Antioxidant, Anti-Inflammatory Activities, and Neuroprotective Behaviors of Phyllanthus emblica L. Fruit Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of P. emblica Extracts
2.3. Antioxidant Activities
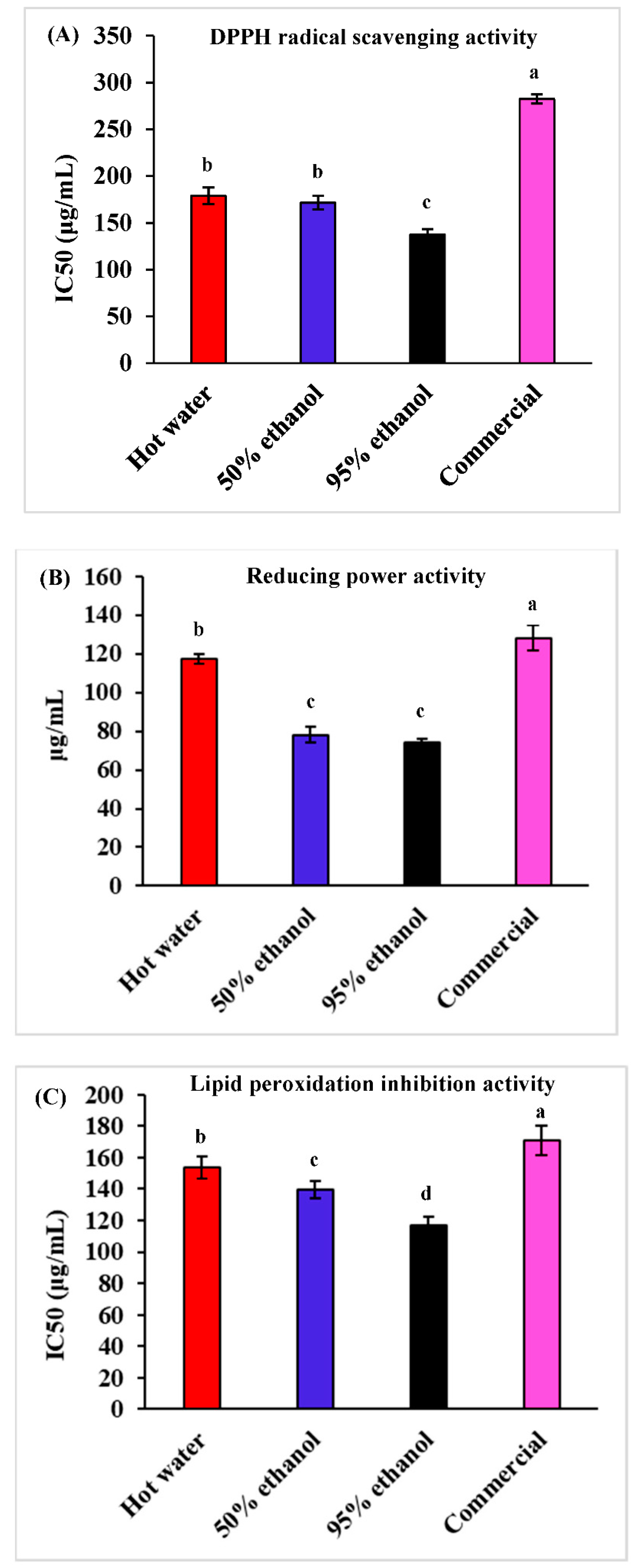
2.3.1. DPPH Radical Scavenging Activity
2.3.2. Reducing Power Activity
2.3.3. Lipid Peroxidation Inhibition Activity
2.4. Anti-Inflammation Activity and Neuroprotection
2.4.1. NO Inhibition
2.4.2. COX-2 Inhibition
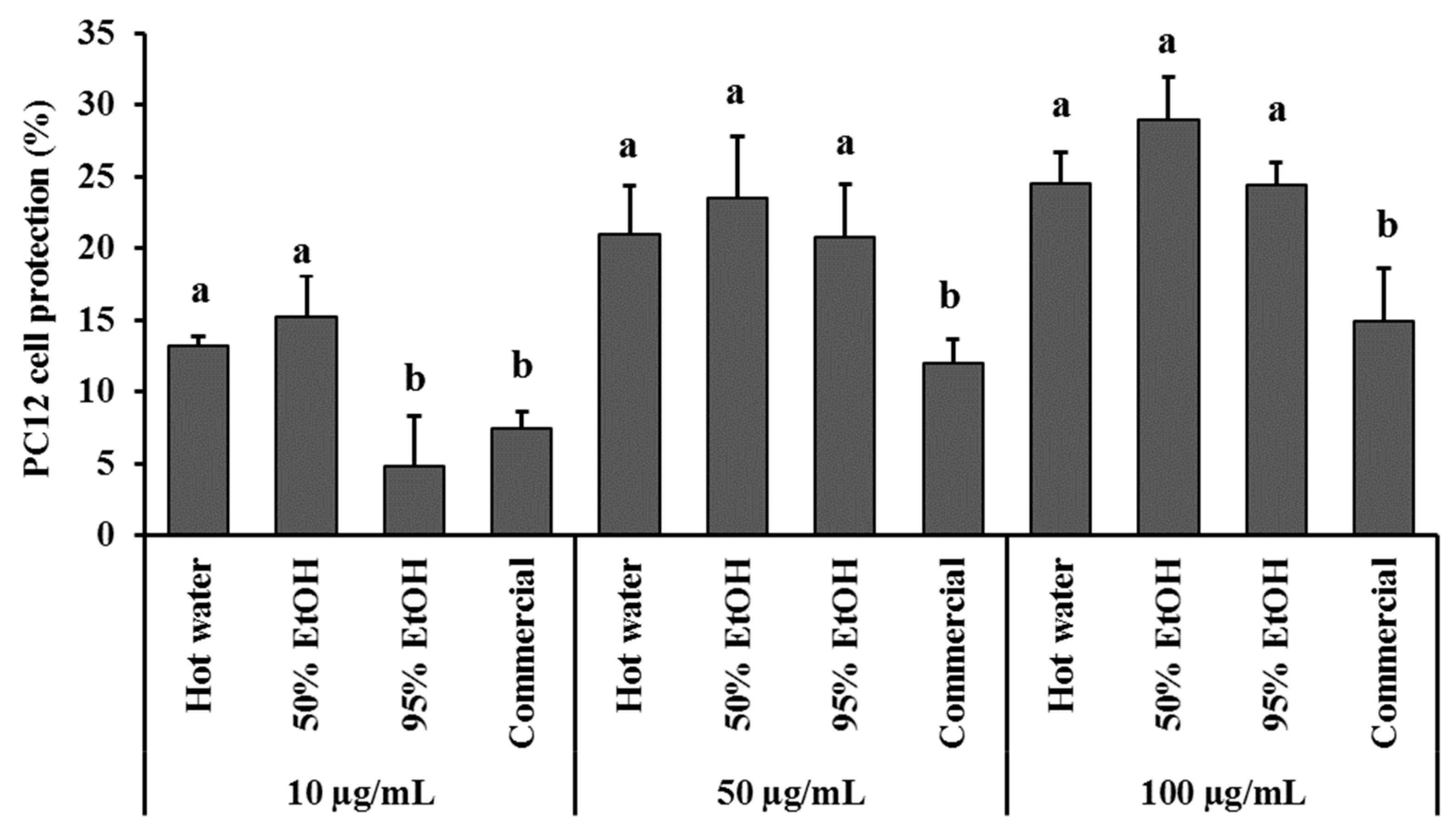
2.4.3. PC12 Cell Protection against H2O2 Damage
2.5. Total Flavonoid Content
2.6. Total Phenolic Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Neuroprotection Effect
3.4. Total Phenolic and Flavonoid Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.J.; Tanaka, T.; Iwamoto, Y.; Yang, C.R.; Kouno, I. Phyllaemblic acid, a novel highly oxygenated norbisabolane from the roots of Phyllanthus emblica. Tetrahedron Lett. 2000, 41, 1781–1784. [Google Scholar] [CrossRef]
- Rani, P.; Khullar, N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. J. Phytother. Res. 2004, 18, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Wang, J.; Yang, B.; Jiang, Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J. Food Compos. Anal. 2008, 21, 219–228. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 2011, 126, 277–282. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Lin, H.H.; Charles, A.L.; Hsieh, C.W.; Lee, Y.C.; Ciou, J.Y. Antioxidant effects of 14 Chinese traditional medicinal herbs against human low-density lipoprotein oxidation. J. Tradit. Complement. Med. 2015, 5, 51–55. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Liu, H.; Yuan, Z.L.; Wei, D.W.; Ye, Y.Z. Evaluation of antioxidant activity of chrysanthemum extracts and tea beverages by gold nanoparticles-based assay. Colloids Surf. B 2012, 92, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ozen, T.; Cöllü, Z.; Korkmaz, H. Antioxidant properties of Urtica pilulifera root, seed, flower, and leaf extract. J. Med. Food 2010, 13, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Miao, M.; Xia, H.; Yang, L.G.; Wang, S.K.; Sun, G.J. Antioxidant activities of aqueous extracts from 12 Chinese edible flowers in vitro and in vivo. Food Nutr. Res. 2016, 61, 1265324. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.J.; Lim, P.E.; et al. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Ceylan, O.; Tepe, B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crop. Prod. 2020, 154, 112632. [Google Scholar] [CrossRef]
- Le, D.H.T.; Chiu, C.; Chan, Y.; Wang, C.R.; Liang, Z.; Hsieh, C.; Lu, W.; Mulio, A.T.; Wang, Y.; Li, P. Bioactive and physicochemical characteristics of natural food: Palmyra palm (Borassus flabellifer Linn.) syrup. Biology 2021, 10, 1028. [Google Scholar]
- Le, D.H.T.; Lu, W.; Li, P. Sustainable Processes and Chemical Characterization of Natural Food Additives: Palmyra Palm (Borassus Flabellifer Linn.) Granulated Sugar. Sustainability 2020, 12, 2650. [Google Scholar]
- Chen, S.J.; Chung, J.G.; Chung, Y.C.; Chou, S.T. In vitro antioxidant and antiproliferative activity of the stem extracts from Graptopetalum paraguayense. Am. J. Chin. Med. 2008, 36, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Guan, X.Q.; Reis, J.C.; Papasian, C.J.; Jabre, S.; Morrison, D.C.; Qureshi, N. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids Health Dis. 2012, 11, 76. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, R.; Li, Y.; Miao, J.; Liu, G.; Lan, Y.; Chen, Y.; Cao, Y. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J. Ethnopharmacol. 2020, 254, 112740. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Yen, G.-C. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Vijayakumar, S.; Praseeth, P.K. Neuroprotective behaviour of Phyllanthus emblica (L) on human neural celllineage (PC12) against glutamate-induced cytotoxicity. Gene Rep. 2019, 17, 100545. [Google Scholar] [CrossRef]
- Li, P.; Chan, Y.; Lu, W.; Huang, D.; Chang, T.; Chang, W.; Nie, X.; Jiang, C.; Zhang, X. Bioresource Utilization of Djulis (Chenopodium formosanum) Biomass as Natural Antioxidants. Sustainability 2020, 12, 5926. [Google Scholar] [CrossRef]
- Mahajan, A.; Sharma, R. COX-2 selective nonsteroidal anti-inflammatory drugs: Current status. J. Assoc. Physicians India 2005, 53, 200–204. [Google Scholar] [PubMed]
- Jayanthi, P.; Lalitha, P. Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. 3), 126–128. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Rikans, L.E.; Hornbrook, K.R. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta 1997, 1362, 116–127. [Google Scholar] [CrossRef]
- Víteček, J.; Lojek, A.; Valacchi, G.; Kubala, L. Arginine-based inhibitors of nitric oxide synthase: Therapeutic potential and challenges. Mediat. Inflamm. 2012, 2012, 22. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.-C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Ganju, L.; Karan, D.; Chanda, S.; Srivastava, K.K.; Sawhney, R.C.; Selvamurthy, W. Immunomodulatory effects of agents of plant origin. Biomed. Pharmacother. 2003, 57, 296–300. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods Hum. Nutr. 2006, 61, 1–5. [Google Scholar] [CrossRef]
- Marinova, G.; Batchvarov, V. Evaluation of the Methods for Determination of the Free Radical Scavenging Activity by Dpph. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Rüegg, C.; Zaric, J.; Stupp, R. Non steroidal anti-inflammatory drugs and COX-2 inhibitors as anti-cancer therapeutics: Hypes, hopes and reality. Ann. Med. 2003, 35, 476–487. [Google Scholar] [CrossRef]
- Smith, E.R.; Daly, M.B.; Xu, X. A Mechanism for Cox-2 Inhibitor Anti-Inflammatory Activity in Chemoprevention of Epithelial Cancers A Mechanism for Cox-2 Inhibitor Anti-Inflammatory Activity in Chemoprevention of Epithelial Cancers. Cancer Epidemiol. Biomark. Prev. 2004, 13, 144–145. [Google Scholar] [CrossRef]
- Kumar, G.P.; Navya, K.; Ramya, E.M.; Venkataramana, M.; Anand, T.; Anilakumar, K.R. DNA damage protecting and free radical scavenging properties of Terminalia arjuna bark in PC-12 cells and plasmid DNA. Free Radic. Antioxid. 2013, 3, 35–39. [Google Scholar] [CrossRef]
- Park, J.B. Protective effects of veskamide, enferamide, becatamide, and oretamide on H2O2-induced apoptosis of PC-12 cells. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 843–847. [Google Scholar] [CrossRef]
- Fujita, Y.; Izawa, Y.; Ali, N.; Kanematsu, Y.; Tsuchiya, K.; Hamano, S.; Yoshizumi, M. Pramipexole protects against H2O2-induced PC12 cell death. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 372, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Golechha, M.; Bhatia, J.; Ojha, S.; Arya, D.S. Hydroalcoholic extract of Emblica officinalis protects against kainic acid-induced status epilepticus in rats: Evidence for an antioxidant, anti-inflammatory, and neuroprotective intervention. Pharm. Biol. 2011, 49, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Mayachiew, P.; Devahastin, S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. LWT—Food Sci. Technol. 2008, 41, 1153–1159. [Google Scholar] [CrossRef]



| Extract Samples | Total Phenol (mg GA/g Extract) | Total Flavonoid (mg RU/g Extract) |
|---|---|---|
| Hot water | 196.9 ± 2.1 c | 2.40 ± 0.01 c |
| 50 % Ethanol | 276.0 ± 0.8 b | 3.40 ± 0.01 b |
| 95 % Ethanol | 354.5 ± 2.5 a | 4.19 ± 0.11 a |
| Commercial | 172.2 ± 4.7 d | 4.17 ± 0.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.-H.; Wang, C.-W.; Lu, W.-C.; Song, T.-Y.; Wang, C.-C.R. Antioxidant, Anti-Inflammatory Activities, and Neuroprotective Behaviors of Phyllanthus emblica L. Fruit Extracts. Agriculture 2022, 12, 588. https://doi.org/10.3390/agriculture12050588
Li P-H, Wang C-W, Lu W-C, Song T-Y, Wang C-CR. Antioxidant, Anti-Inflammatory Activities, and Neuroprotective Behaviors of Phyllanthus emblica L. Fruit Extracts. Agriculture. 2022; 12(5):588. https://doi.org/10.3390/agriculture12050588
Chicago/Turabian StyleLi, Po-Hsien, Chien-Wen Wang, Wen-Chien Lu, Tuzz-Ying Song, and Chiun-C. R. Wang. 2022. "Antioxidant, Anti-Inflammatory Activities, and Neuroprotective Behaviors of Phyllanthus emblica L. Fruit Extracts" Agriculture 12, no. 5: 588. https://doi.org/10.3390/agriculture12050588
APA StyleLi, P.-H., Wang, C.-W., Lu, W.-C., Song, T.-Y., & Wang, C.-C. R. (2022). Antioxidant, Anti-Inflammatory Activities, and Neuroprotective Behaviors of Phyllanthus emblica L. Fruit Extracts. Agriculture, 12(5), 588. https://doi.org/10.3390/agriculture12050588








