Co-Flowering Species Richness Increases Pollinator Visitation to Apple Flowers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Apple Flower Visitor Observations and Sampling
2.3. Do Apple Flower-Visitors Carry Pollen?
2.4. Flower-Visitor Observations and Sampling of Co-Flowering Species
2.5. Effect of Co-Flowering Plant Area on Apple Visitation
2.6. Data Analysis
2.6.1. Construction of Habitat–Pollinator Networks and Calculation of Indices
2.6.2. The Effect of Co-Flowering Species’ Richness and Abundance on Pollinator Visits to Apple
3. Results
3.1. Pollinator Networks on Granny Smith and Pink Lady Apples and Co-Flowering Plants within the Orchard and Wider Vegetation Matrix
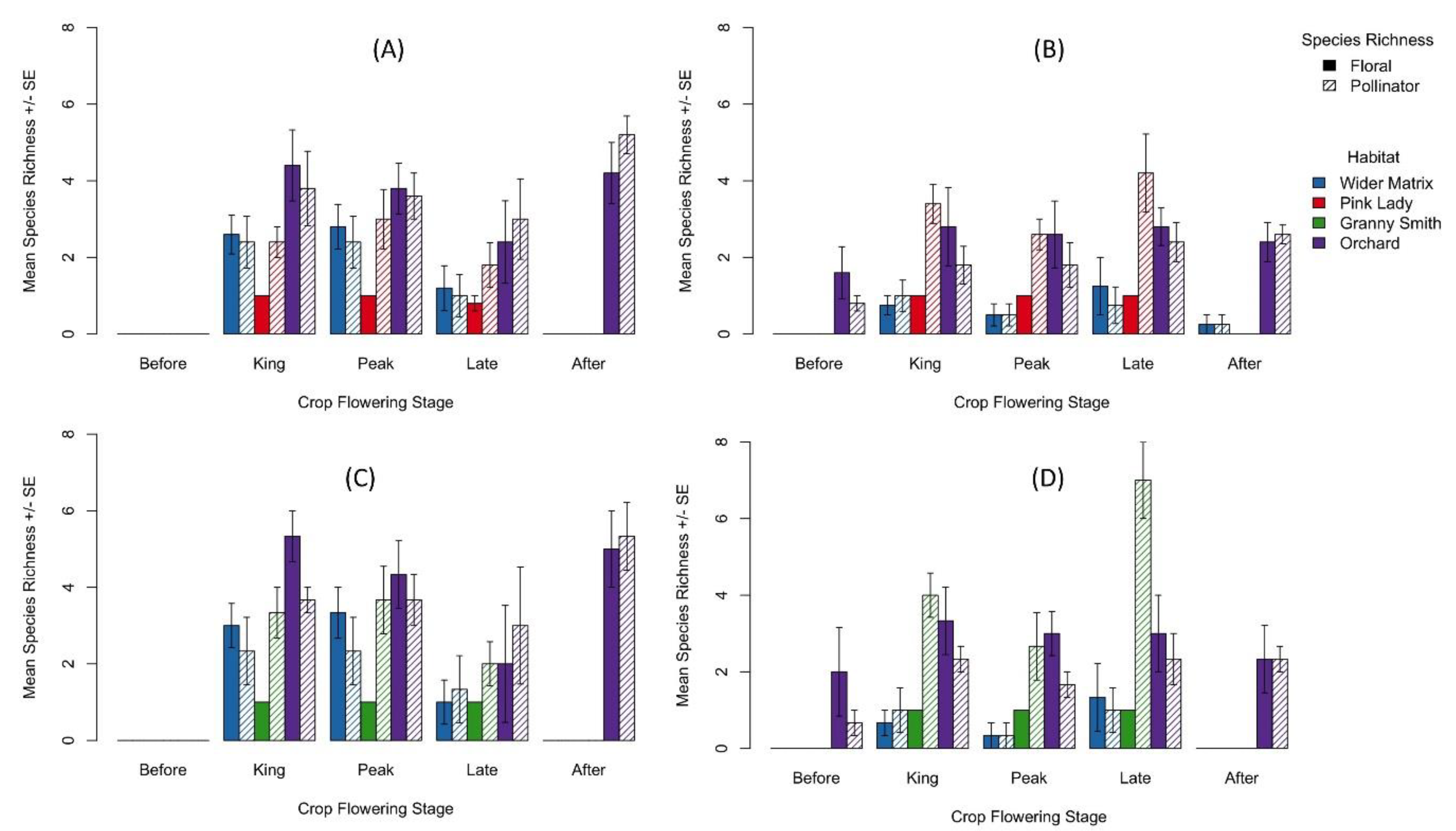
3.2. Species Richness of Pollinators and Plants
3.3. Influence of Co-Flowering Species on the Number of Pollinator, Honeybee and Native-Bee Visits to Apple Flowers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Williams, N.M.; Gaines, H.; Ascher, J.S.; Kremen, C. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J. Appl. Ecol. 2008, 45, 793–802. [Google Scholar] [CrossRef]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, E.J.; Dormann, C.F.; Holzschuh, A.; Klein, A.M.; Rand, T.A.; Tscharntke, T. Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 2012, 146, 34–43. [Google Scholar] [CrossRef]
- Földesi, R.; Kovács-Hostyánszki, A.; Kőrösi, Á.; Somay, L.; Elek, Z.; Markó, V.; Sárospataki, M.; Bakos, R.; Varga, Á.; Nyisztor, K.; et al. Relationships between wild bees, hoverflies and pollination success in apple orchards with different landscape contexts. Agric. For. Entomol. 2016, 18, 68–75. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Leonhardt, S.D.; Aizen, M.A.; Blaauw, B.R.; Isaacs, R.; Kuhlmann, M.; Kleijn, D.; Klein, A.M.; Kremen, C.; et al. From research to action: Enhancing crop yield through wild pollinators. Front. Ecol. Environ. 2016, 12, 439–447. [Google Scholar] [CrossRef]
- Bartholomée, O.; Aullo, A.; Becquet, J.; Vannier, C.; Lavorel, S. Pollinator presence in orchards depends on landscape-scale habitats more than in-field flower resources. Agric. Ecosyst. Environ. 2020, 293, 106806. [Google Scholar] [CrossRef]
- Samnegård, U.; Persson, A.S.; Smith, H.G. Gardens benefit bees and enhance pollination in intensively managed farmland. Biol. Conserv. 2011, 144, 2602–2606. [Google Scholar] [CrossRef]
- Grab, H.; Blitzer, E.J.; Danforth, B.; Loeb, G.; Poveda, K. Temporally dependent pollinator competition and facilitation with mass flowering crops affects yield in co-blooming crops. Sci. Rep. 2017, 7, 45296. [Google Scholar] [CrossRef]
- Lázaro, A.; Lundgren, R.; Totland, Ø. Co-flowering neighbors influence the diversity and identity of pollinator groups visiting plant species. Oikos 2009, 118, 691–702. [Google Scholar] [CrossRef]
- Rathcke, B. Competition and facilitation among plants for pollination. Pollinat. Biol. 1983, 305, 329. [Google Scholar]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Expansion of mass-flowering crops leads to transient pollinator dilution and reduced wild plant pollination. Proc. R. Soc. B Boil. Sci. 2011, 278, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Motten, A.F. The mechanism of competition for pollination between two forest herbs. Ecology 1985, 66, 554–563. [Google Scholar] [CrossRef]
- Morales, C.L.; Traveset, A. Interspecific Pollen Transfer: Magnitude, Prevalence and Consequences for Plant Fitness. Crit. Rev. Plant Sci. 2008, 27, 221–238. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhöffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef]
- Paton, D.C. Honeybees in the Australian Environment. BioScience 1993, 43, 95–103. [Google Scholar] [CrossRef]
- Gilpin, A.-M.; Collette, J.C.; Denham, A.J.; Ooi, M.K.J.; Ayre, D.J. Do introduced honeybees affect seed set and seed quality in a plant adapted for bird pollination? J. Plant Ecol. 2016, 10, 721–729. [Google Scholar] [CrossRef]
- Gilpin, A.-M.; Denham, A.J.; Ayre, D.J. Are there magnet plants in Australian ecosystems: Pollinator visits to neighbouring plants are not affected by proximity to mass flowering plants. Basic Appl. Ecol. 2019, 35, 34–44. [Google Scholar] [CrossRef]
- Whelan, R.J.; Ayre, D.J.; Beynon, F.M. The birds and the bees: Pollinator behaviour and variation in the mating system of the rare shrub Grevillea macleayana. Ann. Bot. 2009, 103, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, L.G.; Seymour, C.L.; Nicolson, S.W.; Veldtman, R. Creating patches of native flowers facilitates crop pollination in large agricultural fields: Mango as a case study. J. Appl. Ecol. 2012, 49, 1373–1383. [Google Scholar] [CrossRef]
- Saunders, M.E.; Luck, G.W.; Mayfield, M.M. Almond orchards with living ground cover host more wild insect pollinators. J. Insect Conserv. 2013, 17, 1011–1025. [Google Scholar] [CrossRef]
- Hanley, M.E.; Franco, M.; Dean, C.E.; Franklin, E.L.; Harris, H.R.; Haynes, A.G.; Rapson, S.R.; Rowse, G.; Thomas, K.C.; Waterhouse, B.R.; et al. Increased bumblebee abundance along the margins of a mass flowering crop: Evidence for pollinator spill-over. Oikos 2011, 120, 1618–1624. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dainese, M.; González-Varo, J.P.; Mudri-Stojnić, S.; Riedinger, V.; Rundlöf, M.; Scheper, J.; Wickens, J.; Wickens, V.; Bommarco, R.; et al. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 2016, 19, 1228–1236. [Google Scholar] [CrossRef]
- Atlas of Living Australia Occurrence. Occurrence Records Download on 2021-04-28. Available online: https://doi.ala.org.au/doi/bb675f48-97da-4203-8061-2b06836f5a8b (accessed on 29 April 2020).
- Atlas of Living Australia Occurrence. Occurrence Records Download on 2021-04-29. Available online: https://doi.ala.org.au/doi/f46d358e-6b9e-4d23-b566-cf05787834d4 (accessed on 29 April 2020).
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 493–525. [Google Scholar]
- Smith, T.J. The Australian Bee Genera: An Annotated, User-Friendly Key; The Rader Community Ecology Lab, University of New England: Armidale, Australia, 2018. [Google Scholar]
- Gilpin, A.M.; Ayre, D.J.; Denham, A.J. Can the pollination biology and floral ontogeny of the threatened Acacia carneorum explain its lack of reproductive success? Ecol. Res. 2013, 29, 225–235. [Google Scholar] [CrossRef]
- Thomson, J.D.; Wilson, P.; Valenzuela, M.; Malzone, M. Pollen presentation and pollination syndromes, with special reference to Penstemon. Plant Species Biol. 2000, 15, 11–29. [Google Scholar] [CrossRef]
- Thorp, R.W. The collection of pollen by bees. Plant Syst. Evol. 2000, 222, 211–223. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 18 October 2021).
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef]
- Bersier, L.; Banašek-Richter, C.; Cattin, M. Quantitative Descriptors of Food-Web Matrices. Ecology 2002, 83, 2394–2407. [Google Scholar] [CrossRef]
- Blüthgen, N.; Fründ, J.; Vázquez, D.; Menzel, F. What do interaction network metrics tell us about specialization and biological traits? Ecology 2008, 89, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Redhead, J.; Woodcock, B.; Pocock, M.; Pywell, R.; Vanbergen, A.; Oliver, T. Potential landscape-scale pollinator networks across Great Britain: Structure, stability and influence of agricultural land cover. Ecol. Lett. 2018, 21, 1821–1832. [Google Scholar] [CrossRef]
- Gilpin, A.-M.; O’Brien, C.; Kobel, C.; Brettell, L.E.; Cook, J.M.; Power, S.A. Co-flowering plants support diverse pollinator populations and facilitate pollinator visitation to sweet cherry crops. Basic Appl. Ecol. 2022, 63, 36–48. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. Tests in linear mixed effects models. J. Stat. Softw. 2016, 2, 33. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.M.; Schumacher, J.; Weisser, W.W.; Tscharntke, T. How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 2008, 117, 1808–1815. [Google Scholar] [CrossRef]
- Hegland, S.J.; Boeke, L. Relationships between the density and diversity of floral resources and flower visitor activity in a temperate grassland community. Ecol. Èntomol. 2006, 31, 532–538. [Google Scholar] [CrossRef]
- Bänsch, S.; Tscharntke, T.; Ratnieks, F.L.W.; Härtel, S.; Westphal, C. Foraging of honey bees in agricultural landscapes with changing patterns of flower resources. Agric. Ecosyst. Environ. 2020, 291, 106792. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Biesmeijer, J.C.; Benadi, G.; Fründ, J.; Stang, M.; Bartomeus, I.; Kaiser-Bunbury, C.N.; Baude, M.; Gomes, S.I.; Merckx, V.; et al. The potential for indirect effects between co-flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecol. Lett. 2014, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Ghazoul, J. Floral diversity and the facilitation of pollination. J. Ecol. 2006, 94, 295–304. [Google Scholar] [CrossRef]
- Smith, J.P.; Heard, T.A.; Beekman, M.; Gloag, R. Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae). Aust. Entomol. 2017, 56, 50–53. [Google Scholar] [CrossRef]
- Ribbands, C.R. The Flight Range of the Honey-Bee. J. Anim. Ecol. 1951, 20, 220–226. [Google Scholar] [CrossRef]
- Watson, J.C.; Wolf, A.T.; Ascher, J.S. Forested Landscapes Promote Richness and Abundance of Native Bees (Hymenoptera: Apoidea: Anthophila) in Wisconsin Apple Orchards. Environ. Èntomol. 2011, 40, 621–632. [Google Scholar] [CrossRef]
- Joshi, N.K.; Otieno, M.; Rajotte, E.G.; Fleischer, S.J.; Biddinger, D.J. Proximity to Woodland and Landscape Structure Drives Pollinator Visitation in Apple Orchard Ecosystem. Front. Ecol. Evol. 2016, 4, 38. [Google Scholar] [CrossRef]
- Wu, P.; Axmacher, J.C.; Li, X.; Song, X.; Yu, Z.; Xu, H.; Tscharntke, T.; Westphal, C.; Liu, Y. Contrasting effects of natural shrubland and plantation forests on bee assemblages at neighboring apple orchards in Beijing, China. Biol. Conserv. 2019, 237, 456–462. [Google Scholar] [CrossRef]
- Marini, L.; Quaranta, M.; Fontana, P.; Biesmeijer, J.C.; Bommarco, R. Landscape context and elevation affect pollinator communities in intensive apple orchards. Basic Appl. Ecol. 2012, 13, 681–689. [Google Scholar] [CrossRef]
- Miñarro, M.; Prida, E. Hedgerows surrounding organic apple orchards in north-west S pain: Potential to conserve beneficial insects. Agric. For. Entomol. 2013, 15, 382–390. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F.L. Do sown flower strips boost wild pollinator abundance and pollination services in a spring-flowering crop? A case study from UK cider apple orchards. Agric. Ecosyst. Environ. 2017, 239, 20–29. [Google Scholar] [CrossRef]
- Ratto, F.; Steward, P.; Sait, S.M.; Pryke, J.S.; Gaigher, R.; Samways, M.J.; Kunin, W. Proximity to natural habitat and flower plantings increases insect populations and pollination services in South African apple orchards. J. Appl. Ecol. 2021, 58, 2540–2551. [Google Scholar] [CrossRef]
- García, R.R.; Miñarro, M. Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agric. Ecosyst. Environ. 2014, 183, 118–126. [Google Scholar] [CrossRef]
- Garratt, M.P.; Breeze, T.D.; Jenner, N.; Polce, C.; Biesmeijer, J.C.; Potts, S.G. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agric. Ecosyst. Environ. 2014, 184, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Heard, T.; Hendrikz, J. Factors Influencing Flight Activity of Colonies of the Stingless Bee Trigona-Carbonaria (Hymenoptera, Apidae). Aust. J. Zool. 1993, 41, 343–353. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Chacoff, N.P.; Aizen, M.A. Edge effects on flower-visiting insects in grapefruit plantations bordering premontane subtropical forest. J. Appl. Ecol. 2006, 43, 18–27. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Seymour, C.L.; Veldtman, R.; Nicolson, S.W. Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J. Appl. Ecol. 2010, 47, 810–820. [Google Scholar] [CrossRef]
- MacInnis, G.; Buddle, C.M.; Forrest, J.R. Small wild bee abundance declines with distance into strawberry crops regardless of field margin habitat. Basic Appl. Ecol. 2020, 44, 14–23. [Google Scholar] [CrossRef]
- Waser, N.M.; Real, L.A. Effective mutualism between sequentially flowering plant species. Nature 1979, 281, 670–672. [Google Scholar] [CrossRef]
- Moeller, D.A. Facilitative interactions among plants via shared pollinators. Ecology 2004, 85, 3289–3301. [Google Scholar] [CrossRef]
- Schüepp, C.; Herzog, F.; Entling, M.H. Disentangling multiple drivers of pollination in a landscape-scale experiment. Proc. R. Soc. B 2014, 281, 20132667. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.A.; Anderson, W.W. Competition for Pollinators between Simultaneously Flowering Species. Am. Nat. 1970, 104, 455–467. [Google Scholar] [CrossRef]
- Timberlake, T.P.; Vaughan, I.P.; Memmott, J. Phenology of farmland floral resources reveals seasonal gaps in nectar availability for bumblebees. J. Appl. Ecol. 2019, 56, 1585–1596. [Google Scholar] [CrossRef]
- Lander, T.A.; Bebber, D.P.; Choy, C.T.; Harris, S.A.; Boshier, D.H. The Circe Principle explains how resource-rich land can waylay pollinators in fragmented landscapes. Curr. Biol. 2011, 21, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Dupont, Y.L.; Hansen, D.M.; Valido, A.; Olesen, J.M. Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biol. Conserv. 2004, 118, 301–311. [Google Scholar] [CrossRef]
- Vaughton, G. Pollination disruption by European honeybees in the Australian bird-pollinated shrub Grevillea barklyana (Proteaceae). Plant. Syst. Evol. 1996, 200, 89–100. [Google Scholar] [CrossRef]
- Fagua, J.C.; Ackerman, J.D. Consequences of floral visits by ants and invasive honeybees to the hummingbird-pollinated, Caribbean cactus Melocactus intortus. Plant. Species Biol. 2011, 26, 193–204. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Gilpin, A.M.; Denham, A.J.; Ayre, D.J. Do mass flowering agricultural species affect the pollination of Australian native plants through localised depletion of pollinators or pollinator spillover effects? Agric. Ecosyst. Environ. 2019, 277, 83–94. [Google Scholar] [CrossRef]

| Location/Orchard | Area of Orchard (ha) | Floral Area within Orchard (ha) | Native Surrounding Floral Area (ha) within 500 m Radius | Apple Cultivar Studied | Approx. Number of Apple Trees |
|---|---|---|---|---|---|
| Bilpin | |||||
| 1 | 2.7 | 2.9 | 42.1 | Pink Lady | 950 |
| Granny Smith | 490 | ||||
| 2 | 1.9 | 2.7 | 32.4 | Pink Lady | 250 |
| Granny Smith | 450 | ||||
| 3 | 3.2 | 3.4 | 27.8 | Pink Lady | 750 |
| Granny Smith | 250 | ||||
| 4 | 7.8 | 9.9 | 46.0 | Pink Lady | 1800 |
| 5 | 1.5 | 2.1 | 47.9 | Pink Lady | 150 |
| Orange | |||||
| 1 | 15.8 | 12.3 | 0.7 | Pink lady | 3500 |
| 2 | 3.2 | 4.8 | 0.3 | Pink Lady | 1320 |
| Granny Smith | 150 | ||||
| 3 | 11.3 | 8.6 | 2.7 | Pink Lady | 1440 |
| Granny Smith | 110 | ||||
| 4 | 4.9 | 6.4 | 15.2 | Pink Lady | 440 |
| Region | Apple Cultivar | Year | Season | Connectance | Interaction Diversity | Interaction Evenness | No. of Pollinator Species | Effective Number of Links |
|---|---|---|---|---|---|---|---|---|
| Bilpin | Granny Smith | 2017 | king | 0.500 | 1.541 | 0.453 | 10 | 4.667 |
| peak | 0.556 | 1.844 | 0.559 | 9 | 6.322 | |||
| late | 0.542 | 1.519 | 0.478 | 8 | 4.568 | |||
| 2018 | king | 0.542 | 1.614 | 0.507 | 8 | 5.020 | ||
| peak | 0.600 | 1.252 | 0.462 | 5 | 3.499 | |||
| late | 0.515 | 1.436 | 0.411 | 11 | 4.202 | |||
| Pink Lady | 2017 | king | 0.667 | 1.804 | 0.531 | 10 | 6.076 | |
| peak | 0.564 | 1.770 | 0.483 | 13 | 5.870 | |||
| late | 0.533 | 1.691 | 0.497 | 10 | 5.426 | |||
| 2018 | king | 0.542 | 1.529 | 0.481 | 8 | 4.612 | ||
| peak | 0.524 | 1.302 | 0.428 | 7 | 3.676 | |||
| late | 0.593 | 1.538 | 0.467 | 9 | 4.656 | |||
| Orange | Granny Smith | 2017 | king | 0.667 | 1.460 | 0.588 | 4 | 4.307 |
| peak | 0.750 | 1.777 | 0.715 | 4 | 5.913 | |||
| late | 0.533 | 1.494 | 0.552 | 5 | 4.456 | |||
| 2018 | king | 0.556 | 1.099 | 0.500 | 3 | 3.000 | ||
| peak | 0.600 | 1.113 | 0.483 | 5 | 3.044 | |||
| late | 0.750 | 0.213 | 0.153 | 2 | 1.237 | |||
| Pink Lady | 2017 | king | 0.611 | 1.521 | 0.526 | 6 | 4.575 | |
| peak | 0.619 | 1.931 | 0.634 | 7 | 6.894 | |||
| late | 0.667 | 1.697 | 0.627 | 5 | 5.458 | |||
| 2018 | king | 0.429 | 0.939 | 0.308 | 7 | 2.556 | ||
| peak | 0.500 | 0.813 | 0.327 | 4 | 2.254 | |||
| late | 0.583 | 0.740 | 0.298 | 6 | 2.095 |
| Intercept | SS Abundance Orchard | SS Abundance Wider Matrix | Total VisitsWider Matrix | Pollinator Richness Orchard | Plant Richness Orchard | Area NV | No. of Apple Trees | FA Wider Matrix | Apple Variety | Region | df | AICc | ΔAICc | R2m | R2c | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model (1): Total visits | 4.24 | 0.30 | + | + | 6 | 773.8 | 0.00 | 0.64 | 0.68 | |||||||
| 4.39 | 0.29 | + | 5 | 774.1 | 0.35 | 0.62 | 0.68 | |||||||||
| 3.91 | 0.31 | 0.54 | 5 | 774.5 | 0.76 | 0.61 | 0.69 | |||||||||
| Model (2): Honeybees | 3.61 | 0.19 | + | + | 6 | 737.5 | 0.00 | 0.41 | 0.45 | |||||||
| 3.62 | 0.13 | 0.15 | + | + | 7 | 737.9 | 0.42 | 0.39 | 0.47 | |||||||
| 3.59 | 0.16 | 0.10 | + | + | 7 | 738.0 | 0.47 | 0.41 | 0.45 | |||||||
| Model (3): Native bees | 3.05 | 0.51 | 0.57 | −0.72 | + | 7 | 500.4 | 0.00 | 0.79 | 0.8 | ||||||
| 1.64 | 0.39 | 0.71 | 1.52 | −0.77 | 7 | 500.7 | 0.25 | 0.78 | 0.812 | |||||||
| 3.18 | 0.49 | 0.36 | −0.40 | −0.56 | + | 8 | 500.7 | 0.25 | 0.82 | 0.82 | ||||||
| Model (4): Richness | 1.22 | + | 3 | 294.7 | 0.00 | 0.25 | 0.25 | |||||||||
| 1.22 | 0.07 | + | 4 | 295.8 | 1.12 | 0.26 | 0.26 | |||||||||
| 0.94 | 0.31 | 3 | 296.0 | 1.31 | 0.23 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilpin, A.-M.; Kobel, C.; Brettell, L.E.; O’Brien, C.; Cook, J.M.; Power, S.A. Co-Flowering Species Richness Increases Pollinator Visitation to Apple Flowers. Agriculture 2022, 12, 1246. https://doi.org/10.3390/agriculture12081246
Gilpin A-M, Kobel C, Brettell LE, O’Brien C, Cook JM, Power SA. Co-Flowering Species Richness Increases Pollinator Visitation to Apple Flowers. Agriculture. 2022; 12(8):1246. https://doi.org/10.3390/agriculture12081246
Chicago/Turabian StyleGilpin, Amy-Marie, Conrad Kobel, Laura E. Brettell, Corey O’Brien, James M. Cook, and Sally A. Power. 2022. "Co-Flowering Species Richness Increases Pollinator Visitation to Apple Flowers" Agriculture 12, no. 8: 1246. https://doi.org/10.3390/agriculture12081246
APA StyleGilpin, A.-M., Kobel, C., Brettell, L. E., O’Brien, C., Cook, J. M., & Power, S. A. (2022). Co-Flowering Species Richness Increases Pollinator Visitation to Apple Flowers. Agriculture, 12(8), 1246. https://doi.org/10.3390/agriculture12081246






