Differential Occurrence of Cuticular Wax and Its Role in Leaf Physiological Mechanisms of Three Edible Aroids of Northeast India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Leaf Samples
2.2. Scanning Electron Microscopy (SEM) of Aroid Leaves
2.3. Extraction and Estimation of Cuticular Wax
2.4. Sun Protection Factor (SPF)
2.5. Contact Angle and Wettability
2.6. Chlorophyll Content (Ch) and Chlorophyll Stability Index (CSI)
2.7. Color Parameters
2.8. Relative Water Content (RWC) and Leaf Moisture Loss
2.9. Cell Membrane Injury (CMI)
2.10. In Vitro Phytophthora colocasiae (Pc) Infectivity Assay
2.11. Statistical Analysis
3. Results and Discussion
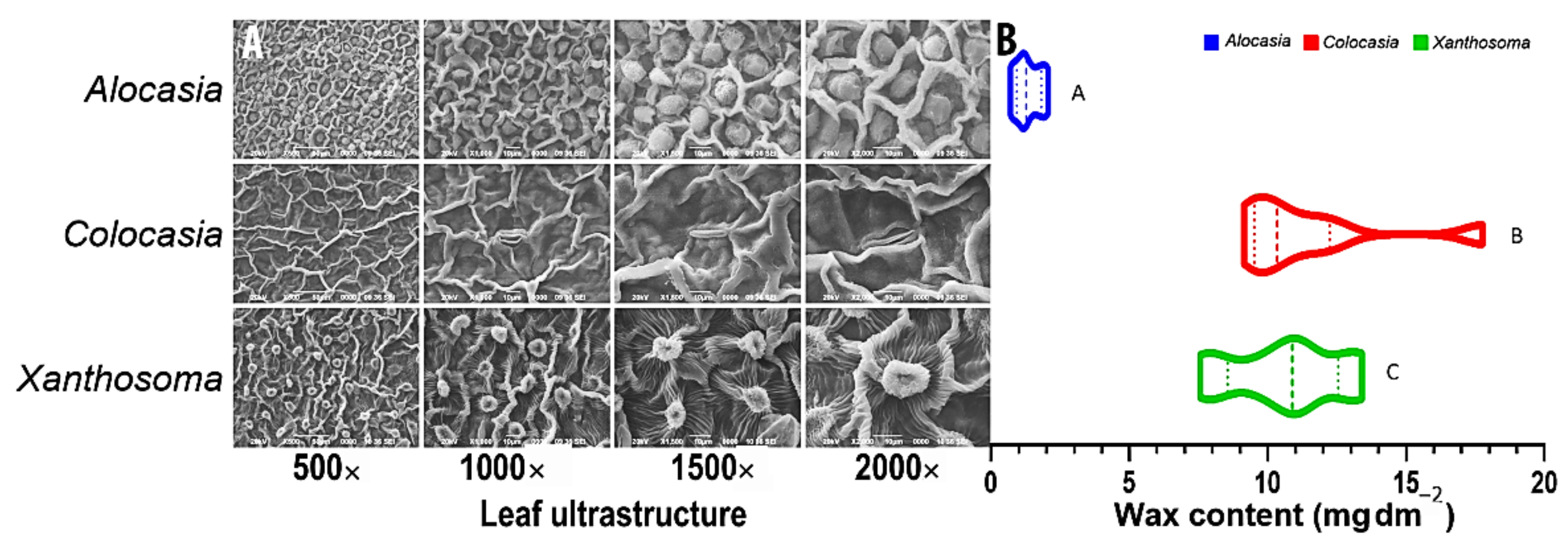
3.1. Surface Properties, Extraction Process, and Estimation of Cuticular Wax
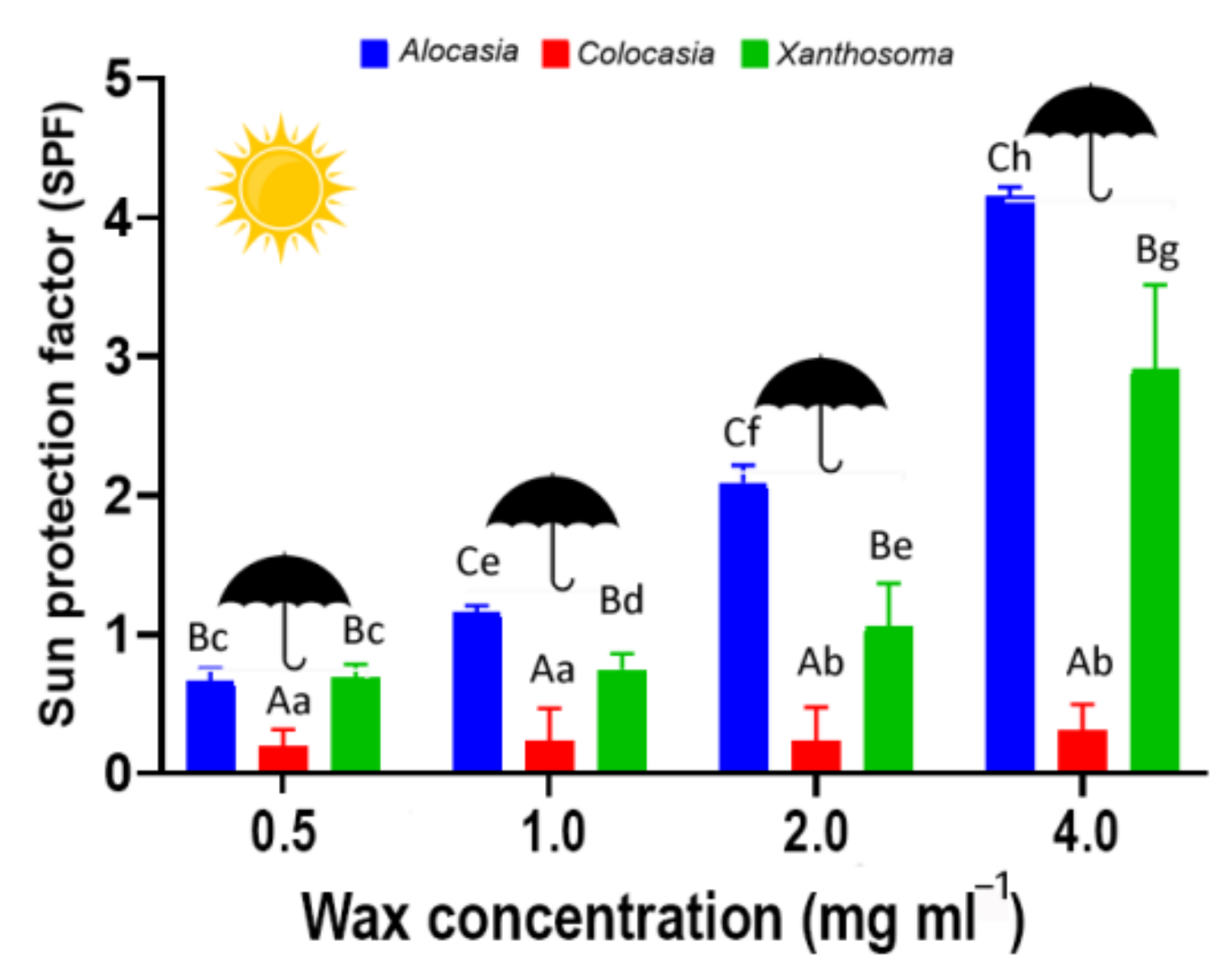
3.2. Sun Protection Factor (SPF)
3.3. Contact Angle (CA)
3.4. Wettability
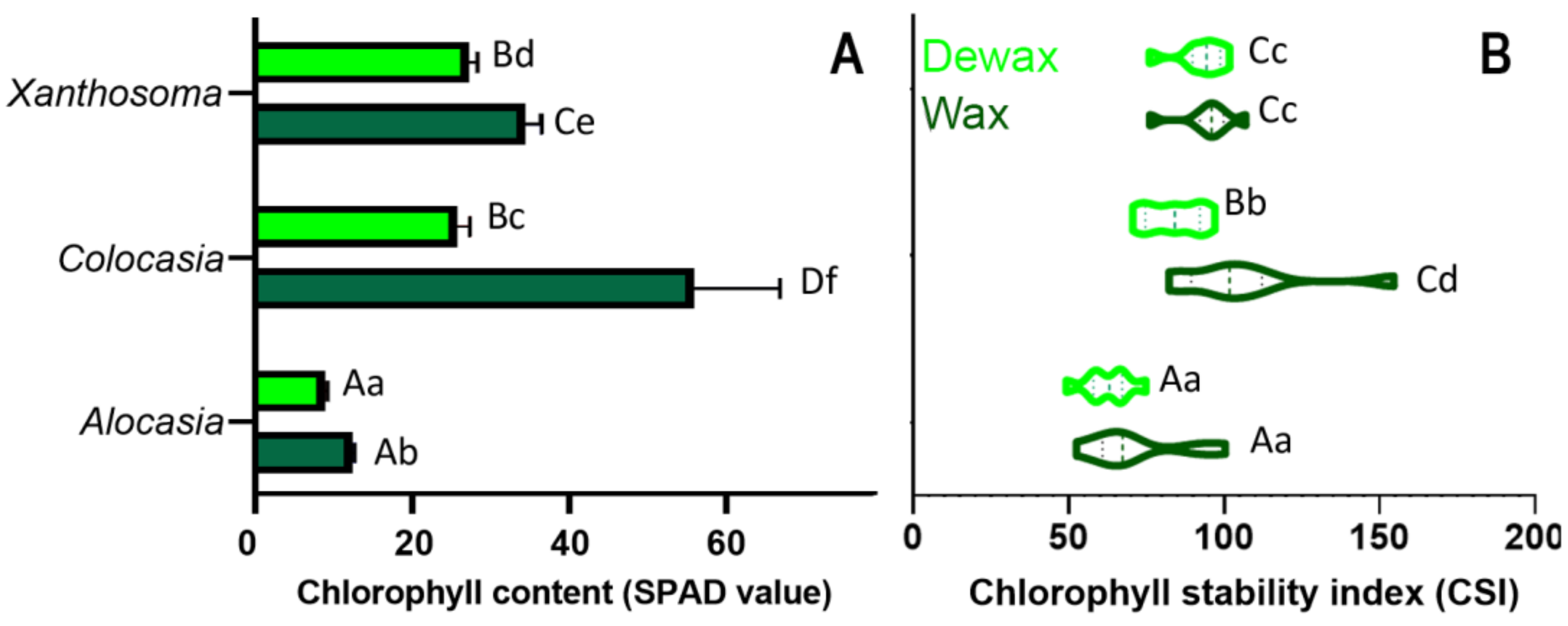
3.5. Chlorophyll Content and Chlorophyll Stability Index (CSI)
3.6. Color Parameters
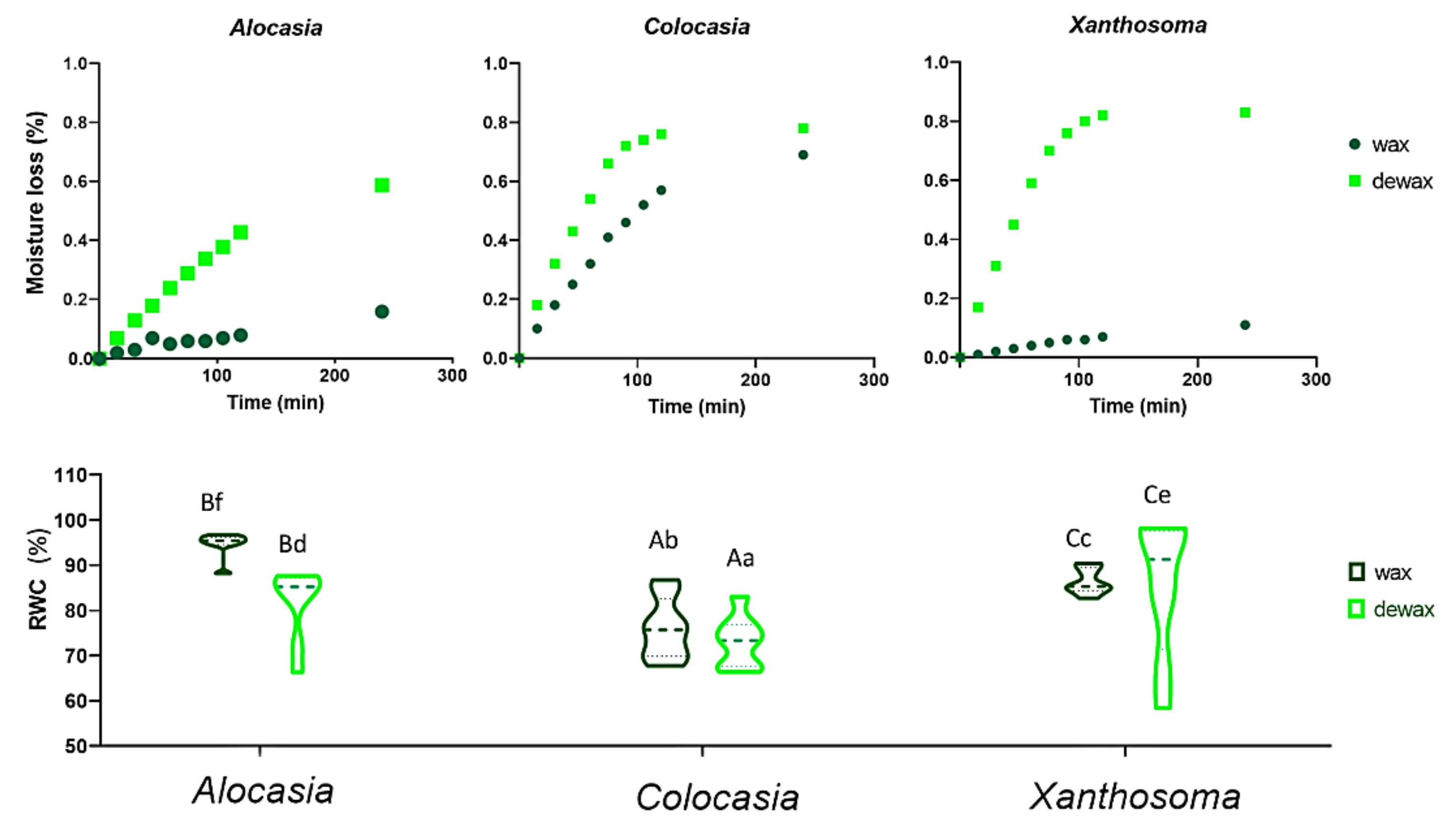
3.7. Relative Water Content (RWC) and Leaf Moisture Loss
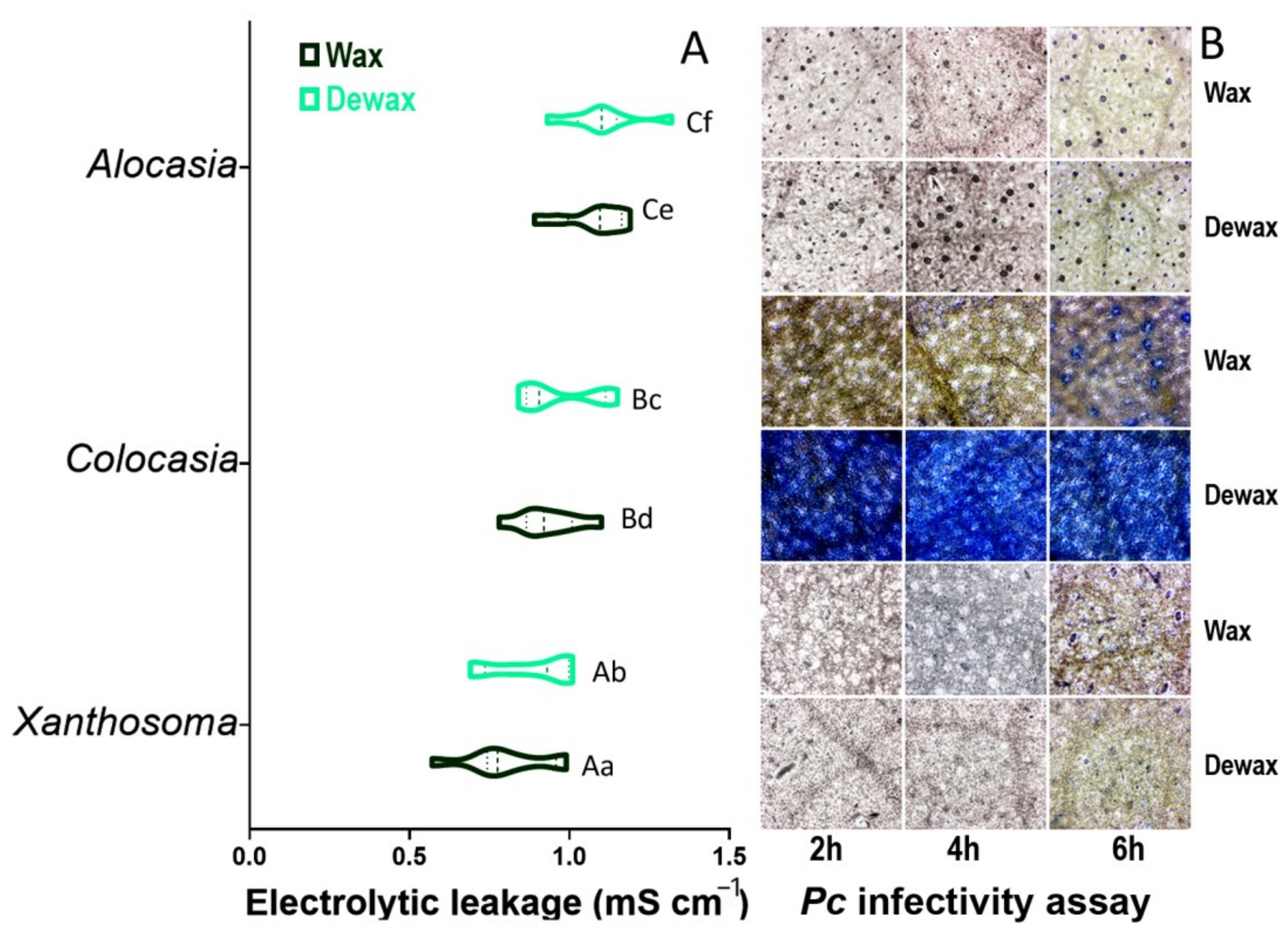
3.8. Cell Membrane Injury (CMI) and In Vitro Phytophthora colocasiae (Pc) Infectivity
3.9. Correlation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irwin, S.V.; Kaufusi, P.; Banks, K.; De la Pena, R.; Cho, J.J. Molecular characterization of taro (Colocasia esculenta) using RAPD markers. Euphytica 1998, 99, 183. [Google Scholar] [CrossRef]
- Opara, L.U.; Mejía, D. Edible Aroids: Post–Harvest Operation. AGST/FAO: Danilo Mejía, PhD, FAO (Technical). 2003. Available online: https://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Edible_aroids.pdf (accessed on 1 January 2020).
- Vieira, G.H.S.; Peterle, G.; Loss, J.B.; Peterle, G.; Poloni, C.M.M.; Colombo, J.N.; Monaco, P.A.V.L. Strategies for taro (Colocasia esculenta) irrigation. J. Exp. Agric. Int. 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Statistics Division; FAO: Rome, Italy, 2021; Available online: http://www.fao.org/statistics/en/ (accessed on 1 February 2021).
- Teron, R. Ethnobotanical study of dietary use and culinary knowledge of Aroids (family Araceae) in Karbi Anglong district, Assam. NeBIO 2019, 10, 80–84. [Google Scholar]
- Matthews, P.J.; Medhi, D. Feasibility study for field research: Ethnobotany and ecology of wild and cultivated aroids in Assam state, northeast India. AREIPGR 2014, 30, 159–183. [Google Scholar]
- Prajapati, R.; Kalariya, M.; Umbarkar, R.; Parmar, S.; Sheth, N. Colocasia esculenta: A potent indigenous plant. Int. J. Nutr. Pharmacol. Neurol. Dis. 2011, 1, 90. [Google Scholar] [CrossRef]
- Huang, W.; Li, C.; Wang, Y.; Yi, X.; He, X. Anti–inflammatory lignanamides and monoindoles from Alocasia macrorrhiza. Fitoterapia 2017, 117, 126132. [Google Scholar] [CrossRef]
- Mulla, W.A.; Kuchekar, S.B.; Thorat, V.S.; Chopade, A.R.; Kuchekar, B.S. Antioxidant, antinociceptive anti–inflammatory activities of ethanolic extract of leaves of Alocasia indica (Schott.). J. Young Pharm. 2010, 2, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Choudhury, M.D.; Paul, S.B. In vitro Antibacterial activity of Alocasia decipiens schott. Int. J. Pharm. Pharm. Sci. 2013, 5, 155–157. [Google Scholar]
- Adewumi, D.F.; Adewole, E.; Ogunmodede, O.T.; Ojo, A. Effect of Chemical Modifications on Pasting Properties of Cocoyam Starch (Xanthosoma Sagittifollium). J. Nat. Sci. Res. 2015, 5, 36–39. [Google Scholar]
- Zhu, F. Structure, properties, and applications of aroid starch. Food Hydrocoll. 2016, 52, 378–392. [Google Scholar] [CrossRef]
- Kalita, A.; Talukdar, N. Colocasia esculenta (L.) leaf bio–wax as a hydrophobic surface coating substance for paper for preparing hydrophobic paper bags. Int. J. Pharm. Biol. Sci. 2018, 8, 583–590. [Google Scholar]
- Acebuche, M.J.A.; Alvarez, M.L.C. Hydrophobic paper from the wax of Colocasia esculenta (taro) leaf and chitin from crab shell. Int. J. Res. Publ. 2019, 30, 1–7. Available online: https://www.ijrp.org (accessed on 1 January 2020).
- De Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.R. Technological, applications, and characteristics of edible films and coatings: A review. SN Appl. Sci. 2019, 1, 632. [Google Scholar] [CrossRef] [Green Version]
- Boakye, A.A.; Wireko–Manu, F.D.; Oduro, I.; Ellis, W.O.; Gudjónsdóttir, M.; Chronakis, I.S. Utilizing cocoyam (Xanthosoma sagittifolium) for food and nutrition security: A review. Food Sci. Nutr. 2018, 6, 703–713. [Google Scholar] [CrossRef]
- Sharma, P.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Verma, K.S.; Verma, S.; Prasad, J.; Kothari, S.L.; Gour, V.S. An appraisal of cuticular wax of Calotropis procera (Ait.) R. Br.: Extraction, chemical composition, biosafety and application. J. Hazard. Mater. 2019, 368, 397–403. [Google Scholar] [CrossRef]
- Sajeevan, R.S.; Parvathi, M.S.; Nataraja, K.N. Leaf wax trait in crops for drought and biotic stress tolerance: Regulators of epicuticular wax synthesis and role of small RNAs. Indian J. Plant Physiol. 2017, 22, 434–447. [Google Scholar] [CrossRef]
- Sharma, P.; Bala, N.; Saini, M.K.; Jain, A.; Kothari, S.L.; Gour, V.S. Assessment of role of cuticular wax in adaptive physiological responses of Calotropis procera and Calotropis gigantea. Plant Physiol. Rep. 2021, 26, 368–373. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition and physiological function of the wax layers coating Arabidopsis Leaves: B–Amyrin negatively affects the intracuticular water barrier. Plant Physiol. 2012, 160, 1120–1129. [Google Scholar] [CrossRef] [Green Version]
- Zeisler–Diehl, V.V.; Barthlott, W.; Schreiber, L. Plant Cuticular Waxes: Composition, Function, and Interactions with Microorganisms. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Wilkes, H., Ed.; Handbook of Hydrocarbon and Lipid Microbiology; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Sahoo, M.R.; Dasgupta, M.; Mukherjee, A.; Kole, P.C. In vitro and in vivo screening of taro [Colocasia esculenta (L.) Schott] for Phytophthora leaf blight disease. In Advances in Fungal Diversity and Host–Pathogen Interaction; Rodrigues, B.F., Gour, H.N., Bhat, D.J., Kamat, N., Eds.; Goa University: Goa, India, 2005; pp. 144–152. [Google Scholar]
- Pieniazek, F.; Messina, V. Texture and color analysis of freeze–dried potato (cv. Spunta) using instrumental and image analysis techniques. Int. J. Food Prop. 2017, 20, 1422–1431. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Bhardwaj, R. Wetting characteristics of Colocasia esculenta (Taro) leaf and a bioinspired surface thereof. Sci. Rep. 2020, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.d.S.; Breder, M.N.R.; Mansur, M.C.d.A. Determination of sun protection factor by spectrophotometry. An. Bras. De Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.M.; Narayanan, S.L.; Ibrahim, S.M. Chlorophyll stability index (CSI): Its impact on salt tolerance in rice. Int. Rice Res. Notes 2000, 25, 38–39. [Google Scholar]
- Afshari–Jouybari, H.; Farahnaky, A. Evaluation of Photoshop software potential for food colorimetry. J. Food Eng. 2011, 106, 170–175. [Google Scholar] [CrossRef]
- Perez–Perez, J.G.; Syvertsen, J.P.; Botía, P.; García–Sánchez, F. Leaf water relations and net gas exchange responses of salinized carrizo citrange seedlings during drought stress and recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, A.; Sancho-Knapik, D.; Gil-Pelegrín, E.; Leide, J.; Peguero-Pina, J.J.; Burghardt, M.; Riederer, M. Cuticular wax coverage and its transpiration barrier properties in Quercus coccifera L. leaves: Does the environment matter? Tree Physiol. 2020, 40, 827–840. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Comparative analysis of the chemical composition and water permeability of the cuticular wax barrier in Welsh onion (Allium fistulosum L.). Protoplasma 2019, 257, 833–840. [Google Scholar] [CrossRef]
- Fernandez–Baustia, N.; Dominguez–Nunez, J.A.; Moreno, M.M.C.; Berrocal–Lobo, M. Plant tissue trypan blue staining during phytopathogen infection. Bio–Protoc. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Jenks, M.A.; Ashworth, E.N. Plant epicuticular waxes: Function, production, and genetics. Hortic. Rev. 1999, 23, 1–68. [Google Scholar]
- Hossain, M.E.; Rahman, M.S.; Ketata, C.; Mann, H.; Islam, M.R. SEM–based structural and chemical analysis of paraffin wax and beeswax for petroleum applications. J. Charact. Dev. Nov. Mater. 2009, 1, 21–38. [Google Scholar]
- Mazumder, M.; Das, K.; Choudhury, A.D.; Khazeo, P. Determination of Sun Protection Factor (SPF) Number of Some Hydroalcoholic Vegetable Extracts. Pharma Tutor 2018, 6, 41–45. [Google Scholar]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Srinivasan, P.; Inala, M.S.R.; Nandini, H.S.; Kutty, A.V.M.; Kiranmayee, P. A pilot study on sun protecting factor of plant extracts: An observational study. Asian J. Pharm. Clin. Res. 2016, 11, 67–71. [Google Scholar] [CrossRef]
- Yasmeen, S.; Gupta, P. In vitro demonstration of Dalbergia sissoo (Indian rosewood) methanolic extracts as potential agents for sun screening and Dan nick prevention. Int. J. Pharm. Sci. 2016, 8, 175–181. [Google Scholar]
- Chen, S.Y.; Mochizuki, T.; Nishi, M.; Takagi, H.; Yoshimura, Y.; Toba, M. Hydrotreating of Jatropha–derived Bio–oil over Mesoporous Sulfide Catalysts to Produce Drop–in Transportation Fuels. Catalysts 2019, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Gomes, D.J.C.; de Souza, N.C.; Silva, J.R. Using a monocular optical microscope to assemble a wetting contact angle analyser. Measurement 2013, 46, e3623–e3627. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Oner, D.; McCarthy, T.J. Ultrahydrophobic Surfaces. Effects of topography length scales on wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- De Carvalho Faria, M.A.; da Silva Sousa, M.; dos Santos, K.F.; de Souza, N.C.; Silva, J.R. Preparation and characterization of epicuticular wax films. Heliyon 2019, 5, e01319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, C.D.; Falcão, H.M.; Almeida–Cortez, J.; Santos, D.Y.A.C.; Oliveira, A.F.M.; Santos, M.G. Leaf epicuticular wax content changes under different rainfall regimes, and its removal affects the leaf chlorophyll content and gas exchanges of Aspidosperma pyrifolium in a seasonally dry tropical forest. S. Afr. J. Bot. 2017, 111, 267–274. [Google Scholar] [CrossRef]
- Ni, Y.; Guo, Y.J.; Han, L.; Tang, H.; Conyers, M. Leaf cuticular waxes and physiological parameters in alfalfa leaves as influenced by drought. Photosynthetica 2012, 50, 458–466. [Google Scholar] [CrossRef]
- Charuvi, D.; Nevo, R.; Shimoni, E.; Naveh, L.; Zia, A.; Farrant, J.M.; Kirchhoff, H.; Reich, Z. Photoprotection conferred by changes in photosynthetic protein levels and organization during dehydration of a homoiochlorophyllous resurrection plant. Plant Physiol. 2015, 167, 1554–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, F.; Zhang, P.; Ke, C.; Zhu, Y.; Cao, W.; Jiang, H. Skewed distribution of leaf color RGB model and application of Skewed parameters in leaf color description model. Plant Methods 2020, 16, 23. [Google Scholar] [CrossRef]
- Mohammadian, M.A.; Watling, J.R.; Hill, R.S. The impact of epicuticular wax on gas–exchange and photoinhibition in Leucadendron lanigerum (Proteaceae). Acta Oecologica 2007, 31, 93–101. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.-J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self–assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Devi, Y.I.; Sahoo, M.R.; Mandal, J.; Dasgupta, M.; Prakash, N. Correlations between antioxidative enzyme activities and resistance to Phytophthora leaf blight in taro. J. Crop Improv. 2020, 35, 250–263. [Google Scholar] [CrossRef]
- Tresina, P.S.; Doss, A.; Mohan, V.R. Nutritional and antinutritional assessment of some underutilized corms, rhizomes and tubers. Trop. Subtrop. Agroecosystems 2020, 23, 1–11. [Google Scholar]
- Cajuste, J.F.; González–Candelas, L.; Veyrat, A.; García–Breijo, F.J.; Reig–Armiñana, J.; Lafuente, M.T. Epicuticular wax content and morphology as related to ethylene and storage performance of “Navelate” orange fruit. Postharvest Biol. Technol. 2010, 55, 29–35. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieniazek, F.; Dasgupta, M.; Messina, V.; Devi, M.P.; Devi, Y.I.; Mohanty, S.; Singh, S.; Sahoo, B.B.; Nongdam, P.; Acharya, G.C.; et al. Differential Occurrence of Cuticular Wax and Its Role in Leaf Physiological Mechanisms of Three Edible Aroids of Northeast India. Agriculture 2022, 12, 724. https://doi.org/10.3390/agriculture12050724
Pieniazek F, Dasgupta M, Messina V, Devi MP, Devi YI, Mohanty S, Singh S, Sahoo BB, Nongdam P, Acharya GC, et al. Differential Occurrence of Cuticular Wax and Its Role in Leaf Physiological Mechanisms of Three Edible Aroids of Northeast India. Agriculture. 2022; 12(5):724. https://doi.org/10.3390/agriculture12050724
Chicago/Turabian StylePieniazek, Facundo, Madhumita Dasgupta, Valeria Messina, Mayengbam Premi Devi, Yumnam Indrani Devi, Sansuta Mohanty, Satyapriya Singh, Bibhuti Bhusan Sahoo, Potshangbam Nongdam, Gobinda Chandra Acharya, and et al. 2022. "Differential Occurrence of Cuticular Wax and Its Role in Leaf Physiological Mechanisms of Three Edible Aroids of Northeast India" Agriculture 12, no. 5: 724. https://doi.org/10.3390/agriculture12050724
APA StylePieniazek, F., Dasgupta, M., Messina, V., Devi, M. P., Devi, Y. I., Mohanty, S., Singh, S., Sahoo, B. B., Nongdam, P., Acharya, G. C., & Sahoo, M. R. (2022). Differential Occurrence of Cuticular Wax and Its Role in Leaf Physiological Mechanisms of Three Edible Aroids of Northeast India. Agriculture, 12(5), 724. https://doi.org/10.3390/agriculture12050724






