Spatiotemporal Variation of Small Mammal Communities in Commercial Orchards across the Small Country
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Small Mammal Trapping
2.3. Data Analysis
3. Results
3.1. Temporal Trends of Small Mammal Diversity and Abundance
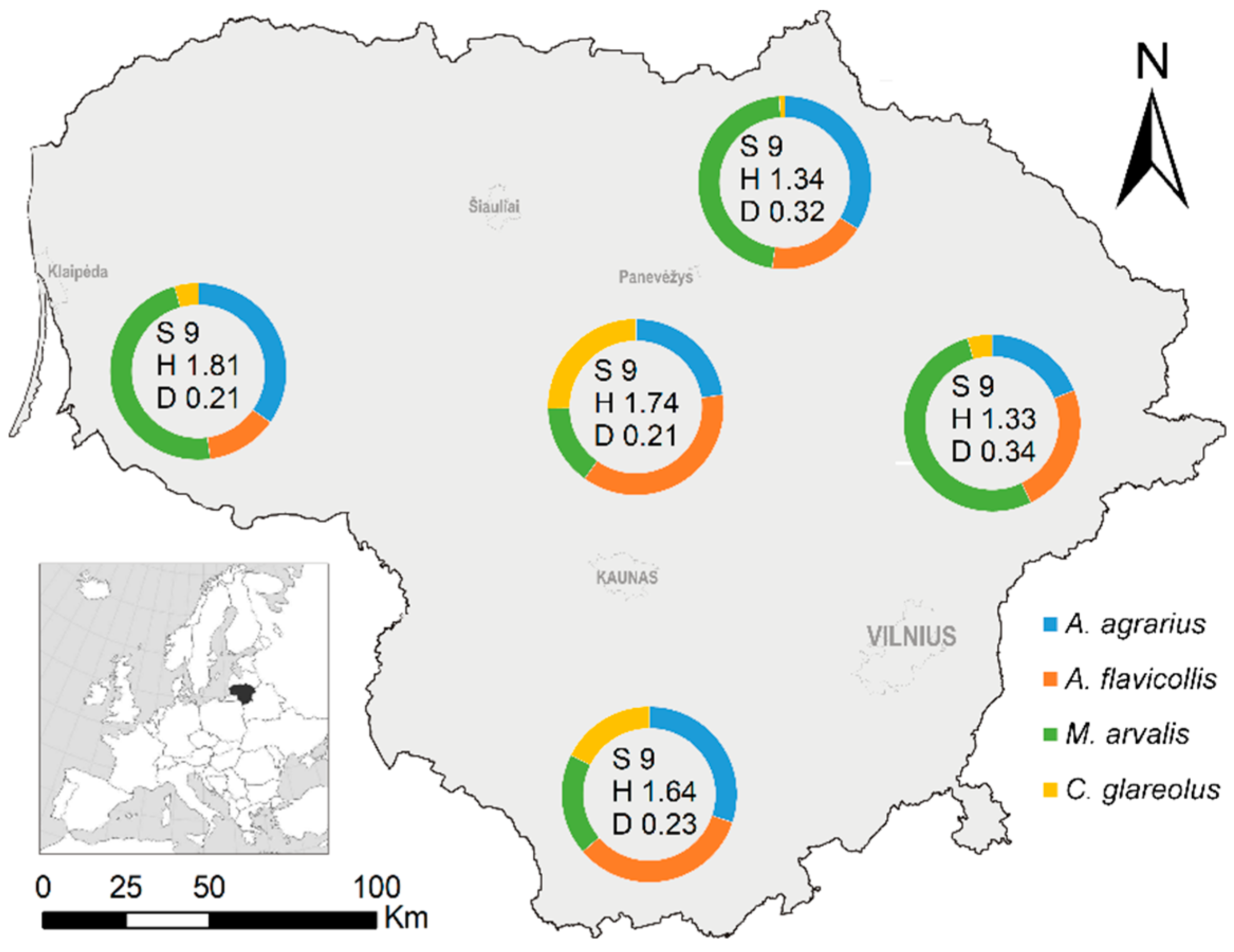
3.2. Location-Based Differences of Small Mammal Diversity and Abundance
3.3. Habitat-Based Differences of Small Mammal Diversity and Abundance
4. Discussion
5. Conclusions
- In Lithuania, the proportions and relative abundances of the most numerous small mammal species and the diversity of their communities in commercial orchards mainly depend on the season and the region within the country, with crop type being less significant.
- In comparison to the summer season, the relative abundance of C. glareolus doubled in autumn, while that of M. arvalis and A. flavicollis tripled and A. agrarius increased by nearly 15 times. Increases of relative abundance show potential of the orchard habitat to support diverse populations of small mammals belonging to different groups (omnivores, herbivores, and granivores).
- The absence of significant year on year differences in the relative abundances of small mammals and the stability in the number of species allows us to conclude that orchards are an important source of biodiversity in the agricultural landscape.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid-Holmes, S.; Drickamer, L.C. Impact of forest patch characteristics on small mammal communities: A multivariate approach. Biol. Conserv. 2001, 99, 293–305. [Google Scholar] [CrossRef]
- Corbalán, V.; Ojeda, R. Spatial and temporal organisation of small mammal communities in the Monte desert, Argentina. Mammalia 2004, 68, 5–14. [Google Scholar] [CrossRef]
- Guidobono, J.S.; Cueto, G.R.; Teta, P.; Busch, M. Effect of environmental factors on the abundance variations of two native rodents in agricultural systems of Buenos Aires, Argentina. Austral. Ecol. 2019, 44, 36–48. [Google Scholar] [CrossRef]
- Yang, L.H. Toward a more temporally explicit framework for community ecology. Ecol. Res. 2020, 35, 445–462. [Google Scholar] [CrossRef]
- White, T.C.R. The role of food, weather and climate in limiting the abundance of animals. Biol. Rev. 2008, 83, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Šipoš, J.; Suchomel, J.; Purchart, L.; Kindlmann, P. Main determinants of rodent population fluctuations in managed Central European temperate lowland forests. Mammal. Res. 2017, 62, 283–295. [Google Scholar] [CrossRef]
- Utrera, A.; Duno, G.; Ellis, B.A.; Salas, R.A.; de Manzione, N.; Fulhorst, C.F.; Tesh, R.B.; Mills, J.N. Small mammals in agricultural areas of the western llanos of Venezuela: Community structure, habitat associations, and relative densities. J. Mammal. 2000, 81, 536–548. [Google Scholar] [CrossRef][Green Version]
- Pearce, J.; Venier, L. Small mammals as bioindicators of sustainable boreal forest management. For. Ecol. Manag. 2005, 208, 153–175. [Google Scholar] [CrossRef]
- Michel, N.; Burel, F.; Butet, A. How does landscape use influence small mammal diversity, abundance and biomass in hedgerow networks of farming landscapes? Acta Oecol. 2006, 30, 11–20. [Google Scholar] [CrossRef]
- Nocera, J.J.; Dawe, K.L. Managing for habitat heterogeneity in grassland agro-ecosystems influences the abundance of masked shrews Sorex cinereus. J. Sustain. Agr. 2008, 32, 379–392. [Google Scholar] [CrossRef]
- Fischer, C.; Schröder, B. Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Magioli, M.; Moreira, M.Z.; Fonseca, R.C.B.; Ribeiro, M.C.; Rodrigues, M.G.; de Barros, K.M.P.M. Human-modified landscapes alter mammal resource and habitat use and trophic structure. Proc. Natl. Acad. Sci. USA 2019, 116, 18466–18472. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Balmford, A.; Green, R.E. The level of threat to restricted-range bird species can be predicted from mapped data on land use and human population. Biol. Conserv. 2005, 123, 317–326. [Google Scholar] [CrossRef]
- Foley, J.A. Can We Feed the World and Sustain the Planet? Sci. Am. 2011, 24, 60–65. [Google Scholar] [CrossRef]
- Norris, K. Agriculture and biodiversity conservation: Opportunity knocks. Conserv. Lett. 2008, 1, 2–11. [Google Scholar] [CrossRef]
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Henle, K.; Alard, D.; Clitherow, J.; Cobb, P.; Firbank, L.; Kull, T.; McCracken, D.; Moritzh, R.F.A.; Niemelä, J.; Rebane, M.; et al. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe—A review. Agr. Ecosyst. Environ. 2008, 124, 60–71. [Google Scholar] [CrossRef]
- EEA. High. Nature Value Farmland: Characteristics; Trends and Policy Challenges. European Environment Agency: Copenhagen, Denmark, 2004. [Google Scholar]
- Büchs, P.D.W. Biotic Indicators for Biodiversity and Sustainable Agriculture. Agr. Ecosyst. Environ. 2003, 98, 603–606. [Google Scholar] [CrossRef]
- Chiatante, G.; Pellitteri-Rosa, D.; Torretta, E.; Marzano, F.N.; Meriggi, A. Indicators of biodiversity in an intensively cultivated and heavily human modified landscape. Ecol. Indic. 2021, 130, 108060. [Google Scholar] [CrossRef]
- Van Vuren, D.; Smallwood, K.S. Ecological management of vertebrate pests in agricultural systems. Biol. Agric. Horti. C 1996, 13, 39–62. [Google Scholar] [CrossRef]
- Ruscoe, W.A.; Brown, P.R.; Henry, S.; van de Weyer, N.; Robinson, F.; Hinds, L.A.; Singleton, G.R. Conservation agriculture practices have changed habitat use by rodent pests: Implications for management of feral house mice. J. Pest. Sci. 2021, 95, 493–503. [Google Scholar] [CrossRef]
- Singleton, G.R.; Lorica, R.P.; Htwe, N.M.; Stuart, A.M. Rodent management and cereal production in Asia–balancing food security and conservation. Pest. Manag. Sci. 2021, 77, 4249–4261. [Google Scholar] [CrossRef] [PubMed]
- Stenseth, N.C.; Leirs, H.; Skonhoft, A.; Davis, S.A.; Pech, R.P.; Andreassen, H.P.; Singleton, G.R.; Lima, M.; Machang’u, R.S.; Makundi, R.H.; et al. Mice, rats, and people: The bio-economics of agricultural rodent pests. Front. Ecol. Environ. 2003, 1, 367–375. [Google Scholar] [CrossRef]
- Imholt, C.; Reil, D.; Plašil, P.; Rödiger, K.; Jacob, J. Long-term population patterns of rodents and associated damage in German forestry. Pest. Manag. Sci. 2016, 73, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Imholt, C.; Esther, A.; Perner, J.; Jacob, J. Identification of weather parameters related to regional population outbreak risk of common voles (Microtus arvalis) in Eastern Germany. Wildl. Res. 2011, 38, 551–559. [Google Scholar] [CrossRef]
- Imholt, C.; Reil, D.; Eccard, J.A.; Jacob, D.; Hempelmann, N.; Jacob, J. Quantifying the past and future impact of climate on outbreak patterns of bank voles (Myodes glareolus). Pest. Manag. Sci. 2014, 71, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Peles, J.D.; Williams, C.K.; Barrett, G.W. Small mammal population dynamics in strip-cropped vs. monoculture agroecosystems. J. Sustain. Agr. 1997, 9, 51–60. [Google Scholar] [CrossRef]
- Pech, R.P.; Davis, S.A.; Singleton, G.R. Outbreaks of rodents in agricultural systems: Pest control problems or symptoms of dysfunctional ecosystems? ACIAR Monogr. Ser. 2003, 96, 311–315. [Google Scholar]
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntke, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2018, 55, 548–558. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Hogue, E.J.; Lautenschlager, R.A.; Wagner, R.G. Population dynamics of small mammals in relation to vegetation management in orchard agroecosystems: Compensatory responses in abundance and biomass. Crop. Prot. 1998, 17, 1–11. [Google Scholar] [CrossRef]
- Riojas-López, M.E. Response of rodent assemblages to change in habitat heterogeneity in fruit-oriented nopal orchards in the Central High Plateau of Mexico. J. Arid Environ. 2012, 85, 27–32. [Google Scholar] [CrossRef]
- Quinn, N.; Baldwin, R.A. Managing Roof Rats and Deer Mice in Nut and Fruit Orchards. ANR Publ. 2014, 8513, 1–7. [Google Scholar] [CrossRef]
- Bertolino, S.; Asteggiano, L.; Saladini, M.A.; Giordani, L.; Vittone, G.; Alma, A. Environmental factors and agronomic practices associated with Savi’s pine vole abundance in Italian apple orchards. J. Pest. Sci. 2015, 88, 135–142. [Google Scholar] [CrossRef]
- Somoano, A.; Ventura, J.; Miñarro, M. Continuous breeding of fossorial water voles in northwestern Spain: Potential impact on apple orchards. Folia Zool. 2017, 66, 29–36. [Google Scholar] [CrossRef]
- Muhammad Aminuddin Baqi, H.F.; Mohamad Iqbal, N.H.; Nur Nabilah, A.R.; Nur Ain Aiman, A.R.; Suganthi, A.; Fong, P.H.; Jayaraj, V.K. The diversity of small mammals in a mixed fruit orchard at Bukit Bekong limestone massif, Merapoh, Pahang, Malaysia. Iop. C Ser. Earth Environ. 2020, 596, 1. [Google Scholar] [CrossRef]
- Caudill, S.A.; Vaast, P.; Husband, T.P. Assessment of small mammal diversity in coffee agroforestry in the Western Ghats, India. Agroforest Syst. 2014, 88, 173–186. [Google Scholar] [CrossRef]
- Caudill, S.A.; DeClerck, F.J.; Husband, T.P. Connecting sustainable agriculture and wildlife conservation: Does shade coffee provide habitat for mammals? Agr. Ecosyst. Environ. 2015, 199, 85–93. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals 2019, 9, 334. [Google Scholar] [CrossRef]
- Juknelienė, D.; Kazanavičiūtė, V.; Valčiukienė, J.; Atkocevičienė, V.; Mozgeris, G. Spatiotemporal Patterns of Land-Use Changes in Lithuania. Land 2021, 10, 619. [Google Scholar] [CrossRef]
- Lithuanian Statistical Yearbook. National Land Service under the Ministry of Agriculture of the Republic of Lithuania. 2000. Available online: http://www.nzt.lt/go.php/lit/Lietuvos-respublikos-zemes-fondas (accessed on 10 December 2021).
- Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Common vole as a focal small mammal species in orchards of the Northern Zone. Diversity 2021, 13, 134. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Isotopic niche of syntopic granivores in commercial orchards and meadows. Animals 2021, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Stable isotopes reveal the dominant species to have the widest trophic niche of three syntopic Microtus voles. Animals 2021, 11, 1814. [Google Scholar] [CrossRef]
- Balčiauskas., L.; Stirkė, V.; Balčiauskienė, L. Rodent fertility in commercial orchards in relation to body mass and fitness. Agric. Ecosyst. Environ. 2022, 329, 107886. [Google Scholar] [CrossRef]
- Balčiauskas, L. Methods of Investigation of Terrestrial Ecosystems. Part I. Animal Surveys; VU leidykla: Vilnius, Lithuania, 2004; p. 183. [Google Scholar]
- Prūsaitė, J. (Comp.). Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; p. 295. [Google Scholar]
- Brown, L.D.; Cat, T.T.; DasGupta, A. Interval Estimation for a proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available online: http://OpenEpi.com (accessed on 19 January 2021).
- G-Test Calculator. Available online: https://elem.com/~btilly/effective-ab-testing/g-test-calculator.html (accessed on 16 March 2021).
- Thomas, J.R.; Salazar, W.; Landers, D.M. What is missing in p < 0.05? Effect size. Res. Q. Exerc. Sport 1991, 62, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Tschumi, M.; Ekroos, J.; Hjort, C.; Smith, H.G.; Birkhofer, K. Rodents, not birds, dominate predation-related ecosystem services and disservices in vertebrate communities of agricultural landscapes. Oecologia 2018, 188, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Nistreanu, V.; Paraschiv, D.; Larion, A.; Sîtnic, V. Structure of small rodent communities in orchards from the central part of the Republic of Moldova and Bacau district, Romania. In Sustainable Use and Protection of Animal World in the Context of Climate Change; Institutul de Zoologie: Chișinău, Moldova, 2021; pp. 334–340. [Google Scholar] [CrossRef]
- Robinson, A.Y. Sustainable agriculture: The wildlife connection. Am. J. Altern. Agr. 1991, 6, 161–167. [Google Scholar] [CrossRef]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does organic farming benefit biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What is sustainable agriculture? A systematic review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Men, X.; Guo, X.; Dong, W.; Ding, N.; Qian, T. Influence of Human Disturbance to the Small Mammal Communities in the Forests. Open J. For. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Bonnet, T.; Crespin, L.; Pinot, A.; Bruneteau, L.; Bretagnolle, V.; Gauffre, B. How the common vole copes with modern farming: Insights from a capture-mark-recapture experiment. Agric. Ecosyst. Environ. 2013, 177, 21–27. [Google Scholar] [CrossRef]
- Jurišić, A.; Kranik, N.; Ivanović, I.; Vuković, S.; Potkonjak, A. Rodents and their control in orchards. Biljn. Lek. 2021, 49, 613–625. [Google Scholar] [CrossRef]
- Heroldová, M.; Šipoš, J.; Suchomel, J.; Zejda, J. Influence of crop type on common vole abundance in Central European agroecosystems. Agr. Ecosyst. Environ. 2021, 315, 107443. [Google Scholar] [CrossRef]
- Jacob, J. Response of small rodents to manipulations of vegetation height in agro-ecosystems. Integr. Zool. 2008, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Heroldová, M.; Bryja, J.; Zejda, J.; Tkadlec, E. Structure and diversity of small mammal communities in agriculture landscape. Agric. Ecosyst. Environ. 2007, 120, 206–210. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I. Responses of small mammal communities to environment and agriculture in a rural mosaic landscape. Mamm. Biol. 2018, 90, 55–65. [Google Scholar] [CrossRef]
- Andreassen, H.P.; Sundell, J.; Ecke, F.; Halle, S.; Marko, M.; Henttonen, H.; Huitu, O.; Jacob, J.; Johnsen, K.; Koskela, E.; et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia 2021, 195, 601–622. [Google Scholar] [CrossRef] [PubMed]
- Steen, H.; Yoccoz, N.G.; Ims, R.A. Predators and small rodent cycles: An analysis of a 79-year time series of small rodent population fluctuations. Oikos 1990, 59, 115–120. [Google Scholar] [CrossRef]
- Jedrzejewski, W.; Jedrzejewska, B. Rodent cycles in relation to biomass and productivity of ground vegetation and predation in the Palearctic. Acta Theriol. 1996, 41, 1–34. [Google Scholar] [CrossRef]
- Hörnfeldt, B.; Hipkiss, T.; Eklund, U. Fading out of vole and predator cycles? Proc. R. Soc. B 2005, 272, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Sullivan, D.S. Plant and small mammal diversity in orchard versus non-crop habitats. Agric. Ecosyst. Environ. 2006, 116, 235–243. [Google Scholar] [CrossRef]
- Jiménez-García, L.; Sánchez-Rojas, G.; Villarreal, O.; Bernal, H.; Jiménez-García, D. Agroecosystems management and biodiversity loss in an intensification gradient in traditional agriculture in Mexico. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 407–420. [Google Scholar] [CrossRef]
- Kalivodová, M.; Kanka, R.; Miklós, P.; Sládkovičová, V.H.; Žiak, D. Importance of wetland refugia in agricultural landscape provided based on the community characteristics of small terrestrial mammals. Ekológia 2018, 37, 358–368. [Google Scholar] [CrossRef]
- Wilson, M.H.; Lovell, S.T. Agroforestry—The next step in sustainable and resilient agriculture. Sustainability 2016, 8, 574. [Google Scholar] [CrossRef]
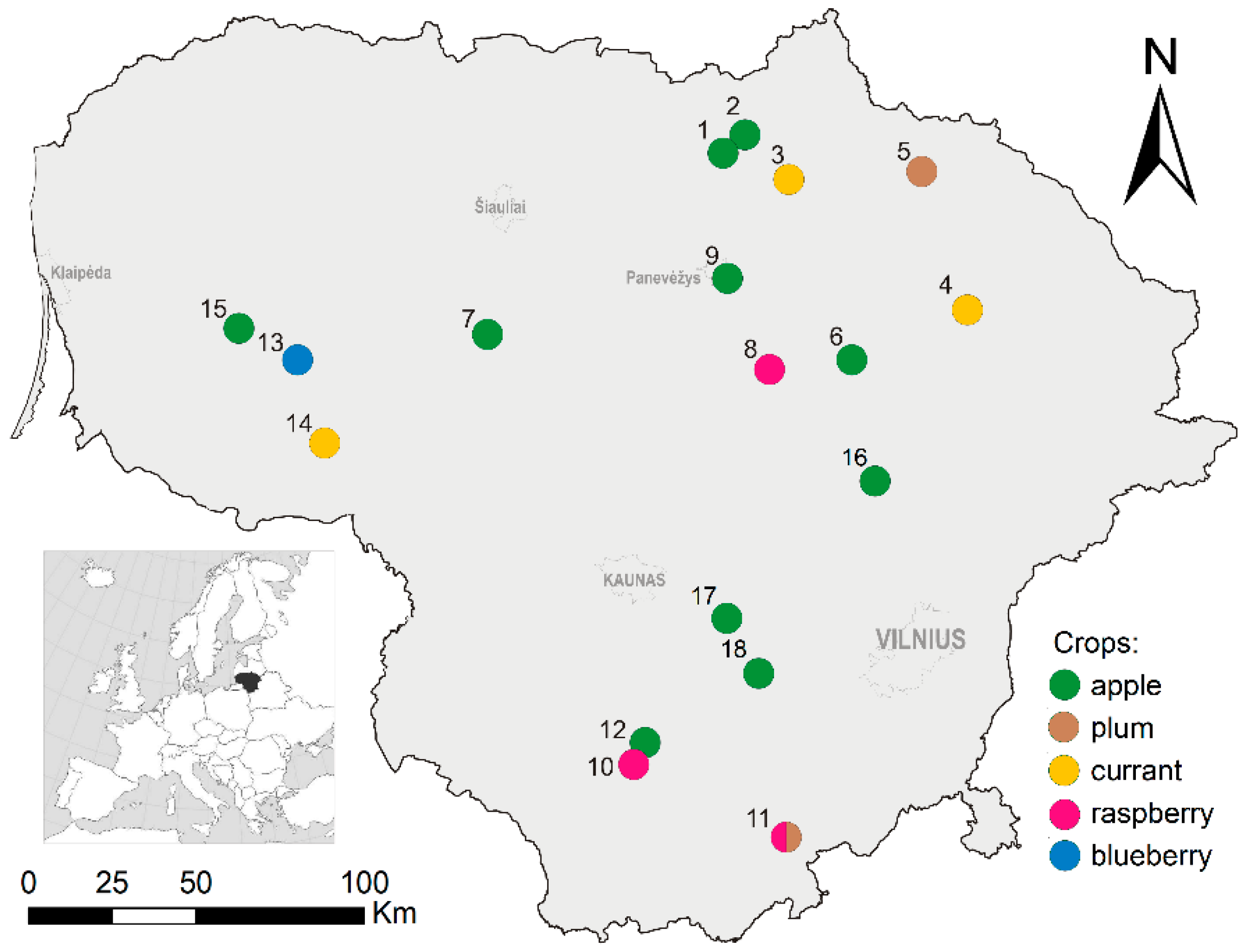

| Parameter | Values | Sites | Trapping Effort |
|---|---|---|---|
| Crop age | old | 1, 2, 6, 7, 9, 12, 16–18 | 9768 |
| medium | 3, 4, 8, 11, 13–15 | 5050 | |
| young | 1, 5, 10, 12 | 1900 | |
| Intensity of agriculture 1 | high | 2, 6, 10–13, 15, 17 | 8218 |
| medium | 1, 5, 9, 14 | 4450 | |
| low | 3, 4, 7, 8, 11, 16, 18 | 4050 | |
| Control | forest | 11, 17 | 525 |
| mowed meadow | 1, 2, 4, 6, 8–10, 12, 13–16 | 5560 | |
| non-mowed meadow | 1, 3, 5, 7–9, 11, 18 | 2700 |
| Relative Abundance | 2018 | 2019 | 2020 | F2,165 | p= | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Max | Mean | SE | Max | Mean | SE | Max | |||
| M. arvalis | 1.5 | 0.3 | 10.0 | 2.4 | 0.7 | 26.7 | 1.2 | 0.4 | 13.3 | 1.81 | 0.17 |
| A. flavicollis | 1.0 | 0.2 | 8.0 | 1.8 | 0.4 | 12.7 | 2.0 | 0.5 | 14.7 | 2.25 | 0.11 |
| A. agrarius | 2.1 | 0.5 | 16.0 | 1.3 | 0.4 | 16.0 | 1.6 | 0.6 | 17.3 | 0.67 | 0.52 |
| C. glareolus | 1.0 | 0.4 | 15.3 | 0.84 | 0.2 | 10.7 | 0.7 | 0.2 | 5.3 | 0.13 | 0.88 |
| Community | 6.2 | 1.1 | 36.0 | 6.9 | 1.1 | 44.7 | 5.8 | 1.2 | 36.0 | 0.23 | 0.79 |
| Index | N Lithuania | E Lithuania | S Lithuania | W Lithuania | C Lithuania | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Max | Mean | SE | Max | Mean | SE | Max | Mean | SE | Max | Mean | SE | Max | |
| RA of the community | 4.3 a | 1.2 | 27.3 | 7.4 a | 1.7 | 44.7 | 7.5 a | 1.4 | 36.0 | 2.0 b | 0.8 | 14.7 | 8.9 a | 1.5 | 36.0 |
| RA of M. arvalis | 1.6 a | 0.5 | 10.0 | 3.5 a | 1.2 | 26.7 | 1.3 ab | 0.3 | 8.0 | 0.7 b | 0.3 | 5.3 | 1.4 ab | 0.5 | 13.3 |
| RA of A. flavicollis | 0.8 b | 0.4 | 12.7 | 1.6 a | 0.4 | 9.3 | 2.0 a | 0.5 | 14.7 | 0.2 b | 0.1 | 2.1 | 2.6 a | 0.6 | 14.0 |
| RA of A. agrarius | 1.6 a | 0.4 | 17.3 | 1.4 a | 0.6 | 16.0 | 2.5 a | 0.7 | 17.3 | 0.5 a | 0.4 | 8.0 | 1.7 a | 0.6 | 16.0 |
| RA of C. glareolus | 0.1 b | 0.03 | 0.7 | 0.5 b | 0.2 | 4.0 | 1.1 a | 0.4 | 0.8 | 0.03 b | 0.03 | 0.7 | 2.1 a | 0.6 | 15.3 |
| Index | Fruit Types | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| AO | PO | CP | RP | MM | NM | FO | ||
| Community | RA | 6.0, SE = 1.1 | 4.3, SE = 1.6 | 6.6, SE = 3.2 | 6.6, SE = 2.2 | 6.7, SE = 1.1 | 6.3, SE = 1.9 | 12.8, SE = 5.0 |
| S | 9 a | 3 c | 6 b | 6 b | 11a | 8 a | 6 b | |
| H | 1.61 b | 0.93 d | 0.86 d | 1.29 c | 1.78 a | 1.63b | 1.27c | |
| D | 0.24 c | 0.45 a | 0.54 a | 0.31 b | 0.21 c | 0.23 c | 0.31 b | |
| M. arvalis | RA | 1.2, SE = 0.3 | 2.7, SE = 1.1 | 4.5, SE = 1.9 | 2.1, SE = 1.0 | 1.9, SE = 0.6 | 1.0, SE = 0.5 | 0.1 |
| % (CI) | 27.7 b (24.2–31.5) | 61.5 a (42.5–77.6) | 71.7 a (64.7–77.7) | 31.6 b (22.2–42.7) | 23.1 b (19.2–27.5) | 14.9 c (9.9–21.9) | 1.5 d (0.3–7.9) | |
| A. flavicollis | RA | 2.0, SE = 0.4 | 0.8, SE = 0.4 | 0.2, SE = 0.2 | 1.86, SE = 0.8 | 1.2, SE = 0.4 | 1.9, SE = 0.7 | 3.7, SE = 2.6 |
| % (CI) | 33.9 a (0.1–37.9) | 19.2 ab (8.5–33.9) | 3.3 b (1.5–7.1) | 26.3 a (17.7–37.2) | 20.6 ab (16.9–24.9) | 32.1 a (24.8–40.4) | 36.8 a (26.3–48.6) | |
| A. agrarius | RA | 1.1, SE = 0.5 | 0.8 | 1.6, SE = 1.2 | 2.4, SE = 1.1 | 2.0, SE = 0.6 | 1.6, SE = 0.9 | 3.7, SE = 1.7 |
| % (CI) | 16.2 b (13.4–19.5) | 19.2 b (8.5–33.9) | 22.8 a (7.3–29.4) | 36.4 a (26.9–48.1) | 32.4 a (28.0–37.2) | 23.9 a (17.4–31.8) | 27.9 a (19.7–39.6) | |
| C. glareolus | RA | 1.2, SE = 0.4 b | – | 0.05b | – | 0.5, SE = 0.2 b | 1.3, SE = 0.6 b | 4.9, SE = 1.7 a |
| % (CI) | 14.3 b (11.7–17.4) | – | 0.6 d (0.1–3.1) | – | 8.3 c (6.0–11.4) | 20.9 ab (14.9–28.5) | 30.9 a (21.2–42.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Spatiotemporal Variation of Small Mammal Communities in Commercial Orchards across the Small Country. Agriculture 2022, 12, 632. https://doi.org/10.3390/agriculture12050632
Stirkė V, Balčiauskas L, Balčiauskienė L. Spatiotemporal Variation of Small Mammal Communities in Commercial Orchards across the Small Country. Agriculture. 2022; 12(5):632. https://doi.org/10.3390/agriculture12050632
Chicago/Turabian StyleStirkė, Vitalijus, Linas Balčiauskas, and Laima Balčiauskienė. 2022. "Spatiotemporal Variation of Small Mammal Communities in Commercial Orchards across the Small Country" Agriculture 12, no. 5: 632. https://doi.org/10.3390/agriculture12050632
APA StyleStirkė, V., Balčiauskas, L., & Balčiauskienė, L. (2022). Spatiotemporal Variation of Small Mammal Communities in Commercial Orchards across the Small Country. Agriculture, 12(5), 632. https://doi.org/10.3390/agriculture12050632








