discrepancy (39.4% > 32.6%), but it was not significant. Moreover, the proline content of “Dai 2.0” was increased by 51.3%, and MDA content was reduced by 48.0% after sand-priming treatment (Figure 1A). In “Dai 1.0”, proline content was increased by 94.6%, whereas MDA content was reduced 57.9% (Figure 1B). In general, the above results showed that sand priming could invigorate the antioxidant capacity of Pinus massoniana.
3.4. Effect of Sand Priming on the Content of Endogenous Phytohormone
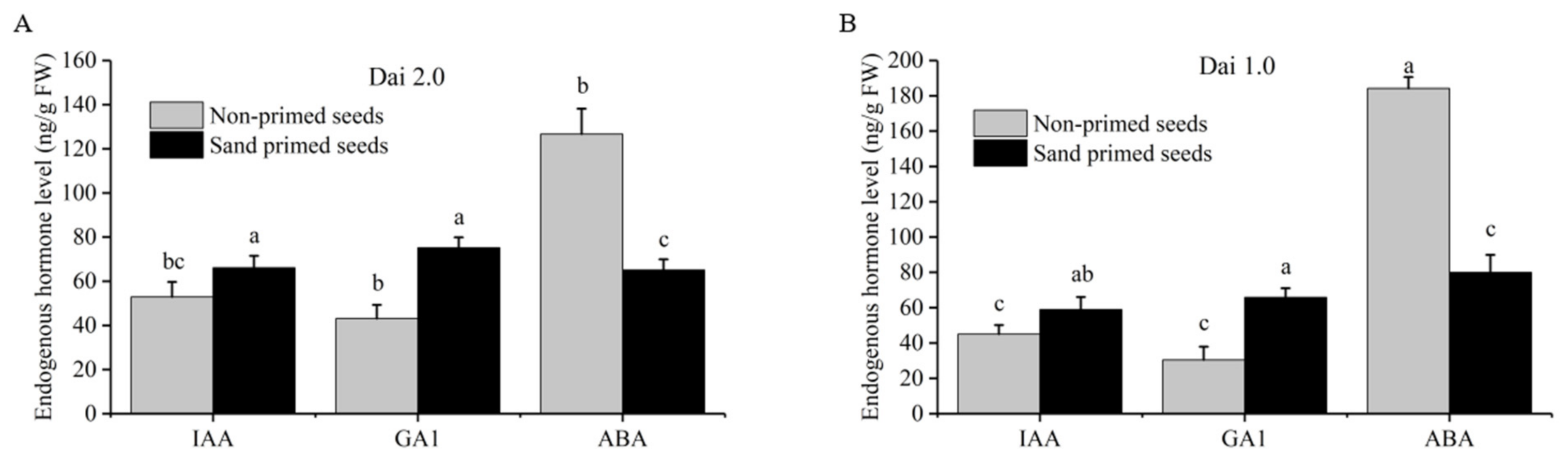
During seed germination and young seedling establishment, GA and ABA played the role of a switch, controlling the seeds’ germination or dormancy, and IAA could also promote seed germination in some content. The content of endogenous phytohormones (IAA, GA1, ABA) of sand-primed seeds and non-primed seeds was determined. Our results showed that the content of IAA and GA1 of “‘Dai 2.0” after sand priming was significantly enhanced by 24.9% and 74.3%, and ABA content was reduced by 48.6% (Figure 2A). For “Dai 1.0”, IAA and GA1 were notably increased by 30.9% and 116.0%, whereas ABA was significantly reduced by 56.6% (Figure 2B). In light of the antagonism or synergistic action among ABA, GA1 and IAA, the investigation demonstrated that sand-priming treatment could promote the synthesis of IAA and GA1 and inhibit the synthesis of ABA.
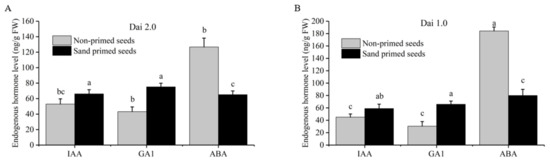
Figure 2.
Effects of sand priming on the content of endogenous phytohormone of Pinus massoniana. (A) The result of Masson pine variety “Dai 2.0”. (B) The result of Masson pine variety “Dai 1.0”. IAA: indole-3-acetic acid; ABA: abscisic acid; GA1: gibberellin A1. Lower-cased letters indicate statistically significant differences (p < 0.05, Duncan multiple range test).
3.5. Effect of Seed Priming on Gene Expression
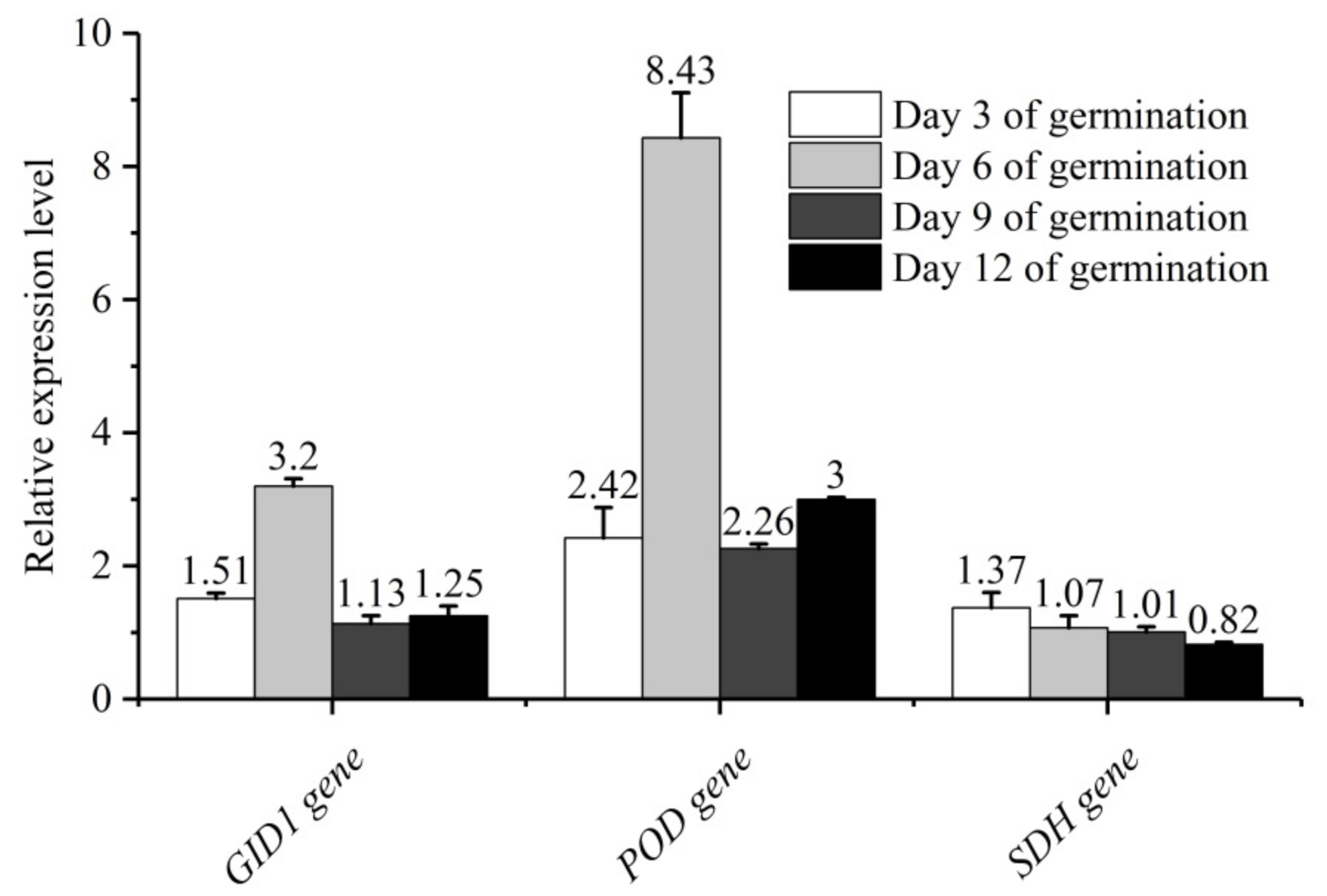
In view of the significant improvement of sand priming on seed germination, antioxidant capacity as well as endogenous hormones content, the change in molecular level aroused our curiosity. Three relatively regulatory genes (POD, SDH and GID1) related to antioxidant capacity, seed respiration and hormone response were selected for quantification analysis. To investigate the dynamic changes of the effect of sand priming on gene expression, “Dai 1.0” seedlings of 3, 6, 9 and 12 days were collected to perform qRT-PCR analysis. The number represents the multiple of the expression level (sand-priming group/non-priming group), and “>1” means upregulation while “<1” means downregulation (Figure 3). The results indicated that all candidate genes were upregulated after sand priming on the third day of germination, ranging from 1.37 to 2.42 folds. SDH peaked on the third day. On the sixth day, the promotion of GID1 and POD reached the peak and were 3.2 and 8.43 folds, respectively. On the 9th and 12th day, the promotion effect was not so obvious. Based on the above results, we speculated that the acceleration of sand priming on genes expression mainly manifested in the early stage of seed germination, and the general trend increased first and then decreased.
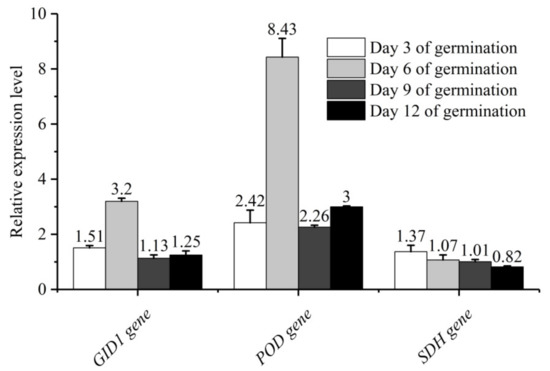
Figure 3.
Effect of sand priming on genes expression in Pinus massoniana “Dai 1.0”. Annotation information of above genes had been listed in Table 1.
4. Discussion
The demand for high-quality seeds has become a necessary priority to face the present requirement for high standards in the afforestation project of Pinus massoniana Lamb. Seed priming is an excellent choice and well-suitable technique to improve seed quality, which could promote seed germination and ensure uniform seedling establishment [26]. Moreover, seeds after priming showed higher activities of antioxidant enzymes and lower levels of ROS [27]. Sand priming had been examined in Oryza sativa L., Medicago sativa L., Zea mays L. and Gossypium hirsutum Linn. [11,12,28,29], but the reports on promoting seed germination of Pinus massoniana were little.
Our study showed that seed germination of Pinus massoniana was improved, and the mean germination time was shortened among all of three varieties (Table 2), which was consistent with those in Oryza sativa and Solanum melongena L. [3,11]. The research [11] reported that the germination rate and germination potential of sand-primed seeds were increased by 4.2~6% and 4.2~8% compared to non-primed controls; meanwhile, the mean germination time was decreased by about 0.01~0.04 d. The research [3] also verified that seed priming could enhance the germination rate and reduce germination time simultaneously. Besides for promoting seed germination and shortening germination time, this study indicated that the stimulation of sand priming was more visible for low-vigor seeds (Dai “1.0”) than high-vigor seeds (“Dai 2.0”).
ABA and GA act as antagonistic elements to determine the seed fate that results, dormancy or germination [30]. IAA can promote seed germination of Cunninghamia lanceolata (Lamb.) Hook. [31]. Additionally, exogenous GA3 could promote seed germination and seedling vigor of Pinus massoniana by strengthening seed respiration, stimulating IAA and GA1 biosynthesis and reducing the ABA level [32]. In the current study, sand priming also influenced these endogenous hormones in a positive manner to promote seed germination. A previous study [33] indicated that seed priming alleviated the disadvantages caused by stress conditions, which resulted in a low ABA concentration and augmentation of salicylic acid (SA), IAA and GA. Collectively, a conclusion can be drawn that plant hormones might play vital roles during seed germination of Pinus massoniana after sand priming.
The study [34] indicated there was an intricate interplay between phytohormones and ROS during abiotic stress conditions. IAA joined in ROS homeostasis by reducing the ROS to promote plant growth [35], while ABA as a stress trigger controlled stomatal closure by H2O2, which was generated in the ROS metabolism [36]. This study indicated that sand priming could increase the antioxidant capacity and stress tolerance of the Masson pine by increasing the activities of antioxidant enzymes (CAT, POD and SOD) and proline content and decreasing MDA content (Figure 1). Similarly, H2O2 priming could cause an enhancement of the activities of APX, SOD, CAT and GPOX and decrease the MDA content [37].
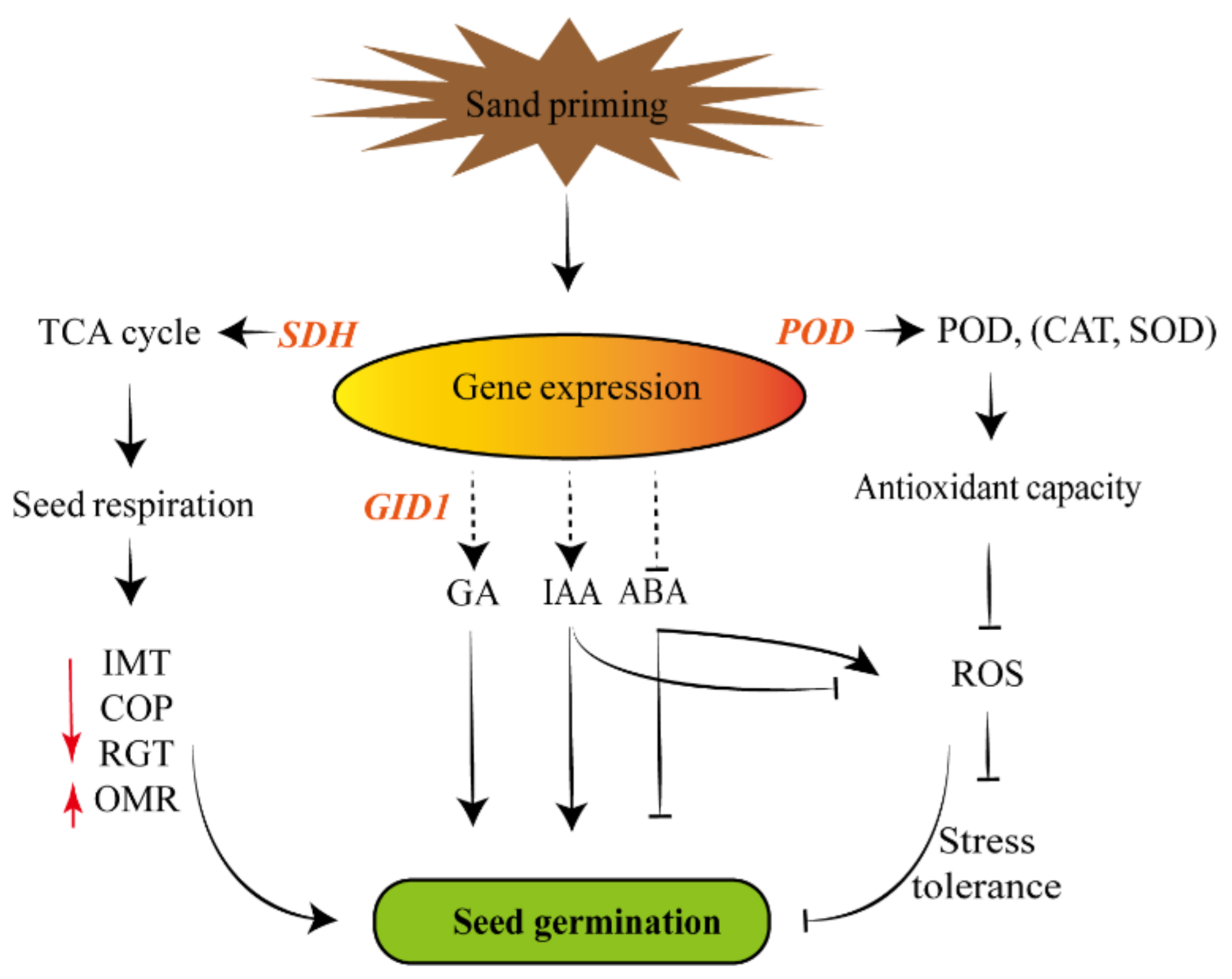
Sand priming played key roles in the regulation of seed germination, antioxidant capacity and physiological indexes of Pinus massoniana, but the molecular events underlying also needed to be made clear. GID1 gene, as a key regulatory element in the GA signaling pathway, affects seed germination induced by GA [38]; POD gene regulates the antioxidant capacity by encoding peroxidase, which participates in scavenging ROS [39]. In this paper, the endogenous GA and IAA (Figure 2) and the activities of POD, CAT and SOD (Figure 1) were increased, which was broadly in line with the highly expressed GID1 and POD (Figure 3). SDH gene participated in the TCA cycle (tricarboxylic acid Cycle) and may function as a regulator of the respiratory metabolism [40]. Both the SDH gene (Figure 3) and SDH activity (Figure 1) were promoted after sand priming, which suggested that seed respiration was strengthened. Interestingly, the reduction of ROS would increase the stress tolerance. Our findings revealed a regulatory mechanism (Figure 4) related to the improvement of sand-priming treatment to seed germination of Pinus massoniana. Sand priming might modulate seed respiration, endogenous hormone and ROS content through affecting the interrelated genes expression, which could facilitate seed germination of Pinus massoniana conjointly.
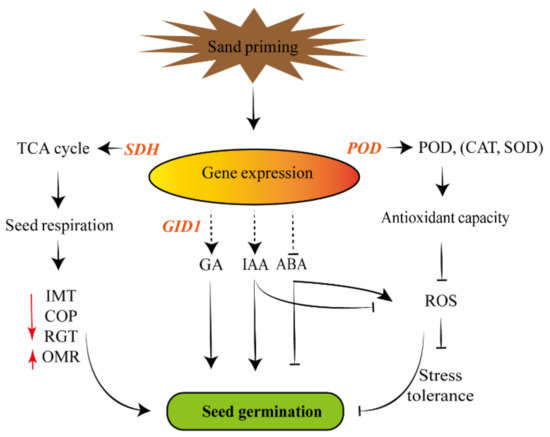
Figure 4.
The regulatory mechanism of promotion of sand priming to seed germination in Pinus massoniana. Sand priming might enhance the relevant gene expression (GID1, POD, SDH, etc.). These genes might further affect the endogenous hormones level, antioxidant capacity and seed respiration. The enhancement of GA, IAA, low ROS and ABA might contribute to seed germination of Pinus massoniana. Simultaneously, it would enhance the stress tolerance, but it should be verified in follow-up research. SDH: succinate dehydrogenase; POD: peroxidase; CAT: catalase; SOD: superoxide dismutase; TCA: tricarboxylic acid; GID1: Gibberellin metabolism protein gene; GA: gibberellin; IAA: indole-3-acetic acid; ABA: abscisic acid; IMT: increased metabolism time; OMR: oxygen metabolism rate; COP: critical oxygen pressure; RGT: relative germination time; ROS: reactive oxygen species.
5. Conclusions
In conclusion, our results show that sand priming can promote the seed germination and field emergence, antioxidant ability and seed respiration of Pinus massoniana. Significant differences in germination rate, mean germination time, the activity of antioxidant enzymes (POD, SOD and CAT), endogenous phytohormone level (GA, IAA and ABA) and seed respiration were observed after sand-priming treatment. Sand as a solid matrix to prime the seeds of Pinus massoniana may contribute to breaking seed dormancy and improving the seed vitality of Pinus massoniana on a deeper layer, which will also provide an effective pretreatment method for the afforestation project.
Author Contributions
K.Z. performed the experiments, analyzed the data and prepared the manuscript. Z.J. performed experiments and helped with data analysis. D.J. participated in the data collection and data analysis. G.Z. and T.Z. designed the study and reviewed the draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the National Natural Science Foundation of China (30800890), the Basic Public Welfare Research Projects of Zhejiang Province of China (LGN21C160015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the graphs and tables provided in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, X.; Ding, G.; Wang, Y. Active constituents of soil extracts from mycorrhizal seedling rhizosphere of Pinus massoniana and their effects on seed germination. Sci. Silvae Sin. 2018, 54, 32–38. (In Chinese) [Google Scholar]
- Wang, Y.; Ding, G. Influence of ectomycorrhiza on nutrient absorption of Pinus massoniana seedlings under water stress. For. Res. 2013, 26, 227–233. (In Chinese) [Google Scholar]
- Ali, M.; Hayat, S.; Ahmad, H.; Ghani, M.I.; Amin, B.; Atif, M.J.; Cheng, Z. Priming of Solanum melongena L. seeds enhances germination, alters antioxidant enzymes, modulates ROS, and improves early seedling growth: Indicating aqueous garlic extract as seed-priming bio-stimulant for eggplant production. Appl. Sci. 2019, 9, 2203. [Google Scholar] [CrossRef] [Green Version]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Tabatabaei, S.A. Effect of osmo-priming on germination and enzyme activity in barley (Hordeum vulgare L.) seeds under drought stress conditions. J. Physiol. Biochem. 2013, 9, 25–31. [Google Scholar]
- Saeedipour, S. Effect of osmo-priming on germination and early seedling growth of Brassica napus L. under salinity conditions. Res. Crops 2012, 13, 139–142. [Google Scholar]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 2020, 168, 361–373. [Google Scholar]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed biopriming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil. 2011, 347, 387–400. [Google Scholar] [CrossRef]
- Madsen, M.D.; Svejcar, L.; Radke, J.; Hulet, A. Inducing rapid seed germination of native cool season grasses with solid matrix priming and seed extrusion technology. PLoS ONE 2018, 13, e0204380. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Huo, W.; Yao, D.; Li, M. Effects of solid matrix priming (SMP) and salt stress on broccoli and cauliflower seed germination and early seedling growth. Sci. Hortic. 2019, 255, 161–168. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Z.Y.; Song, W.J.; Wang, J.C.; Hu, W.M. Effects of sand priming on germination and field performance in direct-sown rice (Oryza sativa L.). Seed Sci. Technol. 2005, 33, 243–248. [Google Scholar] [CrossRef]
- Hu, J.; Xie, X.J.; Wang, Z.F.; Song, W.J. Sand priming improves alfalfa germination under high-salt concentration stress. Seed Sci. Technol. 2006, 34, 199–204. [Google Scholar] [CrossRef]
- Bray, C.M.; Davison, P.A.; Ashraf, M.; Taylor, R.M. Biochemical changes during osmopriming of leek seeds. Ann. Bot. 1989, 63, 185–193. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2005, 59, 206–216. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Amjad, M.; Jahangir, M.M.; Khan, S.M.; Cui, H.; Hu, J. Induction of salt tolerance in tomato (Lycopersicon esculentum Mill.) seeds through sand priming. Aust. J. Crop Sci. 2012, 6, 1199–1203. [Google Scholar]
- Zhai, K.; Zhao, G.; Jiang, H.; Sun, C.; Ren, J. Overexpression of maize ZmMYB59 gene plays a negative regulatory role in seed germination in Nicotiana tabacum and Oryza sativa. Front. Plant Sci. 2020, 11, 564665. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, T. Improving the assessment method of seed vigor in Cunninghamia lanceolata and Pinus massoniana based on oxygen sensing technology(Article). J. For. Res. 2012, 23, 95–101. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.X.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA(4) interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pradhan, C.; Jana, B.B. Acclimation induced responses of SDH activity of tilapia (Oreochromis mossambicus) following Introduction in a new pond habitat. J. Appl. Aquac. 2009, 21, 169–182. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Torres, N.H.; Aguiar, M.M.; Romanholo Ferreira, L.F.; Pine Americo, J.H.; Machado, A.M.; Cavalcanti, E.B.; Tornisielo, V.L. Detection of hormones in surface and drinking water in Brazil by LC-ESI-MS/MS and ecotoxicological assessment with Daphnia magna. Environ. Monit. Assess. 2015, 187, 379. [Google Scholar] [CrossRef]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, J.; Chen, G.; Jiang, Y. Effect of sand priming on aged seed vigor and physiological changes of transgenic insect resistant cotton. Seed 2013, 32, 20–24. (In Chinese) [Google Scholar]
- Zhao, G.; Zhong, T.; Zheng, D. Improving the field emergence performance of super sweet corn by sand priming. Plant Prod. Sci. 2009, 12, 359–364. [Google Scholar] [CrossRef]
- Wang, M.M.; Qu, H.B.; Zhang, H.D.; Liu, S.; Li, Y.; Zhang, C.Q. Hormone and RNA-seq analyses reveal the mechanisms underlying differences in seed vigour at different maize ear positions. Plant Mol. Biol. 2019, 99, 461–476. [Google Scholar] [CrossRef]
- Zhao, G.W.; Zhong, T.L. Influence of exogenous IAA and GA on seed germination, vigor and their endogenous levels in Cunninghamia lanceolata. Scand. J. For. Res. 2013, 28, 511–517. [Google Scholar] [CrossRef]
- Zhao, G.; Jiang, X. Roles of gibberellin and auxin in promoting seed germination and seedling vigor in Pinus massoniana. For. Sci. 2014, 60, 367–373. [Google Scholar]
- Iqbal, M.; Ashraf, M.; Jamil, A.; Ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Plant Biol. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 00420. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wang, P.; Sun, J.K.; Li, M. Cloning of GID1-homologous genes and expression analysis during seed germination in Zanthoxylum dissitum Hemsl. Zhi Wu Sheng Li Xue Bao 2017, 53, 1499–1506. [Google Scholar]
- Nkang, A.; Chandler, G. Changes during embryogenesis in rainforest seeds with orthodox and recalcitrant viability characteristics. J. Plant Physiol. 1986, 126, 243–256. [Google Scholar] [CrossRef]
- Hartman, T.; Weinrick, B.; Vilcheze, C.; Berney, M.; Tufariello, J.; Cook, G.M.; Jacobs, W.R. Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1004510. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).