Abstract
Pest management is being confronted with immense economic and environmental issues worldwide because of massive utilization and over-reliance on pesticides. The non-target toxicity, residual consequence, and challenging biodegradability of these synthetic pesticides have become a serious concern, which urgently requires the alternative and prompt adoption of sustainable and cost-effective pest control measures. Increasing attention in environmental safety has triggered interest in pest control approaches through eco-friendly plant-based pesticides. Botanical pesticidal constituents are effective against myriads of destructive pests and diseases. More importantly, they are widely available, inexpensive, accessible, rapidly biodegradable, and have little toxicity to beneficiary agents. The phytochemical compositions in diverse plant species are responsible for their varying mechanisms of action against pests and diseases. However, difficulties in their formulation and insufficient appropriate chemical data have led to a low level of acceptance and adoption globally. Therefore, the review seeks to highlight the status, phytochemical compositions, insecticidal mechanisms, and challenges of plant-based pesticide usage in sustainable agricultural production.
1. Introduction
In the era of Green Revolution technology, the 1950s and 1960s, crop production was intensified to meet food availability in low-income nations through substantial utilization of inputs such as inorganic fertilizers, synthetic pesticides, and genetically modified organisms [1,2]. About 67,000 species exhibit noticeable benefits on agricultural crops, and more than 70% of agricultural production would suffer colossal losses without proactive and preventive measures by utilizing agrochemicals [2,3]. Oftentimes, farmers prefer to adopt quick-fix pest management solutions, i.e., synthetic pesticides, to protect the crops from pest attack and damage [4]. However, the efficacious attributes and repetitive abuse of synthetic pesticides result in many bottlenecks, especially leading to pest resistance and health-related issues [5]. The indiscriminate use of these synthetic pesticides has caused unwanted consequences, such as environmental degradation, harm to non-target organisms, pesticide residues contaminating food and feed, pest resurgence, genetic variation in plants, and negative impacts on biodiversity [6,7].
The toxic effects of synthetic pesticides on human lives include but are not limited to the bioaccumulation of contaminants in food chains, which are widely spread in terrestrial and aquatic environments [8]. The most common synthetic pesticides (Dichlorodiphenyltrichloroethane and toxaphene) could result in residual effects on the soil for decades, and they could be washed off into groundwater and aquatic biota [9]. The persistence of human illnesses, either due to ingestion or exposure, lead to adverse effects on immune systems, impairment of neurodevelopmental and endocrine systems, metabolic diseases, cancer, and infertility [10,11], and these are key health risks related to the continuous use of synthetic pesticides [12]. In several rural settlements, the quality of synthetic pesticides is often compromised by dilution, improper mixing, and sale beyond expiry dates [13]. The World Health Organization reported that 200,000 people die annually across the world due to the direct impact of pesticides [14]. Notably, a large number of synthetic pesticides persist in the environment, thereby polluting the soil as well as water bodies and depleting the ozone layer [15,16]. It is worth noting that the consumption of synthetic pesticides in Africa was about USD 31 billion, which is 2–4% of the world pesticide market [17]. However, it has been reported that Africa has the highest human fatality rate risks because of the inappropriate use of pesticides [18]. The United Nations Environmental Program reported that the cost of pesticide-related illnesses could reach USD 90 billion in Sub-Saharan Africa between 2005 and 2020 [19]. The adverse consequences related to the inappropriate and overuse of chemical pesticides have necessitated the need for alternative means of pest management [20]. Thus, there is a greater focus on the development and research of botanical pesticides.
Botanical pesticides were widely utilized for millennia in both subsistence and commercial farming before the development of synthetic pesticides. Botanical pesticide ingredients are naturally occurring chemical derivatives of plants that act as repellants, attractants, antifeedants, and growth inhibitors [20,21,22]. When these compounds are extracted with appropriate solvents and/or mixed with necessary pesticide adjuvants, they become botanical pesticides. Due to the potency and efficacy of synthetic pesticides upon destructive crop diseases, the application of plant-based products slowly diminished until recently, when the heavy utilization of synthetic pesticides started posing an imminent threat to environmental safety and human health [23,24]. Botanicals are gaining popularity in organic farming due to their safety profile on crop consumption, and consumers are willing to pay a premium price for organic produce [25].
Several findings have been carried out to examine the known and unexploited plant species with pesticidal properties [26,27]. Hundreds of botanical pesticidal compounds have been discovered from many plants, including wild plants and herbal medicines. Many plants of Rutaceae, Compositae, Meliaceae, Leguminosae, Araceae, Platycodoniaceae, Solanaceae, Chenopodiaceae, Zingiberaceae, Labiatae, Loniceraceae, Umbelliferae, Polygonaceae, and Euphorbiaceae possess pesticidal attributes, and numerous compounds of plant secondary metabolites, including alkaloids, terpenoids, and flavonoids that show pesticidal activities. Interestingly, some commercially plant-based pesticides are mainly extracted from pyrethrum (Tanacetum cinerariifolium), tobacco (Nicotiana tabacum L.), neem (Azadirachta indica A. Juss), sabadilla (Schoenocaulon officinale Gray), and ryania (Ryania speciosa) [28]. The majority of plant-derived pesticides are used to control insect pests [29,30] and also mitigate plant parasites, such as nematodes, fungi, bacteria, and viruses [31,32]. Botanical pesticides could be useful as alternative tools for integrated pest management since they have positive impacts on environmental preservation, low toxicity to mammals, and low risk of developing resistance in target pests [33,34,35]. Thus, this review discusses vital information regarding the status, prospects, and challenges of botanical pesticides and their possible adoption and utilization for sustainable crop management.
2. Status of Botanical Pesticides
Plants offer many bioactive compounds that are vital for the relationship that exists between plants and their environments. There are several reports on ethnobotanical plants with known pest and disease compounds, as shown in Table 1. Globally, there are about 2500 species from 235 plant families that are effective in tackling pests [30,36].

Table 1.
Some botanicals, parts used, and extraction methods against targeted pests and diseases.
Bioactive Compounds from Botanicals
In ancient times, plant species and their secondary metabolites were exploited, especially in herbal traditional cultures [94]. Most times, plant species from different families might have related chemical structures for defense, such as isoflavonoids in the Fabaceae and sesquiterpenes in the Solanaceae [95]. Diverse studies established that botanical pesticidal constituents comprise a variety of isolated secondary metabolites that have behavioral and physiological effects (repellence, oviposition, feeding deterrence, acute toxicity, developmental disruption, and growth suppression) on agriculturally important pests and diseases [96].
Hyoscyamine, Atropine, and Scopolamine are bioactive compounds found in Datura, and they indicate repellent and oviposition deterrent activity against insect pests [97]. Calatropin and Calotoxin are bioactive ingredients present in calotropis, which manifest as antifeedant, repellent, oviposition deterrent, and insect growth regulator activities against insect pests [98,99]. Meanwhile, Vasicine, Vasicinone, and Adhatodin are bioactive ingredients available in the Adhatoda, and they exhibit repellent and insecticidal activity on insect pests [100,101]. Karanjin is the bioactive ingredient present in P. pinnata. It indicates antifeedant, Juvenile Hormone Analogue (JHA), and insecticidal activity against insect pests [102]. Furthermore, Azadiractin, Melantriol, Nimbinin, Nimbidin, Salanin, Nimbin, Nimbolin A, and Nimbolin B are the bioactive ingredients present in the neem leaf extract. These compounds show antifeedant, repellent, oviposition deterrent, and insect growth regulator activity against pests [25,103]. Allicin and diallyl sulfide are the bioactive ingredients present in garlic extract, which have insecticidal activity [104]. Chili extract contains Capsaicin as the active ingredient that has repellent and deterrent activity against insect pests [105]. Meanwhile, Lantanolic acid and Lantic acid are the bioactive compounds present in Lantana, which exhibit growth inhibition and repellent activity against pests [106]. Anonaine and squamocin are bioactive ingredients present in A. squamosa, which show feeding deterrent activity against pests [107]. However, these compounds have considerable benefits and are precursors in sustainable agriculture [108,109].
Botanical pesticidal compounds have noticeable effects against various agricultural pest species because of their varied mechanisms of action [110,111]. It has been shown that plant-based pesticides act specifically in the insect nervous system; affect γ-aminobutyric acid (GABA), gated chloride channels, and sodium channels; and competitively combine acetylcholinesterase, nicotinic acetylcholine receptors (nAChR), octopamine, and tyramine receptors [112,113]. All these possible mechanisms are shown in Table 2 and Table 3.

Table 2.
Some commercial botanical pesticides, bioactive compounds, and biological effects.

Table 3.
Mechanism of action of some botanicals.
3. Application of Botanical Pesticides
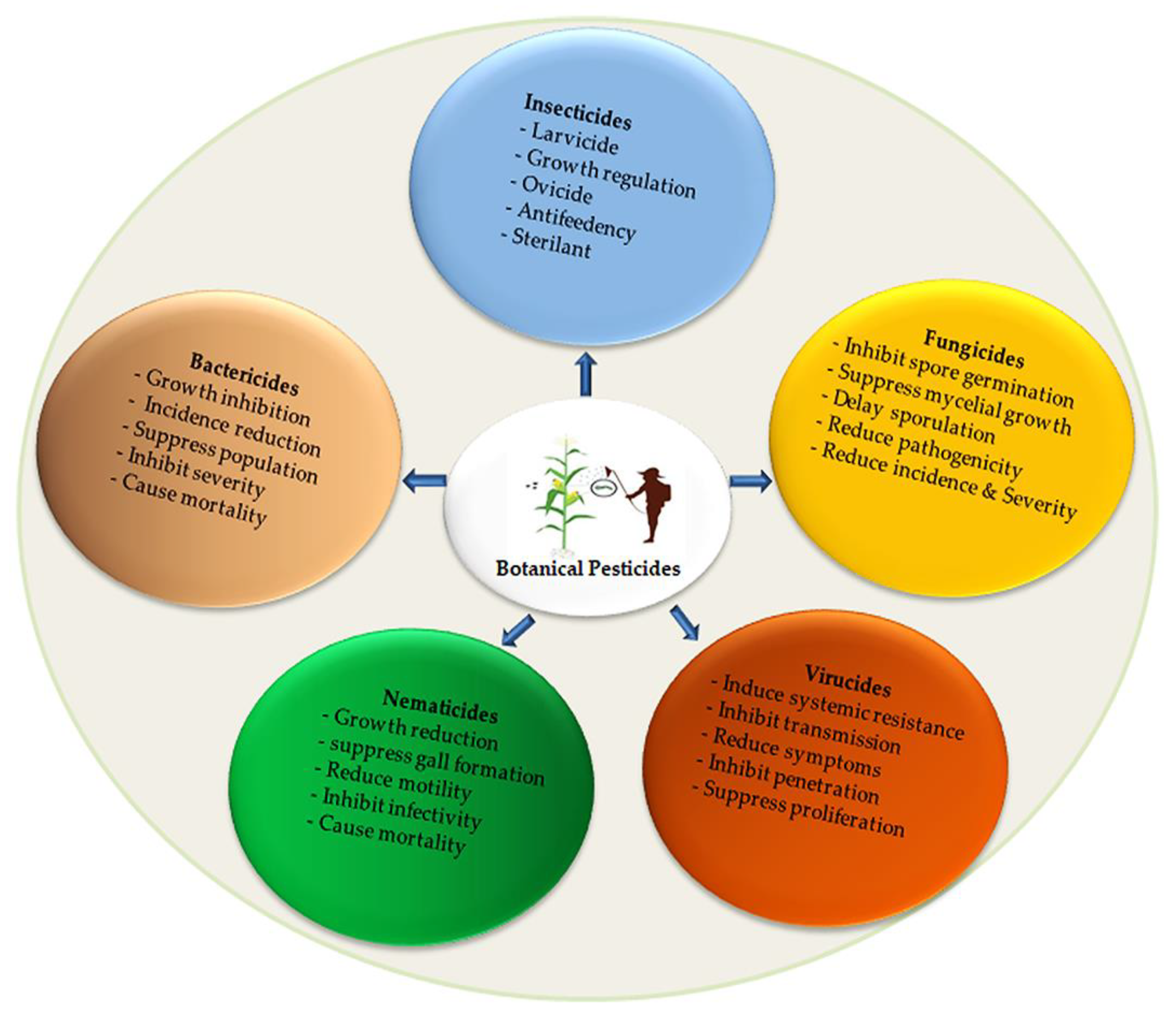
Many plant-based pesticides have been discovered, but significant amounts have yet to be isolated and analyzed to identify their bioactive ingredients. These sizeable sources of botanicals have been underutilized and neglected as pesticidal agents to manage a plethora of destructive pests and diseases. Thus, the application of botanicals as pesticidal agents and their effectiveness as alternative pest management in sustainable agricultural and in related fields have been researched. The products are utilized as insect repellents, antifeedants, insecticides, and insect growth inhibitors. Moreover, these botanical bioactive ingredients are applied as nematicides, fungicides, bactericides, and virucides, as shown in Figure 1. Apparently, several plants have pesticidal potential, vast research has been carried out, and several kinds of efficacies have been confirmed.
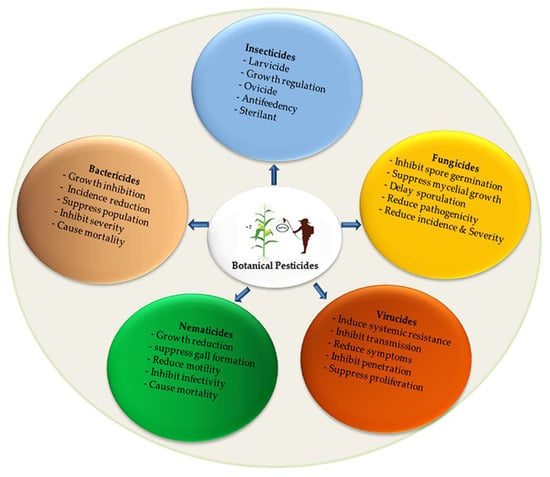
Figure 1.
Illustration of botanical pesticides application in agriculture.
3.1. Insecticidal Activities
Botanical pesticides, such as A. indica, A. sativum, C. cinerariaefolium, Datura metel, Hyptis suaveolens, L. camara, Mirabilis jalapa, R. speciose, and Tagetes minuta have been utilized to manage common bean (Phaseolus vulgaris L.) pests, including aphids, armyworms, bean leaf spot, bollworm, cabbage loopers, caterpillars, common grasshoppers, bruchid beetle, pink stalk borer, and thrips [134]. Carica papaya L. and T. minuta extracts were the most potent in suppressing the abundance of aphids and damages caused to the leaves. This might be because of the different insecticidal constituents contained in these plant materials [135]. The C. papaya leaf extract contains groups of cysteine protease enzymes such as papain, alkaloids, terpenoids, flavonoids, and non-protein amino acids that are deleterious to plant-sucking insect pests, including aphids, spotted bollworms, and whiteflies [136,137]. T. minuta leaf extracts possess different insecticidal compounds, such as phenylpropanoids, carotenoids, flavonoids, phototoxin alphaterthieenyl, and thiophenes, which are effective in controlling insect pests [138].
Azadirachtin exhibits multiple modes of action, which include antifeedancy, detrimental effects on morphology, alteration in biological fitness, fecundity suppression, decreased growth, oviposition repulsive, and even sterilization [139,140]. Azadirachtin induced harmful metamorphic effects, such as a slow rate of pupation time as well as reduced growth from larva-to-pupa, in D. melanogaster, S. frugiperda, and Callosobruchus maculatus [141,142]. Moreover, extract from neem bark exhibited anti-lepidopteran potency that was highly significant relative to neem leaf extracts because of its higher azadirachtin and nimbin contents [143]. Interestingly, there was an increase in the level of mortality and morphological impairments of the wings, legs, and scutellum based on the increase in the concentration of neem oil [144]. Leaf extracts from A. indica, O. sanctum, and Eucalyptus globulus had significant insecticidal activity against Aphis gossypi and Phenacoccus solenopsis in vitro [145]. Furthermore, neem leaf extract resulted in a significant decrease in the number of eggs laid and survival rates of adults of grains/seeds storage pests at a concentration of 1.5 mg/100 g [143]. An enrichment of organic fertilizers with neem leaf powder and boiler ash was observed to significantly improve the resistance of plants against aphids [146]. Azadirachtin induced lethal toxicity during the pupal phase, causing significant morphological impairments and prolonging the adult formation in D. melanogaster [140]. In a previous study, it was confirmed that 20 uM azadirachtin induced robust developmental caused a delay in the larva-to-pupae phase and resulted in molting defect [147]. Furthermore, higher concentrations of azadirachtin (0.1 and 1.0μg) hindered growth in the third instar of Lutzomyia longipalpis (Diptera) [148]. In addition, azadirachtin caused deformity of larvae, pupae, and adult, reduction in protein synthesis of pupae, low hemolymph volume during last instar larvae, inhibit enzyme activities of larvae gut, and lower cuticular protein level changes of larvae of S. litura [149,150]. Furthermore, azadirachtin had strong antifeedant impacts on D. melanogaster, Plutella xylostella, and Galleria mellonella [151,152]. Sublethal effects of azadirachtin, i.e., alteration in mating and post-mating behavior [153] and low rate of fecundity in D. melanogaster [154], were also reported. Several findings revealed that azadirachtin disrupted juvenile hormone and 20-hydroxyecdysone pathways, which resulted in partial larval development, sterile eggs, and decreased fecundity [155,156]. Azadirachtin led to 100% mortality after treating tobacco whitefly B. tabaci for 72 h through oral ingestion [142]. The mortality of the pollinator Bombus terrestris (Hymenoptera) ranged from 32 to 100% once exposed to azadirachtin at concentrations between 3.2 and 320 mg/L after 11 weeks [157].
Under laboratory conditions, 10% turmeric dust induced 80% mortality in rice pests (Cnaphalocrosis medinalis, Oxya nitidula), eggplant pests (Aphis gossypii, Coccidohystrix insolitus, Epilachna vigintioctopunctata, and Urentius hystricellus), okra pests (Amrasca devastans, Anomis flava, Dysdercus cingulatus, Earias vittella, Oxycarenus hyalinipennis, S. litura, and Tetranychus neocaledonicus), respectively [158]. Moreover, there was an inhibitory effect on the feeding activity at the first and second instar of S. frugiperda caterpillars when exposed to the turmeric, clove, and palmarosa plant oils [159].
Furthermore, essential oils could act as antifeedants, repellants, and oviposition deterrents which serve as larvicide, ovicide, and insecticide, thereby exhibiting compounds that interfere in all phases of insect metamorphosis [160]. The essential oil extracted from turmeric leaves and applied as contact or fumigant is efficacious against three main stored-product beetles, R. dominica, Sitophilus oryzae, and Tribolium castaneum [161]. Again, some essential oils, such as eucalyptus and rosemary, exhibit repellent effects against several insect species, including vectors [162]. The toxicity of eucalyptus and rosemary essential oils caused a neurotoxic mode of action in some insects, which caused hyperactivity and was followed by hyperexcitation, leading to a rapid knockdown and immobilization [163]. Allicin extracted from garlic bulbs (A. sativum) resulted in the suffocation of insect pests due to its negative effects on neurotransmitter receptors [164]. Ethanolic extracts of 2.08% to 5.80% (w/w) from Tagetes spp. inhibited growth and development of S. frugiperda [165]. Leaf powder of E. globulus showed insecticidal activity against Prostephanus trunatus [166]. The leaves powdered from Dalbergia saxatilis possessed protectant properties against cowpea bruchid, C. maculatus, which served as a respiratory poison [167]. For Piper guineense, P. longum, and P. retrofractum extracts caused 96–100% mortality rate in 48 hours of the management of C. maculatus, Zonocerus variegatus, and mosquito larvae [168]. O. sanctum oil (1000 ppm) caused 13.33% larval mortality in fourth instar larvae of S. litura [169,170].
In addition, saunf and khas oil led to the highest mortality (100%) and followed by clove oil (93.33%). The lowest larvae weight was seen in clove oil (−0.025 g and −0.06 g) at 1 and 2%, respectively [171]. Findings revealed that rhizome and its aerial part extract offered dose mortality activity towards T. castaneum adults [172]. Isodon rugosus and Daphne mucronata plant extracts act as possible pesticides against pea aphid, Acyrthosiphon pisum [173]. Onion (A. cepa) and ginger (Z. officinale) were assessed for effectiveness on tomato fruit worm (Helicoverpa zea), which resulted in a 70–80% decrease compared to control [42]. Over 50% rape aphids (Brevicoryne brassicae) and tomato red spider mites (Tetranychus evansi) were decreased by powder extracts from Lippia javanica and Solanum delaguense [174]. Extracts from Curcuma longa, A. sativum, and Ferula assafoetida significantly decreased both larvae and pupa of H. armigera [175]. Artemisia herbaalba, Eucalyptus camaldulensis, and R. officinalis extracts were applied on broad bean leaves, which led to 60–100% mortality of Myzus persicae after 24 hours of exposure in dose-dependent in vitro experiments [176]. An increase in concentrations of extracts from C. longa and A. sativum resulted in high mortality rates, reduced weight, and serve as anti-molting properties on larvae, pupa, and adults of T. castaneum [177]. Similarly, Eucalyptus terreticonis, Tagetes minuta, and L. camara extracts induced mortality of S. zeamais adults [178].
3.2. Fungi Management
Extracts from many plant species are discovered to be efficacious against several plant pathogenic microorganisms without imposing ill side effects. In addition to inhibiting germ tube elongation and mycelial development, plant-based bioactive compounds, including alcohols, alkaloids, phenols, tannins, and terpenes delay sporulation, DNA, and protein synthesis [179,180]. Moreover, they altered the structure of hypha and mycelia, inhibiting the production of toxic substances from mycotoxin-producing fungal such as Aspergillus spp. and Fusarium spp.; thus, reducing their pathogenicity [181]. Furthermore, curcumin had fungicidal activity against P. infestans, P. recondita, and R. solani with 100 and 63% at 500 mg/L, and 85%, 76%, and 45% at 250 mg/L, respectively [182]. Extracts of pawpaw leaves at concentrations of 20%, 40%, 60%, and 80% were more potent against A. solani [183]. Extracts from Ocimum gratissimum and E. globules inhibit wilting in cowpea seedlings induced by Sclerotium rolfsil from 39.6% for untreated to 4–12% for treated plants [64]. Methanol extract of turmeric rhizomes controlled the growth of anthracnose on red pepper caused by Colletotrichum coccodes [184]. The curcuminoids effectively inhibited the mycelial growth of three red pepper anthracnose pathogens, C. coccodes, C. gloeosporioides, and C. acutatum, in a range of 0.4–100 μg/mL [183]. Reports revealed turmeric essential oil and curcumin against plant pathogenic bacteria and fungus effectively [185,186]. Extracts from Ricinus communis effectively inhibited the growth of post-harvest pathogens Penicillium oxalicum and A. niger of yams [187].
Additionally, the prevalence of tomato fruit rot in many parts of Africa could be curtailed significantly with extracts from Cassia alata, Alchornea cordifolia, and Moringa oleifera as postharvest agents [188]. Post-harvest fungi, including F. solani, Rhizopus arrhizus, and Sclerotium rolfsii, were controlled after using extracts from Duranta erecta and Lasonia ineruis [189]. Fusarium verticillioides was treated with different plant extracts, causing cell wall disruption, loss of cellular components, and suppressed fumonisin and ergosterol production [190]. Whereas alcoholic extracts from suicide tree (Cerbera odollam), clove (S. aromaticum), and mahogany (Swietenia macrophyllai) at 3000 ppm repressed the growth of A. niger, Penicillium digitatum, and Fusarium sp. by 40–90% in citrus [145]. Botanical extracts have been shown to successfully mitigate late blight of potato (Phytophthora infestans) and Fusarium wilt of legumes [191,192]. Again, ginger extracts reduced the cytoplasm and changed the microconidia morphology of Fusarium sp. [193]. Extracts from Piper nigrum, Cinnamomum zeylanium, and C. cassia were reported as strong repellents to reduce the population of Megalurothrips sjostedti [194]. F. oxysporum f. sp. ciceris, a pathogen causing wilt of chickpea (Cicer arietinum) by up to 50%, could be controlled with methanolic extracts of Chenopodium ambrosioides [195].
A study found that neem (A. indica) and Mexican sunflower (T. diversifolia) extracts declined the growth of tomato rot disease, A. niger, F. oxysporum, and G. candidium up to 100% [49]. Allicin was reported to cause morphological abnormalities, including collapsing, thinning, and damaging of hyphal strands, thus, inhibiting germination of spores and hyphal growth [196]. A. sativum, Copaifera langsdorfii, C. zeylanicum, and Eugenia caryophyllata essential oils significantly reduced anthracnose of banana via immersion [197].
3.3. Bacteria Management
The botanical compositions also have multiple antibacterial properties. A study showed that acetone extracts of Aloe vera influenced growth of P. aeruginosa, while its methanolic extracts inhibited E. coli and B. subtilis. The phytochemicals that denature microbe proteins and disrupt their functionality were supposed to result in the antimicrobial activity of A. vera. For instance, cinnamic acid inhibits glucose uptake and ATP production [198]. Some botanical pesticides inhibited cellular processes against bacteria, and increased permeability of plasma membrane could lead to leakage of cell contents and cell death [199]. As for the essential oils of T. vulgaris, the presence of thymol causes membrane permeability and depolarization, resulting in the interference of cell mechanisms of Bacillus cereus, Klebsiella pneumonia, S. aureus, Salmonella typhimurim, and Escherichia coli [79,200].
D. metel substantially reduced the growth of X. oryzae pv. oryzae in vitro, and decreased bacterial leaf blight of rice in greenhouse, which mainly resulted from daturilin presence [201]. Utilization of D. metel as a seed treatment, root dip at transplanting time, and foliar spray influenced superior effectiveness against X. oryzae pv. oryzae in rice intensity by 59.17% in greenhouse and 46.02% in microplots as compared to control [202]. Cold and hot water extracts of leaves from Datura, Garlic, and Nerium applied two days pre-inoculation and two days post-inoculation on Super Marmande tomato cultivars considerably lowered the disease index of R. solanacearum wilt in greenhouse experiments [203]. Furthermore, [201] discovered that several plant extracts, including D. metel, A. indica, Ipoma cornea, Vitex negunda, and Zizyphus jujuba, had antibacterial properties against Xanthomona campestris pv. oryzae.
Under greenhouse and field trials, aqueous extracts of Hibiscus sabdariffa, Punica granatum, and E. globulus were found to effectively protect potato plants against bacterial wilt (R. solanacearum) [204]. A. sativum extract was extremely effective in safeguarding tomato plants against P. syringae pv., Xanthomonas vesicatoria, and Clavibacter michiganensis subsp., respectively [205]. A. sativum extract reduced the incidence of tomato bacterial wilt R. solanacearum population when applied to soil in vitro experiments [203]. Similarly, when compared to the leaf extract from L. camera and the bulb extract from A. sativum, the aqueous leaf extract from A. vasica was found to be extremely effective in preventing the growth of X. oryzae pv. oryzae [206].
Thymol and palmarosa oil were utilized for preplant soil fumigation and successfully reduced the incidence of tomato bacterial wilt (R. solanacearum) [207]. Oils of palmarosa and lemongrass were found to inhibit the growth of R. solanacearum race 4 (phylotype I) in potting media, as well as the occurrence of bacterial wilt in edible ginger [208]. In both greenhouse and field, soil biofumigation using palmarosa essential oil inhibited the severity of bacterial wilt of sweet peppers [209]. In vitro conditions, extract from A. fistulosum of 50 and 100% decreased growth of R. solanacearum, and preplant treatment of the soil with A. fistulosum considerably declined the R. solanacearum population compared to control [210]. C. longa decoctions were the most effective in controlling bacteria leaf blight (BLB) in rice plants, both in the lab and field [211]. Again, [212] ascertained that using neem gold (20 mL/L) decreased bacterial blight severity and increased rice yield.
3.4. Nematodes Management
Death in second-stage juveniles of root-knot nematode (Heterodera cajani) was influenced by essential oils derived from pesticidal plants [213]. Again, lipophilic phytochemicals could easily disintegrate the cytoplasmatic membrane of nematodes by hindering protein structures that serve to enhance growth, development, and survival [214]. The crushed leaves of African 17 marigolds have been utilized by some farmers to manage nematodes [215]. Some plant constituents have affected the soil microbes population, which led to a reduction in eggs and survivability of the larvae of nematodes [216]. Whereas some compounds caused second-stage larvae mortality and toxicity, reduced egg mass and galling, which suppressed nematode accumulation level [217]. Under controlled conditions, the use of L. camara and Trichoderma harzianum inhibited reproduction rate, egg masses, and gall formation in root-knot nematodes in tomato crops [31]. Meanwhile, a decrease in the number of eggs hatched, mobility, and death of juveniles of root-knot nematode at the second stage was ascribed to bioactive compounds, including alkaloids, tannins, and glycosides presence [218,219]. It has been discovered that active compounds extracted from pesticidal plants could cause paralysis and limit infectivity potential of juvenile root-knot nematodes [220].
Under laboratory study, aqueous extracts from C. grandis, C. benghalensis, L. cephalotes, P. amarus, and T. portulacastrum recorded high nematicidal efficacy on egg hatchability and juvenile mortality of M. incognita [221]. The aqueous extracts from bark, leaf, and seed of Cassia siamea, Cassia sieberiana, Dolnix regia, Isoberlinia doka, Tamarindus indica, Ocimum gratisimmum, and H. suaveolens, drastically reduced egg hatching of M. incognita [222,223]. According to [224], their result showed that 24 h exposure of extract from dry leaves from neem influenced juvenile mortality (100%) compared to untreated. More so, freshly chopped leaves from P. amarus at a rate of 100 g/pot induced a high root gall index and affected the population of egg masses and M. incognita in the soil [221].
3.5. Viruses Management
Some plant compounds are found to induce systemic resistance of the host plants with antiviral properties by inhibiting the transmission of viruses and killing insect vectors [32]. Moreover, studies have reported that these plant compounds inhibit virus penetration and replication, hemagglutination, and enzymatic activity [225,226]. Under laboratory conditions, Tobacco Mosaic Virus (TMV) was highly affected by acetone extracts from cottonseed oil. Additionally, under field conditions, Rice Stripe Virus (RSV) and Southern Rice Black Streaked Dwarf Virus (SRBSDV) diseases were reduced. Gossypol and sitosterol compounds were identified antivirals in sludge from cottonseed oil because the derivatives from gossypol and sitosterol inhibited the formation of cell fusion-activated cores and apoptosis, respectively [68]. Furthermore, extract from T. orientalis suppressed the proliferation of Mosaic Virus of Watermelon through a reduction in virus infection on the hypocotyls as a result of the obstruction of the release of nucleic acids [89].
At 6 g/L, extracts from T. orientalis and Artemisia campestris inhibited potato leafroll virus proliferation by 81.72 and 63.6%, respectively [227]. The study disclosed extracts from Paronychia argentea extraction (10 g/mL) via foliar spray within 24 hours prior to viral inoculation decreased disease symptoms, improved plant growth, and lowered Tobacco Mosiac Virus accumulation levels in treated tomato plants under greenhouse conditions [228]. Plant extracts from Eucaluptus camaldulensis, Clerodendrum aculeatum, Haplophyllum tuberculatum, M. jalapa, Potentilla arguta, Boerhaavia diffusa, Sambucus racemose, and T. orientalis inhibited plant virus infection [229,230].
Under field conditions, resistance was induced against Bean Common Mosaic Virus (BCMV) by extracts from A. indica, Plectranthus tenuiflorus, Schinus terebinthifolius, and Clerodendrum inerme [231]. Thuja, Tamarix, and Henna extracts inhibited Tomato Yellow Leaf Curl Virus proliferation within the 10–12 day protection period in tomato plants [232].
4. Challenges of Botanical Pesticides
Despite the stiff competition between plant-based and synthetic pesticides, the former is not common in the market [233]. Moreover, plant-based pesticide effectiveness in the field is extremely contingent on prevailing environmental and weather conditions as they are easily degradable [43]. Several problems related to contamination, preparation potency, attenuation of pesticide activity, and shelf life are evident. It is often challenging to standardize botanical pesticide dosages due to variance growth habitations, varietal differences, harvest duration, extraction methods, and storage conditions [115]. The appropriate formulation is gravely challenging because multiple bioactive constituents are evident in one plant species that differ in chemical properties [234].
There are major obstacles faced with the commercialization of plant-based pesticides viz: (a) limited supply of botanical raw materials; (b) poor standardization and quality control of the required active ingredients; (c) issues with regulatory approval, i.e., costly toxicological evaluation of the botanical pesticide [96,117]. Regardless of the minimal toxicity displayed by botanical pesticides, their regulatory implementations in agricultural production are not different from synthetic products, particularly in low-income nations [235]. Considering bottlenecks, these products need to pass through rigorous registration, environmental, and toxicological assessments processes [113,236].
5. Conclusions and Recommendations
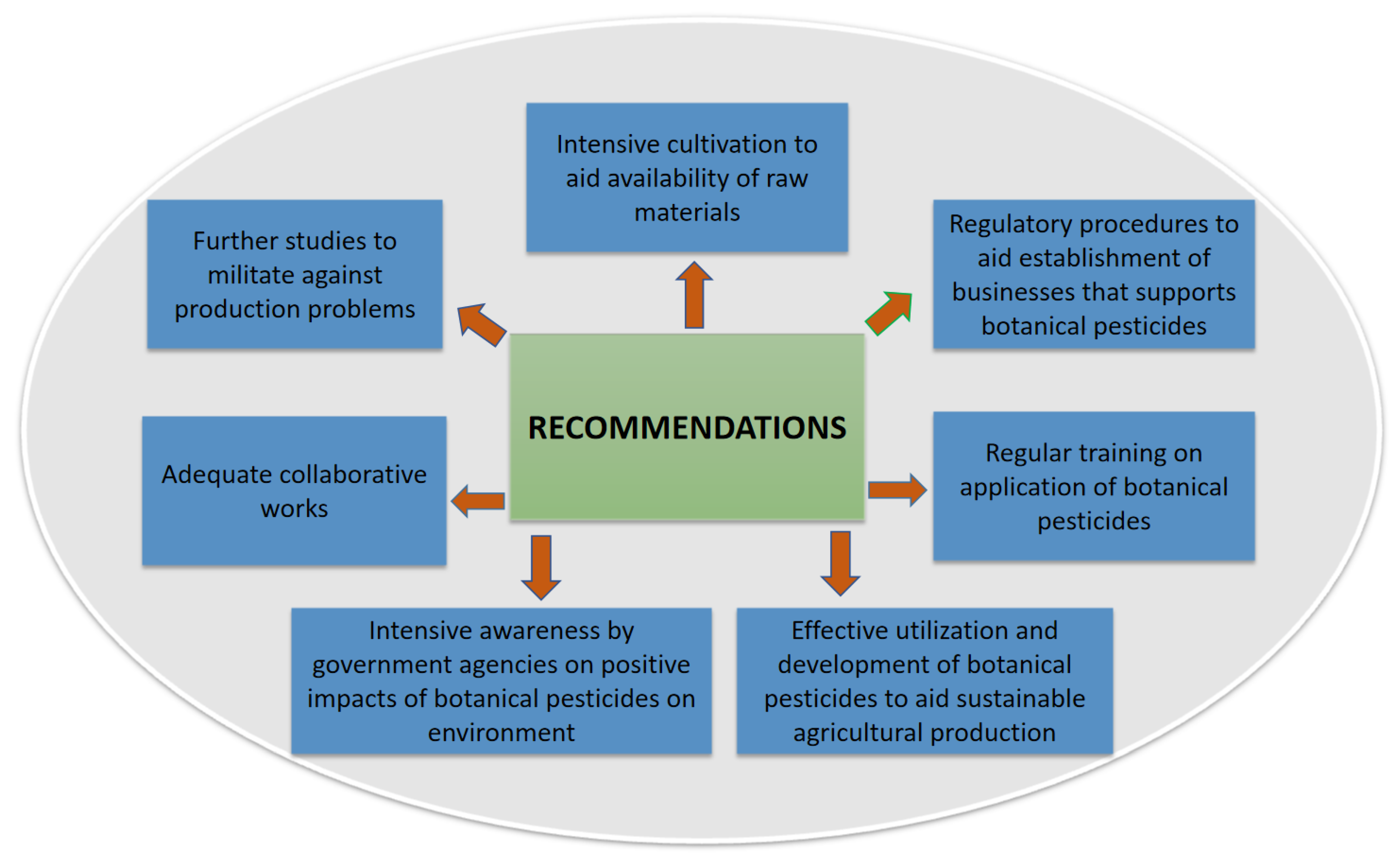
The effectiveness of botanical pesticides to manage agriculturally economic pests is vital due to their renewable tendency, significant environmental safety, and human welfare. Plant-based pesticides are predominantly used in low-income and emerging nations to control pests because of their cost-effectiveness, availability, accessibility, and easy-to-use. Nevertheless, the discovery of the active ingredients from pesticidal plants is still at a rudimentary level. Current strives in enhancing the characterization of efficient phytochemicals and their concentrations in final products are challenging as precision and standardization are still bottlenecks. Thus, in order to enhance and promote the full utilization and adoption of botanical pesticides as safer and sustainable pest management options in integrated pest management systems, this review proposes recommendations as follows.
- Considering the huge quantum of raw materials required to produce plant-based pesticides, intensive cultivation of plant sources should be done to ensure the availability of raw materials for industrial uses.
- Further studies to address the limitations confronted with botanical pesticides bordering on formulations, active ingredients, application rates, storage stability, and volatility under ultraviolet light may enhance the significant commercialization of botanical pesticides.
- In a bid to facilitate market penetration of botanical pesticides, there is a need for collaboration among researchers, investors, manufacturers, marketers, and farmers with the sole aim of sustainable benefits that must be established.
- Addressing the huge constraints of regulatory procedures might promote feasibility and affordability of businesses that would encourage entrepreneurs, public funding of agro-programs, investors, and large pesticide companies to enhance the enterprising of botanical pesticides.
- Regular training on easy production and application techniques by low-income farmers and extension workers to disseminate botanical pesticide usage is essential for better adoption.
- Considering the concerns for environmental safety across the globe, there is a need to promote intensive awareness by government agencies among farmers and manufacturers by sensitizing them to the importance of switching over to botanical pesticides for a sustainable pest management approach.
- Thus, various aspects bordering on constraints, prospects, and regulatory networks towards effective utilization, research, and development of botanical pesticides in sustainable agricultural production should be reviewed often.
The summary of the recommendations is illustrated in Figure 2 below.
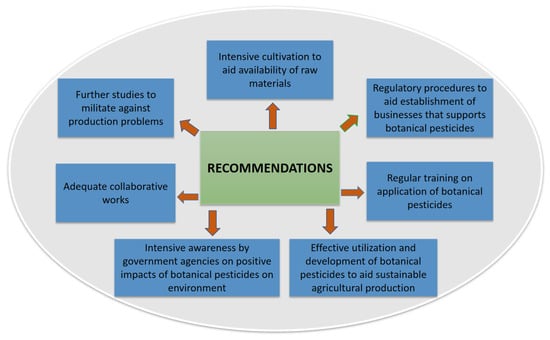
Figure 2.
Recommendations to improve the use of botanical pesticides.
Author Contributions
Conceptualization: G.Z and P.M.N.; G.C. and P.M.N. reviewed the literature and wrote the initial draft of the paper with assistance from M.Z.K.; G.Z. contributed to revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangdong Province Science and Technology Plan Project (No. 2014B020206003) and Basic and Applied Basic Research Foundation of Guangdong Province, China (No. 2019A1515110311).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef] [PubMed]
- Archana Singh, S.K. Biopesticides for integrated crop management: Environmental and regulatory aspects. J. Biofertil. Biopestic. 2014, 5, e121. [Google Scholar] [CrossRef]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Nkechi, E.F.; Ejike, O.G.; Ihuoma, N.J.; Maria-Goretti, O.C.; Francis, U.; Godwin, N.; Njokuocha, R. Effects of aqueous and oil leaf extracts of Pterocarpus santalinoides on the maize weevil, Sitophilus zeamais, pest of stored maize grains. Afr. J. Agric. Res. 2018, 13, 617–626. [Google Scholar] [CrossRef]
- Shabana, Y.M.; Abdalla, M.E.; Shahin, A.A.; El-Sawy, M.M.; Draz, I.S.; Youssif, A.W. Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina. Egypt. J. Basic Appl. Sci. 2017, 1, 67–73. [Google Scholar] [CrossRef]
- Kumar, S. Biopesticides: A Need for Food and Environmental Safety. J. Biofertil. Biopestici. 2012, 3, e107. [Google Scholar] [CrossRef]
- Fountain, E.D.; Wratten, S.D. Conservation biological control and biopesticides in agricultural. Encycl. Ecol. 2013, 1, 377–381. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, M.; Husak, V.V. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Kumari, K.A.; Kumar, K.N.R.; Rao, C.N. Adverse effects of chemical fertilizers and pesticides on human health and environment. J. Chem. Pharm. Res. 2014, 3, 150–151. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Nyirenda, S.P.; Mvumi, B.M.; Sola, P.; Kamanula, J.F.; Sileshi, G.W.; Belmain, S.R. Pesticidal plants: A viable alternative insect pest management approach for resource-poor farming in Africa. In Botanicals in Environment and Food Security; Koul, O., Dhaliwal, G.S., Khokhar, S., Singh, R., Eds.; Scientific Publishers: Jodhpur, India, 2012; pp. 212–238. [Google Scholar]
- Belmain, S.R.; Haggar, J.; Holt, J.; Stevenson, P.C. Managing Legume Pests in Sub-Saharan Africa: Challenges and Prospects for Improving Food Security and Nutrition through Agro-Ecological Intensification; Natural Resources Institute, University of Greenwich: Chatham Maritime, UK, 2013; p. 34. [Google Scholar]
- Sande, D.; Mullen, J.; Wetzstein, M.; Houston, J. Environmental impacts from pesticide use: A case study of soil fumigation in Florida tomato production. Int. J. Environ. Res. Public Health 2011, 12, 4649–4661. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.A.; Wimalawansa, S.J. Agrochemical-related environmental pollution: Effects on human health. Glob. J. Biol. Agric. Health Sci. 2014, 3, 72–83. [Google Scholar]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Secur. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Williamson, S.; Ball, A.; Pretty, J. Trends in pesticide use and drivers for safer pest management in four African countries. Crop Prot. 2008, 27, 1327–1334. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). 2011. Available online: http://www.unep.org/chemicalsandwaste/UNEPsWork/Pesticides/tabid/298/Default.aspx (accessed on 20 September 2021).
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baeshen, R.S.; Said-AlAhl, H.A. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Stankovic, S.; Kostic, M.; Kostic, I.; Krnjajic, K. Practical Approaches to Pest Control: The Use of Natural Compounds. In Pests-Classification, Management and Practical Approaches; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Raja, N. Botanicals: Sources for eco-friendly biopesticides. J. Biofertil. Biopestic. 2014, 5, e122. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Mohammad, B.; Habibi, N.; Farhoosh, R. Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef]
- Misra, H.P. Role of botanicals, biopesticides and bioagents in integrated pest management. Odisha Rev. 2014, 2, 62–67. [Google Scholar]
- Erenso, T.F.; Berhe, D.H. Effect of neem leaf and seed powders against adult maize weevil (Sitophilus zeamais Motschulsky) mortality. Agric. Res. 2016, 2, 90–94. [Google Scholar] [CrossRef]
- Jawalkar, N.; Zambare, S.; Zanke, S. Insecticidal property of Datura stramonium L. seed extracts against Sitophilus oryzae L. (Coleoptera: Curculionidae) in stored wheat grains. J. Entomol. Zool. Stud. 2016, 4, 92–96. [Google Scholar]
- Arnason, J.T.; Sims, S.R.; Scott, I.M. Natural products from plants as insecticides. Encycl. Life Support Syst. 2012, pp. 1–8. Available online: http://www.eolss.net/sample-chapters/c06/e6-151-13.pdf (accessed on 25 September 2021).
- Mkenda, P.A.; Stevenson, P.C.; Ndakidemi, P.; Farman, D.I.; Belmain, S.R. Contact and fumigant toxicity of five pesticidal plants against Callosobruchus maculatus (Coleoptera: Chrysomelidae) in stored cowpea (Vigna unguiculata). Int. J. Trop. Insect Sci. 2015, 35, 172–184. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Indust. Crops Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Feyisa, B.; Lencho, A.; Selvaraj, T.; Getaneh, G. Evaluation of some botanicals and Trichoderma harzianum for the management of tomato root-knot nematode (Meloidogyne incognita (Kofoid and White) Chit Wood). Adv. Crop Sci. Technol. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Waziri, H.M.A. Plants as antiviral agents. J. Plant Pathol. Microbiol. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Ahmad, S.; Ansari, M.S.; Moraiet, M.A. Demographic changes in Helicoverpa armigera after exposure to neemazal (1%EC azadirachtin). Crop Prot. 2013, 50, 30–36. [Google Scholar] [CrossRef]
- Neeraj, G.S.; Kumar, A.; Ram, S.; Kumar, V. Evaluation of nematicidal activity of ethanolic extracts of medicinal plants to Meloidogyne incognita (kofoid and white) chitwood under lab conditions. Int. J. Pure Appl. Biosci. 2017, 1, 827–831. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, L.; Sun, T.; Fu, S.J.; Hu, M.Y.; Zhong, G.H. Inhibition of Echinochloa crusgalli using bioactive components from the stems and leaves of Camellia oleifera. Int. J. Agric. Biol. 2017, 195, 1031–1038. [Google Scholar] [CrossRef]
- Roy, S.; Handique, G.; Muraleedharan, N.; Dashor, K.; Roy, S.M.; Mukhopadhyay, A.; Babu, A. Use of plant extracts for tea pest management in India. Appl. Microbiol. Biotechnol. 2016, 100, 4831–4844. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, W.; Yang, C.; Hua, H. Supercritical fluid CO2 extraction of Acorus calamus L. (Arales: Araceae) and its contact toxicity to Sitophilus zeamais Motschusky (Coleoptera: Curculionidae). Nat. Prod. Res. 2012, 26, 1498–1503. [Google Scholar] [CrossRef]
- Madhiazhagan, K.; Ramadoss, N.; Anuradha, R. Effect of botanicals on bacterial blight of rice. J. Mycol. Plant Pathol. 2002, 32, 68–69. [Google Scholar]
- Ponnanna, K.M.; Adiver, S.S. Fungicidal management of grey mildew of cotton. Plant Pathol. Newsl. 2001, 19, 6–8. [Google Scholar]
- Jha, M.M.; Kumar, S.; Hasan, S. Effect of botanicals on maydis leaf blight of maize in vitro. Ann. Biol. 2004, 20, 173–176. [Google Scholar]
- Abd-El-Khair, H.; Wafaa, M.H. Application of Some Egyptian Medicinal Plant Extracts Against Potato Late and Early Blights. Res. J. Agric. Biol. Sci. 2007, 3, 166–175. [Google Scholar]
- Sumitra, A.; Kanojia, A.K.; Kumar, A.; Mogha, N.; Sahu, V. Biopesticide formulation to control tomato lepidopteran pest menace. Curr. Sci. 2014, 7, 1051–1057. [Google Scholar]
- Sales, M.D.C.; Costa, H.B.; Patrícia, M.B.F.; Jose, A.V.; Debora, D.M. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 1, 26–31. [Google Scholar] [CrossRef]
- Muthomi, J.; Fulano, A.M.; Wagacha, J.M.; Mwang’ombe, A.W. Management of snap bean insect pests and diseases by use of antagonistic fungi and plant extracts. Sustain. Agric. Res. 2017, 6, 52. [Google Scholar] [CrossRef]
- Mougou, I.; Boughalleb-M’hamdi, N. Biocontrol of Pseudomonas syringae pv. Syringae affecting citrus orchards in Tunisia by using indigenous Bacillus spp. and garlic extract. Egypt. J. Biol. Pest Control 2018, 28, 60. [Google Scholar] [CrossRef]
- Suryawanshi, A.P.; Ladkat, G.M.; Dhoke, P.K.; Surayawanshi, S.D.; Pensalwar, S.N. Evaluation of some plant extracts against Sclerotium rolfsii on pigeonpea. J. Plant Dis. Sci. 2007, 2, 32–33. [Google Scholar]
- Okwute, S.K. Plants as potential sources of pesticidal agents: A review. In Pesticides—Advances in Chemical and Botanical Pesticides; Soundararajan, R.P., Ed.; InTech: San Francisco, CA, USA, 2012; pp. 207–323. [Google Scholar]
- Chougule, P.M.; Andoji, Y.S. Antifungal activity of some common medicinal plant extracts against soil borne phytopathogenic fungi Fusarium oxysporum causing wilt of tomato. Int. J. Dev. Res. 2016, 6, 7030–7033. [Google Scholar]
- Ngegba, P.M.; Kanneh, S.M.; Bayon, M.S.; Ndoko, E.J.; Musa, P.D. Fungicidal effect of three plants extracts in control of four phytopathogenic fungi of tomato (Lycopersicum esculentum L.) fruit rot. Int. J. Environ. Agric. Biotechnol. 2018, 1, 112–117. [Google Scholar] [CrossRef]
- Ntalli, N.; Bratidou Parlapani, A.; Tzani, K.; Samara, M.; Boutsis, G.; Dimou, M.; Monokrousos, N. Thymus citriodorus (Schreb) botanical products as ecofriendly nematicides with bio-fertilizing properties. Plants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.H.; Locke, J.C. Effect of botanical extracts on the population density of Fusarium oxysporum in soil and control of Fusarium wilt in the greenhouse. Plant Dis. 2000, 84, 300–305. [Google Scholar] [CrossRef]
- Izah, S.C.; Etim, N.G.; Ilerhunmwuwa, I.A.; Silas, G. Evaluation of crude and ethanolic extracts of Capsicum frutescens var. minima fruit against some common bacterial pathogens. Int. J. Complement Alt. Med. 2019, 12, 105–108. [Google Scholar] [CrossRef]
- Ogundele, R.A.; Oyedele, D.J.; Adekunle, O.K. Management of Meloidogyne incognita and other phytonematodes infecting Amaranthus cruentus and Telfairia occidentalis with African marigold (Tagetes erecta) and Siam weed (Chromolaena odorata). Australas. Plant Pathol. 2016, 45, 537–545. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.W.; Zhou, Z. Antifungal Activity of Citrus Essential Oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Gamboa-Angulo, M.M.; Cristóbal-Alejo, J.; Medina-Baizabal, I.L.; Chí-Romero, F.; Méndez-González, R.; Simá-Polanco, P.; May-Pat, F. Antifungal properties of selected plants from the Yucatan peninsula, Mexico. World J. Microbiol. Biotechnol. 2008, 24, 1955–1959. [Google Scholar] [CrossRef]
- Acedo, A.L., Jr.; Acedo, J.Z.; Evangelio, M.F.N. Post harvest biocontrol of bacterial soft rot of cabbage using botanicals. Philipp. J. Crop Sci. 1999, 24, 12. [Google Scholar]
- Cui, G.F.; Yuan, H.Q.; He, W.; Deng, Y.K.; Sun, R.R.; Zhong, G.H. Synergistic effects of botanical curcumin-induced programmed cell death on the management of Spodoptera litura Fabricius with avermectin. Ecotoxicol. Environ. Saf. 2022, 229, 113097. [Google Scholar] [CrossRef] [PubMed]
- Veeran, S.; Cui, G.F.; Shu, B.S.; Yi, X.; Zhong, G.H. Curcumin-induced autophagy and nucleophagy in Spodoptera frugiperda Sf9 insect cells occur via PI3K/AKT/TOR pathways. J. Cell. Biochem. 2019, 120, 2119–2137. [Google Scholar] [CrossRef] [PubMed]
- Veeran, S.; Shu, B.S.; Cui, G.F.; Fu, S.J.; Zhong, G.H. Curcumin induces autophagic cell death in Spodoptera frugiperda cells. Pestic. Biochem. Physiol. 2017, 139, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Chhillar, A.K.; Yadav, V.; Kamal, P.K.; Gupta, J.; Sharma, G.L. In vitro antifungal activity of 2-(3,4-dimethyl-2,5-dihydro-1H-pyrrol-2-yl)-1-methylethyl pentanoate, a dihydropyrrole derivative. J. Med. Microbial. 2005, 54, 549–552. [Google Scholar] [CrossRef]
- Pande, P.C.; Chauhan, J. Screening of in vitro antifungal activity of plant extract of Datura stramonium, Solanum nigrum and Withania somnifera. J. Plant Dev. Sci. 2011, 3, 315–316. [Google Scholar]
- Patil, V.S.; Kulkarni, S. Bio-Efficacy of certain botanicals against colony growth and spore germination of Exserohilum hawaiiensis, a causal agent of leaf blight of wheat. Karnataka J. Agric. Sci. 2002, 15, 391–396. [Google Scholar]
- Prasad, C.S.; Gupta, V.; Tyagi, A.; Pathak, S. Biological control of Sclerotium rolfsii Sacc., the incitant of cauliflower collar rot. Ann. Plant Prot. Sci. 2003, 11, 61–63. [Google Scholar]
- Patni, C.S.; Kolte, S.J.; Awasthi, R.P. Inhibitory effect of some plant extracts against Alternaria brassicae, causing Alternaria blight of mustard. J. Res. SKUAST-J. 2005, 4, 71–79. [Google Scholar]
- Alabi, D.A.; Oyero, J.A.; Amusa, N.A. Fungitoxic and Phytotoxic Effects of vernonia amygdalina (L.), Bryophyllum pinnantus Kurz Ocimum gratissimum (Closium) L. and Eucalyptna globules (Caliptos) Labill Water Extracts on Cowpea and Cowpea seedling pathogens in Ago-Iwoye, South Western Nigeria. World J. Agric. Sci. 2005, 1, 70–75. [Google Scholar]
- Katooli, N.; Maghsodlo, R.; Razavi, S.E. Evaluation of eucalyptus essential oil against some plant pathogenic fungi. J. Plant Breed. Crop Sci. 2011, 3, 41–43. [Google Scholar]
- Zhao, L.; Feng, C.; Hou, C.; Hu, L.; Wang, Q.; Wu, Y. First discovery of acetone extract from cottonseed oil sludge as a novel antiviral agent against plant viruses. PLoS ONE 2015, 2, e0117496. [Google Scholar] [CrossRef] [PubMed]
- Jantasorn, A.; Boontida, M.; Tida, D. In vitro antifungal activity evaluation of fve plant extracts against fve plant pathogenic fungi causing rice and economic crop diseases. J. Biopestic. 2016, 1, 1–7. [Google Scholar]
- Hsieh, T.F. Effect of leaf extract of Hydnocarpus on control of anthracnose of Chinese cabbage caused by Colletotrichum higginsianum. Acad. J. Med. Plants 2018, 6, 255–261. [Google Scholar] [CrossRef]
- Fayaz, M.; Bhat, M.H.; Fayaz, M.; Kumar, A.; Jain, A.K. Antifungal activity of Lantana camara L. leaf extracts in different solvents against some pathogenic fungal strains. Pharmacologia 2017, 8, 105–112. [Google Scholar] [CrossRef][Green Version]
- Sreeramulu, A.; Arunakumari, M.; Lakshmi, N.R.P. Antifungal Activity of Wild Sage (Lantana camara) against Colletotrichum falcatum. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1847–1852. [Google Scholar] [CrossRef]
- Feng, W.; Zheng, X. Essential oils to control Alternaria alternata in vitro and in vivo. Food Control 2007, 18, 1126–1130. [Google Scholar] [CrossRef]
- Mermer-Doğu, D.; Zobar, D. Effects of some plant essential oils against Botrytis cinerea and Tetranychus urticae on grapevine. Turk. J. Agric. Nat. Sci. 2014, 1, 1268–1273. [Google Scholar]
- Suleiman, M.N. Antifungal properties of leaf extract of neem and tobacco on three fungal pathogens of tomato (Lycopersicon esculentum Mill). Adv. Appl. Sci. Res. 2011, 2, 217–220. [Google Scholar]
- Ogbebor, N.; Adekunle, A.T. Inhibition of conidial germination and mycelial growth of Corynespora cassiicola (Berk and Curt) of rubber (Hevea brasiliensis muell. Arg.) using extracts of some plants. Afr. J. Biotechnol 2005, 4, 996–1000. [Google Scholar]
- Ushamalini, C.; Rajappan, K.; Gangadharan, K. Suppression of charcoal rot and wilt pathogens of cowpea by botanicals. Plant Dis. Res. 1997, 12, 113–117. [Google Scholar]
- Pramanick, T.C.; Phookan, A.K. Effect of plant extracts in the management of sheath rot of rice. J. Agric. Sci. Soc. North East India 1998, 11, 85–87. [Google Scholar]
- Tomar, M.; Chandel, S. Use of phytoextracts in the management of Gladiolus wilt. J. Mycol. Plant Pathol. 2006, 36, 142–144. [Google Scholar]
- Weng, Q.F.; Zhong, G.H.; Hu, M.Y.; Luo, J.J.; Li, X.G. Bioactivities and physiological effects of extracts of Peganum harmala against Bursaphelenchus xylophilus. Sci. Agric. Sin. 2005, 38, 2014–2022. [Google Scholar] [CrossRef]
- Sharoba, A.M.; El Mansy, H.A.; El Tanahy, H.H.; El Waseif, K.H.; Ibrahim, M.A. Chemical composition, antioxidant and antimicrobial properties of the essential oils and extracts of some aromatic plants. Middle East J. Appl. Sci. 2015, 2, 344–352. [Google Scholar]
- Jnaid, Y.; Yacoub, R.; Al-Biski, F. Antioxidant and antimicrobial activities of Origanum vulgare essential oil. Int. Food Res. J. 2016, 4, 1706–1710. [Google Scholar]
- Satish, S.; Raveesha, K.A.; Janardhana, G.R. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett. Appl. Microbiol. 1999, 28, 145–147. [Google Scholar] [CrossRef]
- Patel, P.; Joshi, C.; Birdi, T.; Kothari, V. Anti-infective efficacy of Psidium guajava L. leaves against certain pathogenic bacteria. F1000Research 2019, 3, 8–12. [Google Scholar] [CrossRef]
- Zhong, G.H.; Hu, M.Y.; Weng, Q.F.; Ma, A.Q.; Xu, W.S. Laboratory and field evaluations of extracts from Rhododendron molle flowers as insect growth regulator to imported cabbage worm, Pieris rapae L. (Lepidoptera:Pieridae). J. Appl. Entomol. 2001, 125, 563–569. [Google Scholar] [CrossRef]
- Doltsinis, S.K.; Markellou, E.; Kasselaki, A.M.; Fanouraki, M.N.; Koumaki, C.; Schmitt, M.A.; Tsakalidis, A.L.; Malathrakis, N.E. Efficacy of Milsana®, a Formulated Plant Extract from Reynoutria sachalinensis, against Powdery Mildew of Tomato (Leveillula taurica). BioControl 2006, 51, 375–392. [Google Scholar] [CrossRef]
- Widmer, T.L.; Laurent, N. Plant extracts containing caffeic acid and rosmarinic acid inhibit zoospore germination of Phytophthora spp. pathogenic to Theobroma cacao. Eur. J. Plant Pathol. 2006, 115, 377–388. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Enikuomehin, O.A.; Afolabi, C.G.; Akintokun, A.K.; Egbontan, A.O.; Kanneh, S.M.; Samura, A.E. Evaluation of some Plant Extracts on Mycelial Growth and Sporulation Density of Fungal Pathogens of Groundnut (Arachis hypogaea L.) In-Vitro. Int. J. Dev. Res. 2017, 7, 13808–13814. [Google Scholar]
- Elbeshehy, E.K.F.; Metwali, E.M.R.; Almaghrabi, O.A. Antiviral activity of Thuja orientalis extracts against Watermelon Mosaic Virus (WMV) on Citrullus lanatus. Saudi J. Biol. Sci. 2015, 22, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mathur, K.; Gurjar, R.B.S. Evaluation of different fungal antagonists, plant extracts and oilcakes against Rhizoctonia solani, causing stem rot of chilli. Ann. Plant Prot. Sci. 2002, 10, 319–322. [Google Scholar]
- Purnima, D.; Saxena, S.K. Effect of Withania somnifera on fruit rot of tomato caused by Aspergillus niger in presence of Drosophila busckii. Indian Phytopathol. 2002, 55, 112–113. [Google Scholar]
- Al-Samarrai, G.; Singh, H.; Syarhabil, M. Evaluating eco-friendly botanicals (natural plant extracts) as alternatives to synthetic fungicides. Ann. Agric. Environ. Med. 2012, 19, 673–676. [Google Scholar]
- Rawal, P.; Adhikari, R.S. Evaluation of antifungal activity of Zingiber officinale against Fusarium oxysporum f.sp. lycopersici. Adv. Appl. Sci. Res. 2016, 7, 5–9. [Google Scholar]
- Singh, R.; Anuradha, S. Plant secondary metabolites in the sustainable diamondback moth (Plutella xylostella L.) management. Indian J. Fundam. Appl. Life Sci. 2011, 1, 295–309. [Google Scholar]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Fischer, D.; Imholt, C.; Pelz, H.J.; Wink, M.; Prokop, A.; Jacob, J. The repelling effect of plant secondary metabolites on water voles, Arvicola amphibius. Pest Manag. Sci. 2013, 69, 437–443. [Google Scholar] [CrossRef]
- Devi1, M.R.; Meenakshi Bawari1 Paul, S.B.; Sharma, G.D. Neurotoxic and Medicinal Properties of Datura stramonium L. Review. Assam Univ. J. Sci. Technol. Biol. Environ. Sci. 2011, 7, 139–144. [Google Scholar]
- Jency, B.J.; George Jeevitha, M.V.; Sisssy, C. Pharmacological and biological overview on Calotropis gigantea: A comprehensive review. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 219–223. [Google Scholar]
- Suresh Kumar, P.; Suresh, E.; Kalavathy, S. Review on a potential herb Calotropis gigantea (L.) R. Br. Sch. Acad. J. Pharm. 2013, 2, 135–143. [Google Scholar]
- Emimal Victoria, E. Pest infestation on the biochemical modulation of Adhatoda vasica. J. Biopestic. 2010, 3, 413–419. [Google Scholar]
- Nandre, B.N.; Bakliwal, S.R.; Rane, B.R.; Pawar, S.P. A Review on Adhatoda vasica. Pharma Science Monitor. Int. J. Pharm. Sci. 2012, 3, 3232–3245. [Google Scholar]
- Kumar, V.; Chandrashekar, K.; Sidhu, O.P. Efficacy of karanjin and different extracts of Pongamia pinnata against selected insect pests. J. Entomol. Res. 2006, 30, 103–108. [Google Scholar]
- Subbalakshmi Lokanadhan Muthukrishnan, P.; Jeyaraman, S. Neem products and their agricultural applications. J. Biopestic. 2012, 5, 72–76. [Google Scholar]
- Prowse, G.M.; Galloway, T.S.; Andrew, F. Insecticidal activity of garlic juice in two dipteran pests. Agric. For. Entomol. 2006, 8, 1–6. [Google Scholar] [CrossRef]
- Madhumathy, A.P.; Ali-Ashraf Aivazi Vijayan, A. Larvicidal efficacy of Capsicum annum against Anopheles stephensi and Culex quinquefasciatus. J. Vector Borne Dis. 2007, 44, 223–226. [Google Scholar]
- Nirmal, S.A.; Shashikant, R.; Pattan Shaikh, M.H.; Dhasade, V.V.; Mandal Subhash, C. An overview of Lantana camara: Chemistry and pharmacological profile. Pharmacol. Online Newsl. 2009, 3, 520–531. [Google Scholar]
- Singh, R.N.; Sharatchandra, B. The development of botanical products with special reference to seri-ecosystem. Casp. J. Environ. Sci. 2005, 3, 1–8. [Google Scholar]
- Dar, S.A.; Dar, N.A.; Bhat, M.A.; Bhat, M.H. Prospects, utilization and challenges of botanical pesticides in sustainable agriculture. Int. J. Mol. Biol. Biochem. 2014, 2, 1–14. [Google Scholar]
- Xu, H.X.; Zheng, X.S.; Yang, Y.J.; Tian, J.C.; Lu, Y.H.; Tan, K.H.; Heong, K.L.; Lu, Z.X. Methyl eugenol bioactivities as a new potential botanical insecticide against major insect pests and their natural enemies on rice (Oriza sativa). Crop Prot. 2015, 72, 144–149. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Santana, A.S.; Santana, E.D.R.; Lima, A.P.S.; Faro, R.R.N.; Nunes, R.S.; Lima, A.D.; Blank, A.F.; Araújo, A.P.A.; Cristaldo, P.F.; et al. Nanoformulation prototype of the essential oil of Lippia sidoides and thymol to population management of Sitophilus zeamais (Coleoptera: Curculionidae). Ind. Crops Prod. 2017, 107, 198–205. [Google Scholar] [CrossRef]
- Štefanidesová, K.; Škultéty, L.; Sparagano, O.A.E.; Špitalská, E. The repellent efficacy of eleven essential oils against adult Dermacentor reticulatus ticks. Ticks Tick-Borne Dis. 2017, 8, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Lowrisk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors—A review. Exp. Parasitol. 2016, 167, 103–108. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Dayan, F.G.M.; Tellez, A.; Duke, S. Managing weeds with natural products. Pestic. Outlook 1992, 10, 185–188. [Google Scholar]
- Isman, M.B.; Machial, C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. In Naturally Occuring Bioactive Compounds; Carpinella, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 29–44. [Google Scholar] [CrossRef]
- Isman, M.B.; Paluch, G. Chapter 7. Needles in the haystack: Exploring chemical diversity of botanical insecticides. In RSC Green Chemistry; Òscar, L., Fernàndez-Bolaños, J.G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 248–265. [Google Scholar] [CrossRef]
- Akaike, A.; Izumi, Y. Overview. In Nicotinic Acetylcholine Receptor Signaling in Neuroprotection; Akaike, A., Shimohama, S., Misu, Y., Eds.; Springer: Singapore, 2018; pp. 1–15. [Google Scholar]
- Blotnick-Rubin, E.; Anglister, L. Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction. Front. Mol. Neurosci. 2018, 11, 123. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Bufo, S. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Ware, G.W. An introduction to insecticides. In Radcliffe’s IPM World Textbook, 3rd ed.; Radcliffe, E.B., Hutchison, W.D., Eds.; MeisterPro Information Resources: Willoughby, OH, USA, 2000. [Google Scholar]
- Ware, G.W.; Whitacre, D.M. The Pesticide Book, 6th ed.; MeisterPro Information Resources: Willoughby, OH, USA, 2004; p. 488. [Google Scholar]
- Casida, J.; Durkin, K. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Shivanandappa, T.; Rajashekar, Y. Mode of Action of Plant-Derived Natural Insecticides. In Advances in Plant Biopesticides, 1st ed.; Singh, D., Ed.; Springer: New Delhi, India, 2014; pp. 323–345. [Google Scholar]
- Hare, J.; Morse, J. Toxicity, Persistence, and Potency of Sabadilla Alkaloid Formulations to Citrus Thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 1997, 90, 326–332. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Park, J.H.; Jeon, Y.J.; Lee, C.H.; Chung, N.; Lee, H.S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci. Rep. 2017, 7, 40902. [Google Scholar] [CrossRef]
- Mordue, A.J.; Nisbet, A.J. Azadirachtin from the neem tree Azadirachta indica: Its action against insects. An. Soc. Entomol. Bras. 2000, 29, 615–632. [Google Scholar]
- Chengala, L.; Nandita, S. Review of Mode of action of some major botanical pesticides. Int. Res. J. Sci. Eng. 2018, 6, 2. [Google Scholar]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Protect. Sci. 2016, 4, 229–241. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Olumuyiwa, T.A.; Olasehinde, T.A.; Ademiluyi, A.O.; Adeyemo, A.C. Insecticidal activity of essential oil from orange peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and its inhibitory effects on acetylcholinesterase and Na+/K+-ATPase activities. Phytoparasitica 2017, 45, 501–508. [Google Scholar] [CrossRef]
- Karani, A.O.; Ndakidemi, P.A.; Mbega, E.R. Botanical pesticides in management of common bean pests: Importance and possibilities for adoption by small scale farmers in Africa. J. Appl. Life Sci. Int. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Murovhi, J.; Phophi, M.M.; Mafongoya, P. Efficacy of Plant Materials in Controlling Aphids on Okra (Abelmoschus esculentus L. Moench) in Limpopo Province of South Africa. Agronomy 2020, 10, 1968. [Google Scholar] [CrossRef]
- Ahmad, T.; Fauzy, Z.M.; Yoshia Utami, T.; Arbianti, R.; Hermansyah, H. Production of bio-insecticide from extracted Carica papaya using NADES solvent with ultrasound-assisted extraction (UAE). In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 67, p. 03007. [Google Scholar] [CrossRef]
- Zobayer, N.; Hasan, R. Effects of manually processed Bio-pesticides on crop production and pest managements in okra (Abelmoschus esculentus (L.) Moench). J. Nat. Sci. Res. 2013, 3, 112–118. [Google Scholar]
- Dunkel, F.V.; Jaronski, S.T.; Sedlak, C.W.; Meiler, S.U.; Veo, K.D. Effects of Steam-Distilled Shoot Extract of Tagetes minuta L. (Asterales: Asteraceae) and Entomopathogenic Fungi on Larval Tetanops myopaeformis. Environ. Entomol. 2010, 39, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, T.; Sun, Z.P.; Li, H.Y.; Qi, X.X.; Zhong, G.H.; YI, X. Azadirachtin acting as a hazardous compound to induce multiple detrimental effects in Drosophila melanogaster. J. Hazard. Mater. 2018, 359, 338–347. [Google Scholar] [CrossRef]
- Boulahbel, B.; Aribi, N.; Kilani-Morakchi, S.; Soltani, N. Insecticidal activity of azadirachtin on Drosophila melanogaster and recovery of normal status by exogenous 20-hydroxyecdysone, Afr. Entomol. 2015, 23, 224–233. [Google Scholar] [CrossRef]
- Lai, D.; Jin, X.; Wang, H.; Yuan, M.; Xu, H. Gene expression profile change and growth inhibition in Drosophila larvae treated with azadirachtin. J. Biotechnol. 2014, 185, 51–56. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Shim, J.K.; Lee, S.; Lee, K.Y. Azadirachtin ingestion is lethal and inhibits expression of ferritin and thioredoxin peroxidase genes of the sweet potato whitefly Bemisia tabaci. J. Asia-Pac. Entomol. 2016, 19, 1–4. [Google Scholar] [CrossRef]
- Ahmad, K.; Adnan, M.; Khan, M.A.; Hussain, Z.; Junaid, K.; Saleem, N. Bioactive neem leaf powder enhances the shelf life of stored mungbean grains and extends protection from pulse beetle. Pak. J. Weed Sci. Res. 2015, 21, 71–81. [Google Scholar]
- Zanuncio, J.C.; Mourão, S.A.; Martínez, L.C.; Wilcken, C.F.; Ramalho, F.S.; Plata-Rueda, A. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2016, 6, 30261. [Google Scholar] [CrossRef]
- Singh, A.; Kataria, R.; Kumar, D. Repellence property of traditional plant leaf extracts against Aphis gossypii Glover and Phenacoccus solenopsis Tinsley. Afr. J. Agric. Res. 2012, 7, 1623–1628. [Google Scholar] [CrossRef]
- Brotodjojo, R.R.; Arbiwati, D. Effect of application of granular organic fertilizer enriched with boiler ash and neem leaves powder on plant resistance against insect pests. Int. J. Biosci. Biochem. Bioinform. 2016, 6, 152–157. [Google Scholar] [CrossRef][Green Version]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Aribi, N. Larval exposure to azadirachtin affects fitness and oviposition site preference of Drosophila melanogaster. Pestic. Biochem. Physiol. 2016, 133, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.A.A.; de Souza, N.A.; Feder, M.D.; d Silva, C.E.; Garcia, E.S.; Azambuja, P.; Gonzalez, M.S.; Rangel, E.F. Effects of Azadirachtin on the development and mortality of Lutzomyia longipalpis Larvae (Diptera: Psychodidae: Phlebotominae). J. Med. Entomol. 2006, 43, 262–266. [Google Scholar] [CrossRef]
- Jeyasankar, A.; Raja, N.; Ignacimuthu, S. Insecticidal compound isolated from Syzygium lineare Wall. (Myrtaceae) against Spodoptera litura (Lepidoptera: Noctuidae). Saudi J. Biol. Sci. 2011, 18, 329–332. [Google Scholar] [CrossRef]
- Yooboon, T.; Pluempanupat, W.; Koul, O.; Bulangpoti, V. Effects of azadirachtin on cuticular proteins of Spodoptera litura (Lepidoptera: Noctuidae) vis-à-vis the modes of application. Commun. Agric. Appl. Biol. Sci. 2015, 80, 169–177. [Google Scholar]
- Huang, T.T.; Ali, S.; Long, X.Z. Joint application of Isaria fumosorsea chitinase and azadirachtin as biopesticides against the diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae). Egypt. J. Biol. Pest Control 2016, 26, 539–543. [Google Scholar]
- Kilani-Morakchi, S.; Bezzar-Bendjazia, R.; Ferdenache, M.; Aribi, N. Preimaginal exposure to azadirachtin affects food selection and digestive enzymes in adults of Drosophila melanogaster (Diptera:Drosophilidae). Pestic. Biochem. Physiol. 2017, 140, 58–64. [Google Scholar] [CrossRef]
- Aribi, N.; Oulhaci, M.C.; Kilani-Morakchi, S.; Sandoz, J.C.; Kaiser, L.; Denis, B.; Joly, D. Azadirachtin impact on mate choice, female sexual receptivity and male activity in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 95–101. [Google Scholar] [CrossRef]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Aribi, N. Growth and molting disruption effects of azadirachtin against Drosophila melanogaster (Diptera: Drosophilidae). J. Entomol. Zool. Stud. 2016, 4, 363–368. [Google Scholar]
- Poland, T.M.; Haack, R.A.; Patrice, T.R.; Miller, D.L.; Bauer, L.S. Laboratory evaluation of the toxicity of systemic insecticides for control of Anoplophora glabripennis and Plectrodera scalator (Coleoptera: Cerambycidae). J. Econ. Entomol. 2006, 99, 85–93. [Google Scholar] [CrossRef]
- Abedi, Z.; Saber, M.; Vojoudi, S.; Mahdavi, V.; Parsaeyan, E. Acute, sublethal, and combination effects of azadirachtin and Bacillus thuringiensis on the cotton bollworm, Helicoverpa armigera. J. Insect Sci. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.F.; De Meyer, L.R.; Guedes, N.C.; Smagghe, G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera:Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Sankari, S.A.; Narayanasamy, P. Bio-efficacy of flash-based herbal pesticides against pests of rice and vegetables. Curr. Sci. 2007, 92, 811–816. [Google Scholar]
- Sousa, B.M.; Barbosa, D.B.; Santana, G.M.; Vieira, C.; Haralampidou, G. Applying plant oils to control fall army worm (Spodoptera frugiperda) in corn. Aust. J. Crop Sci. 2018, 12, 557–562. [Google Scholar]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 9471. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Verma, N.; Bahl, J.R.; Bansal, R.P.; Khanuja, S.P.S.; Kumar, S. Bioactivities of the leaf essential oil of Curcuma longa (var. Ch-66) on three species of stored-product beetles (Coleoptera). J. Econ. Entomol. 2002, 95, 183–189. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal and repellent activity of selected essential oils against of the pollen beetle, Meligethes aeneus (Fabricius) adults. Ind. Crops Prod. 2011, 34, 888–892. [Google Scholar] [CrossRef]
- Enan, E.E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Baidoo, P.K.; Mochiah, M.B. Comparing the Effectiveness of Garlic (Allium sativum L.) and Hot Pepper (Capsicum frutescens L.) in the Management of the Major Pests of Cabbage Brassica oleracea (L.). Sustain. Agric. Res. 2016, 2, 83–91. [Google Scholar] [CrossRef]
- Tavares, W.S.; Cruz, I.; Petacci, F.; Assis Júnior, S.L.; Freitas, S.S.; Zanuncio, J.C.; Serrão, J.E. Potential use of Asteraceae extracts to control Spodoptera frugiperda (Lepidoptera: Noctuidae) and selectivity to their parasitoids Trichogramma pretiosum (Hymenoptera: Trichogramma tidae) and Telenomus remus (Hymenoptera: Scelionidae). Ind. Crop. Prod. 2009, 30, 384–388. [Google Scholar] [CrossRef]
- Mukanga, M.; Deedat, Y.; Mwangala, F.S. Toxic effect of five plant extract against larger grain borer, Prostephanus truncates. Afr. J. Agric. Res. 2010, 5, 3369–3378. [Google Scholar]
- Okwute, S.K.; Onyia, R.; Anene, C.; Amodu, O.P. Protectant, insecticidal and antimicrobial potentials of Dalbergia saxatilis Hook f (Fabaceae). Afr. J. Biotechnol. 2009, 8, 6556–6560. [Google Scholar] [CrossRef]
- Dyer, L.A.J.; Richard Dodson, C.D. Isolation, synthesis, and evolutionary ecology of Piper Amides. In A Model Genus for Studies of Phytochemistry, Ecology, and Evolution; Dyer, L.A., Palmer, A.O.N., Eds.; Springer: Boston, MA, USA, 2004; pp. 117–139. [Google Scholar] [CrossRef]
- Elumalai, K.; Krishnappa, K.; Anandan, A.; Govindarajan, M.; Mathivanan, T. Larvicidal and ovicidal activity of seven essential oil against lepidopteran pest S. litura (Lepidoptera: Noctuidae). Int. J. Recent Sci. Res. 2010, 1, 8–14. [Google Scholar]
- Baskaran, J.; Arshid, G.; Krishnappa, K.; Elumalai, K. Larvicidal activity of selected plant essential oils against tobacco cut worm Spodoptera litura (Fab) (Lepidoptera: Noctuidae). Int. J. Curr. Adv. Res. 2012, 1, 13–17. [Google Scholar]
- Priyanka, B.; Srivastava, R.P. Larvicidal and growth regulatory activities of some essential oils against Asian army worm, Spodoptera litura (Fab.). J. Biopestic. 2012, 5, 186–190. [Google Scholar]
- Abida, Y.; Tabassum, F.; Zaman, S.; Chhabi, S.B.; Islam, N. Biological screening of Curcuma longa L. for insecticidal and repellent potentials against Tribolium castaneum (Herbst) adults. Univ. J. Zool. Rajshahi Univ. 2010, 28, 69–71. [Google Scholar] [CrossRef]
- Khan, S.; Taning, C.N.T.; Bonneure, E.; Mangelinckx, S.; Smagghe, G.; Shah, M.M. Insecticidal activity of plant-derived extracts against different economically important pest insects. Phytoparasitica 2017, 45, 113–124. [Google Scholar] [CrossRef]
- Muzemu, S.; Mvumi, B.M.; Nyirenda, S.P.M.; Sileshi, G.W.; Sola, P.; Chikukura, L.; Kamanula, J.F.; Belmain, S.R.; Stevenson, P.C. Pesticidal Effects of Indigenous Plant Extracts against Rape Aphids and Tomato Red Spider Mites. Afr. Crop Sci. Conf. Proc. 2011, 10, 171–173. [Google Scholar]
- Shah, J.A.; Inayatullah, M.; Sohail, K.; Shah, S.F.; Shah, S.; Iqbal, T.; Usman, M. Efficacy of Botanical Extracts and a Chemical Pesticide against Tomato Fruit Worm, Helicoverpa armigera. Sarhad J. Agric. 2013, 1, 93–96. [Google Scholar]
- Nia, B.; Frah, N.; Azoui, I. Insecticidal Activity of Three Plants Extracts against Myzuspersicae (Sulzer, 1776) and Their Phytochemical Screening. Acta Agric. Slov. 2015, 2, 261–267. [Google Scholar] [CrossRef]
- Ali, S.; Muhammad, S.M.H.; Muneer, A.; Faisal, H.; Muhammad, F.; Dilbar, H.; Muhammad, S.; Abdul, G. Insecticidal activity of turmeric (Curcuma longa) and garlic (Allium sativum) extracts against red flour beetle, Tribolium castaneum: A safe alternative to insecticides in stored commodities. J. Entomol. Zool. Stud. 2014, 3, 201–205. [Google Scholar]
- Parwada, C.; Chikuvire, T.J.; Kamota, A.; Mandumbu, R.; Mutsengi, K. Use of botanical pesticides in controlling Sitophilus zeamais (maize weevil) on stored Zea mays (maize) grain. Mod. Concep. Deve. Agron. 2018, 4. [Google Scholar] [CrossRef]
- Yoon, M.; Cha, B.; Kim, J. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Lengai, G.M.; Muthomi, J.W. Biopesticides and their role in sustainable agricultural production. J. Biosci. Med. 2018, 6, 7–41. [Google Scholar] [CrossRef]
- Martnez, A. Natural Fungicides Obtained from Plants, Fungicides for Plant and Animal Diseases. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Ed.; IntechOpen: London, UK, 2012; pp. 953–978. [Google Scholar]
- Damalas, C.A. Potential uses of turmeric (Curcuma longa) products as alternative means of pest management in crop production. Review Article. Plant Omics J. 2011, 4, 136–141. [Google Scholar]
- Suleiman, M.N. Fungitoxic activity of Neem and Pawpaw leaves extracts on Alternaria solani, casual organism of Yam Rots. Adv. Environ. Biol. 2010, 4, 159–161. [Google Scholar]
- Cho, J.Y.; Choi, G.J.; Lee, S.W.; Jang, K.S.; Lim, H.K.; Lim, C.H.; Lee, S.O.; Cho, K.Y.; Kim, J.C. Antifungal activity against Colletotrichum spp. of curcuminoids isolated from Curcuma longa L. rhizomes. J. Microbiol. Biotechnol. 2006, 16, 280–285. [Google Scholar]
- Ferreira, F.D.; Mossini, S.A.G.; Ferreira, F.M.D.; Arrotéia, C.C.; da Costa, C.L.; Nakamura, C.V.M.; Machinski, M., Jr. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci. World J. 2013, 2013, 343804. [Google Scholar] [CrossRef]
- Sivaranjani, M.; Krishnan, S.R.; Kannappan, A.; Ramesh, M.; Ravi, A.V. Curcumin from Curcuma longa affects the virulence of Pectobacterium wasabiae and P. carotovorum subsp. carotovorumvia quorum sensing regulation. Eur. J. Plant Pathol. 2016, 146, 793–806. [Google Scholar] [CrossRef]
- Patrice, A.K.; Séka, K.; Francis, Y.K.; Théophile, A.S.; Fatoumata, F.; Diallo, H.A. Effects of Three Aqueous Plant Extracts in the Control of Fungi Associated with Post-harvest of Yam (Dioscorea alata). Int. J. Agron. Agric. Res. 2017, 3, 77–87. [Google Scholar]
- Enikuomehin, O.A.; Oyedeji, E.O. Fungitoxic effects of some plant extracts against tomato fruit rot pathogens. Arch. Phytopathol. Plant Prot. 2010, 43, 233–240. [Google Scholar] [CrossRef]
- Devi, K.B.; Pavankumar, P.; Bhadraiah, B. Antifungal Activity of Plant Extracts against Post-Harvest Fungal Pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 669–679. [Google Scholar] [CrossRef]
- Bomfim, S.N.; Lydiana, P.N.; Pinheiro, J.F.O.; Cassia, Y.K.; Galerani, S.A.M.; Renata, G.; Samuel, B.N.; Carlos, A.M.; Benicio, A.A.F.; Miguel, M.J. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2014, 166, 330–336. [Google Scholar] [CrossRef]
- Karimi, K.; Amini, J.; Harighi, B.; Bahramnejad, B. Evaluation of biocontrol potential of Pseudomonas and Bacillus spp against Fusarium wilt of chickpea. Aust. J. Crop Sci. 2012, 6, 695–703. [Google Scholar]
- Islam, R.M.D.; Mondal, C.; Hossain, I.; Meah, M.B. Organic management: An alternative to control late blight of potato and tomato caused by Phytophthora infestans. Int. J. Theor. Appl. Sci. 2013, 5, 32–42. [Google Scholar]
- Yamamoto-Ribeiro, M.M.; Grespan, R.; Kohiyama, C.Y.; Ferreira, F.D.; Mossini, S.A.; Silva, E.L.; Filho, B.A.; Mikcha, J.M.; Machinski, M., Jr. Effect of Zingiber officinale Essential Oil on Fusarium verticillioides and Fumonisin Production. Food Chem. 2013, 141, 3147–3152. [Google Scholar] [CrossRef]
- Abtew, A.; Subramanian, S.; Cheseto, X.; Kreiter, S.; Garzia, G.T.; Martin, T. Repellency of plant extracts against the legume flower thrips Megalurothrips sjostedti (Thysanoptera: Thripidae). Insects 2015, 6, 608–625. [Google Scholar] [CrossRef]
- Minz, S.; Samuel, C.O.; Tripathi, C.S. The Effect of Plant Extracts on the Growth of Wilt Causing Fungi Fusarium oxysporum. J. Pharm. Biol. Sci. 2012, 1, 13–16. [Google Scholar] [CrossRef]
- Perelló, A.; Noll, U.; Slusarenko, A.J. In vitro efficacy of garlic extract to control fungal pathogens of wheat. J. Med. Plants Res. 2013, 24, 1809–1817. [Google Scholar] [CrossRef]
- Cruz, M.E.S.; Schwan-Estrada, K.R.F.; Clemente, E.; Itako, A.T.; Stangarlin, J.R.; Cruz, M.J.S. Plant extracts for controlling the post-harvest anthracnose of banana fruit. Rev. Bras. De Plantas Med. 2013, 4, 727–733. [Google Scholar] [CrossRef]
- Djeussi, D.E.; Noumedem, J.A.K.; Seukep, J.A.; Fankam, A.G.; Voukeng, I.K.; Tankeo, S.B.; Nkuete, A.H.L.; Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complementary Altern. Med. 2013, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.; Ali, S.M.; Siddiqui, M.; Khan, A.U. Antimicrobial activity of five herbal extracts against multi drug resistant (mdr) strains of bacteria and fungus of clinical origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Plant, J.; Stephens, B. Evaluation of the antibacterial activity of a sizable set of essential oils. Med. Aromat. Plants. 2015, 4, 185. [Google Scholar] [CrossRef]
- Sateesh, K.; Marimuthu, B.; Thayumanavan, R.; Nandakumar, R.; Samiyappan, R. Antibacterial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. Oryzae. Physiol. Mol. Plant Pathol. 2004, 65, 91–100. [Google Scholar]
- Meena, C.; Gopalakrishnan, J.; Dureja, P. Antibacterial Withametelin B from Datura metel against Xanthomonas oryzae pv. oryzae. Biopestic. Int. 2013, 9, 31–37. [Google Scholar]
- Abo-Elyousr, K.A.M.; Asran, M.R. Antibacterial activity of certain plant extracts against bacterial wilt of tomato. Arch. Phytopathol. Plant Prot. 2009, 42, 573–578. [Google Scholar] [CrossRef]
- Hassan, M.A.E.; Bereika, M.F.F.; Abo-Elnaga, H.I.G.; Sallam, M.A.A. Direct antimicrobial activity and induction of systemic resistance in potato plants against bacterial wilt disease by plant extracts. Plant Pathol. J. 2009, 25, 352–360. [Google Scholar] [CrossRef]
- Balestra, G.M.; Heydari, A.; Ceccarelli, D.; Ovidi, E.; Quattrucci, A. Antibacterial effect of Allium sativum and Ficus carica extracts on tomato bacterial pathogens. Crop Prot. 2009, 28, 807–811. [Google Scholar] [CrossRef]
- Govindappa, M.; Umersha, S.; Lokesh, S. Adathoda vasica leaf extract induces resistance in rice against bacterial leaf blight disease (Xanthomonas oryzae pv. oryzae). Int. J. Plant Physiol. Biochem. 2011, 3, 6–14. [Google Scholar]
- Ji, P.; Momol, M.T.; Olson, S.M.; Pradhanang, P.M.; Jones, J. Evaluation of thymol as biofumigant for control of bacterial wilt of tomato under field conditions. Plant Dis. 2005, 89, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Paret, M.L.; Cabos, R.; Kratky, B.A.; Alvarez, A.M. Effect of plant essential oil on Ralstonia solancearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010, 94, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.O.; Santos, M.M.B.; Santos, T.C.G.; Souza, E.B.; Mariano, R.L.R. Biofumigation for managing bacterial wilt of sweet peppers. J. Plant Pathol. 2014, 96, 363–367. [Google Scholar]
- Deberdt, P.; Perrin, B.; Coranson-Beaudu, R.; Duyck, P.F.; Wicker, E. Effect of Allium fistulosum extract on Ralstonia solanacearum populations and tomato bacterial wilt. Plant Dis. 2012, 96, 687–692. [Google Scholar] [CrossRef]
- Rukhsana, J.; Tehreema, I.; Huma, B. Purification and bioassays of bioactive fraction from Curcuma longa against Xanthomonas oryzae pv. oryzae causing BLB disease in rice. Pak. J. Bot. 2011, 43, 1335–1342. [Google Scholar]
- Sinha, B.B.P.; Sinha, R.K.P. Integrated disease management of bacterial blight of rice with special reference to neem products. Ann. Plant Prot. Sci. 2000, 8, 206–261. [Google Scholar]
- Singh, U.P.; Prithiviraj, B.; Sarma, B.K.; Singh, M.; Ray, A.B. Role of garlic (Allium sativum L.) in human and plant diseases. Indian J. Exp. Biol. 2001, 39, 310–322. [Google Scholar]
- Pavaraj, M.; Bakavathiappan, G.; Baskaran, S. Evaluation of some plant extracts for their nematicidal properties against root-knot nematode, Meloidogyne incognita. J. Biopestic. 2012, 5, 106–110. [Google Scholar]
- Pesticide Action Network (PAN). Field Guide to Non-Chemical Pest Management in Tomato Production, Hamburg. 2005. Available online: www.osiat.org (accessed on 25 September 2021).
- Khan, A.; Sayed, M.; Shaukat, S.S.; Handoo, Z.A. Efficacy of four plant extracts on nematodes associated with papaya in Sindh, Pakistan. Nematol. Mediterr. 2008, 36, 93–98. [Google Scholar]
- Kepenekçi, I.; Erdoğuş, D.; Erdoğan, P. Effects of some plant extracts on root-knot nematodes in vitro and in vivo conditions. Turk. J. Entomol. 2016, 40, 3–14. [Google Scholar] [CrossRef]
- Akyazi, F. Effect of some plant methanol extracts on egg hatching and juvenile mortality of root-knot nematode Meloidogyne incognita. Am. J. Exp. Agric. 2014, 11, 1471–1479. [Google Scholar] [CrossRef]
- Asif, M.; Tariq, M.; Khan, A.; Siddiqui, M.A. Biocidal and antinemic properties of aqueous extracts of Ageratum and Coccinia against root-knot nematode, Meloidogyne incognita in vitro. J. Agric. Sci. 2017, 2, 108–122. [Google Scholar] [CrossRef]
- Oka, Y.; Ben-Daniel, B.; Cohen, Y. Nematicidal activity of the leaf powder and extracts of Myrtus communis against the root-knot nematode Meloidogyne javanica. Plant Pathol. 2012, 61, 1012–1020. [Google Scholar] [CrossRef]
- Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In vitro and greenhouse studystar. Curr. Plant Biol. 2019, 20, 100115. [Google Scholar] [CrossRef]
- Bello, L.Y.; Chindo, P.S.; Marley, P.S.; Alegbejo, M.D. Effects of some plant extracts on larval hatch of the root-knot nematode, Meloidogyne incognita. Arch. Phytopathol. Plant Prot. 2006, 39, 253–257. [Google Scholar] [CrossRef]
- Olabiyi, T.I. Pathogenicity study and nematoxic properties of some plant extracts on the root-knot nematode pest of tomato, Lycopersicon esculentum (L.) Mill. Plant Pathol. J. 2008, 7, 45–49. [Google Scholar] [CrossRef][Green Version]
- Agbenin, N.O.; Emechebe, A.M.; Marley, P.S. Evaluations of neem seed powder for Fusarium wilt and Meloidogyne control on tomato. Arch. Phytopathol. Plant Prot. 2004, 37, 319–326. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Palombo, E.A.; Yeo, T.C.; Ley, D.L.S.; Tu, C.L.; Malherbe, F.; Grollo, L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE 2013, 8, e79293. [Google Scholar] [CrossRef]
- Kohn, L.K.; Foglio, M.A.; Rodrigues, R.A.; Sousa, I.M.; Martini, M.C.; Padilla, M.A.; Neto, L.D.F.; Arns, C.W. In-vitro antiviral activities of extracts of plants of the Brazilian Cerrado against the avian Metapneumovirus (aMPV). Braz. J. Poult. Sci. 2015, 3, 275–280. [Google Scholar] [CrossRef]
- Al-Ani, R.A.; Diwan, S.N.H.; Adhab, M.A. Efficiency of Thuja orientalis and Artimisia campestris extracts to control of Potato leaf roll virus (PLRV) in potato plants. Agric. Biol. J. N. Am. 2010, 1, 579–583. [Google Scholar]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Salem, M.Z.M.; Ali, H.M.; Kordy, A.M.; Salem, A.Z.M.; Behiry, S.I. Antiviral, antifungal, and insecticidal activities of Eucalyptus bark extract: HPLC analysis of polyphenolic compounds. Microb. Pathog. 2020, 147, 104383. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Salem, M.Z.M.; Hafez, E.; Behiry, S.I.; Qari, S.H. The Phytochemical, Antifungal, and First Report of the Antiviral Properties of Egyptian Haplophyllum tuberculatum Extract. Biology 2020, 9, 248. [Google Scholar] [CrossRef]
- El-Sawy, M.M.; Elsharkawy, M.M.; Abass, J.M.; Kasem, M.H. Antiviral activity of 2-nitromethyl phenol, zinc nanoparticles and seaweed extract against cucumber mosaic virus (CMV) in Eggplant. J. Virol. Antivir. Res. 2017, 6, 2. [Google Scholar] [CrossRef]
- Al-Ani, A.R.; Adhab, M.A.; Hassan, K.A. Antiviral activity of Vit-org, 2-nitromethyl phenoland Thuja extract against eggplant blister mottled virus (EBMV). Afr. J. Microbiol. Res. 2011, 5, 3555–3558. [Google Scholar]
- Kekuda, P.T.R.; Akarsh, S.; Nawaz, S.A.N.; Ranjitha, M.C.; Darshini, S.M.; Vidya, P. In Vitro Antifungal Activity of Some Plants against Bipolaris sarokiniana (Sacc.) Shoem. Int. J. Curr. Microbiol. Appl. Sci. 2016, 6, 331–337. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present Status and the Future Prospects. J. Fertil. Pestic. 2015, 6, e129. [Google Scholar] [CrossRef]
- Black R for AATF African Agricultural Technology Foundation. A Guide to the Development of Regulatory Frameworks for Microbial Biopesticides in Sub-Saharan Africa. Nairobi: African Agricultural Technology Foundation. 2013. Available online: https://www.aatf-africa.org/Regulatory-Frameworks-for-Microbial-Biopesticides-InSub-Saharan-Africa (accessed on 5 October 2021).
- Pavela, R. Limitation of plant biopesticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: New Delhi, India, 2014; pp. 347–359. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).