The Exogenous Application of Brassinosteroids Confers Tolerance to Heat Stress by Increasing Antioxidant Capacity in Soybeans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
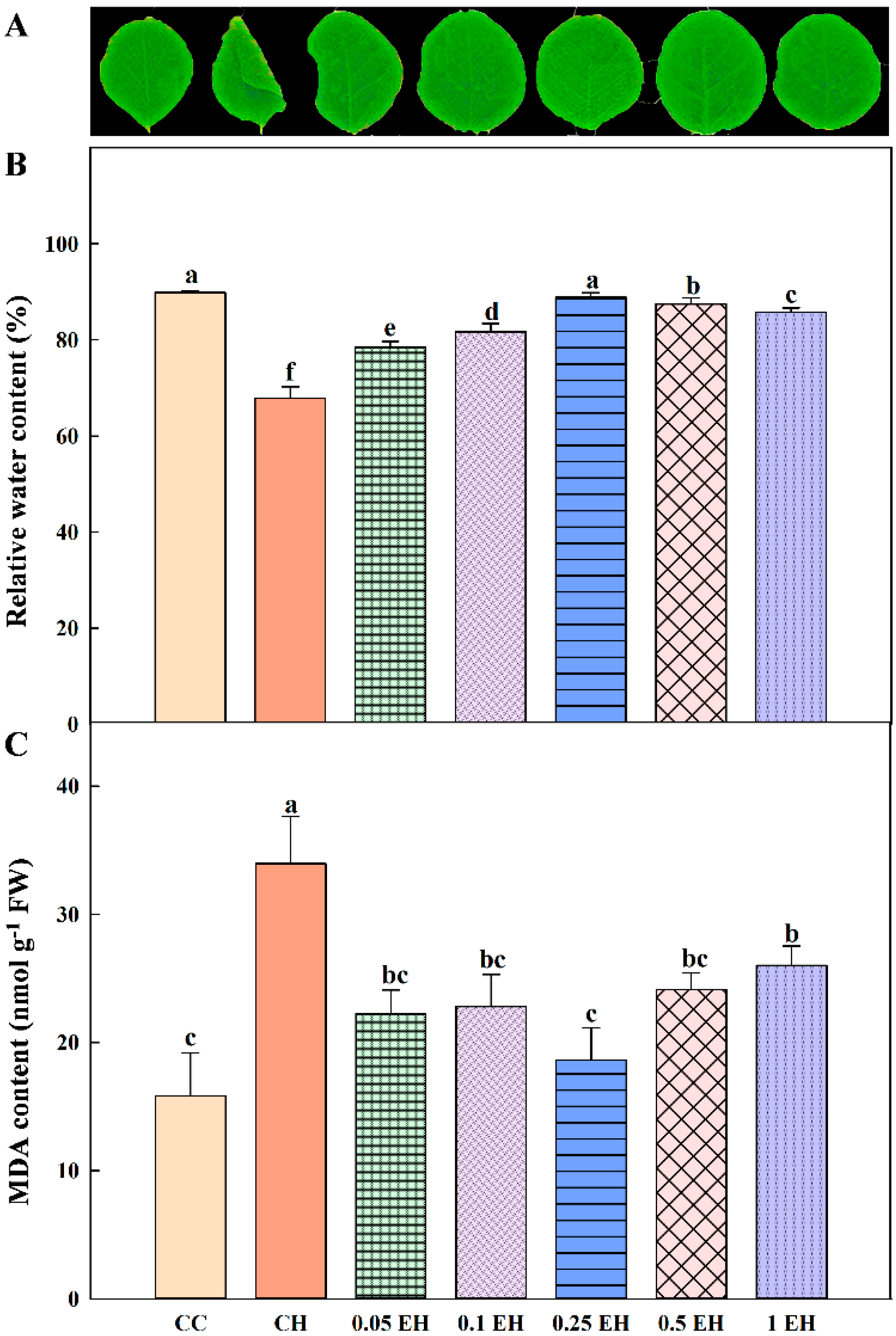
2.2. Leaf Relative Water Content
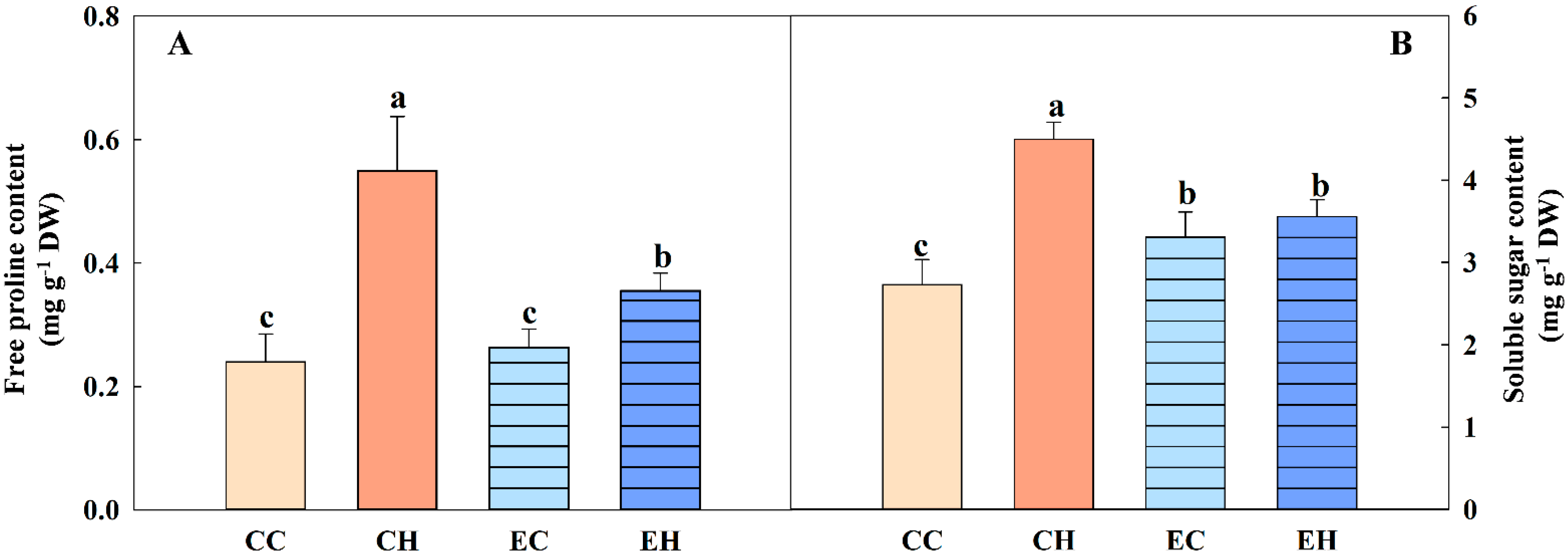
2.3. Osmolyte Content
2.4. Antioxidant System
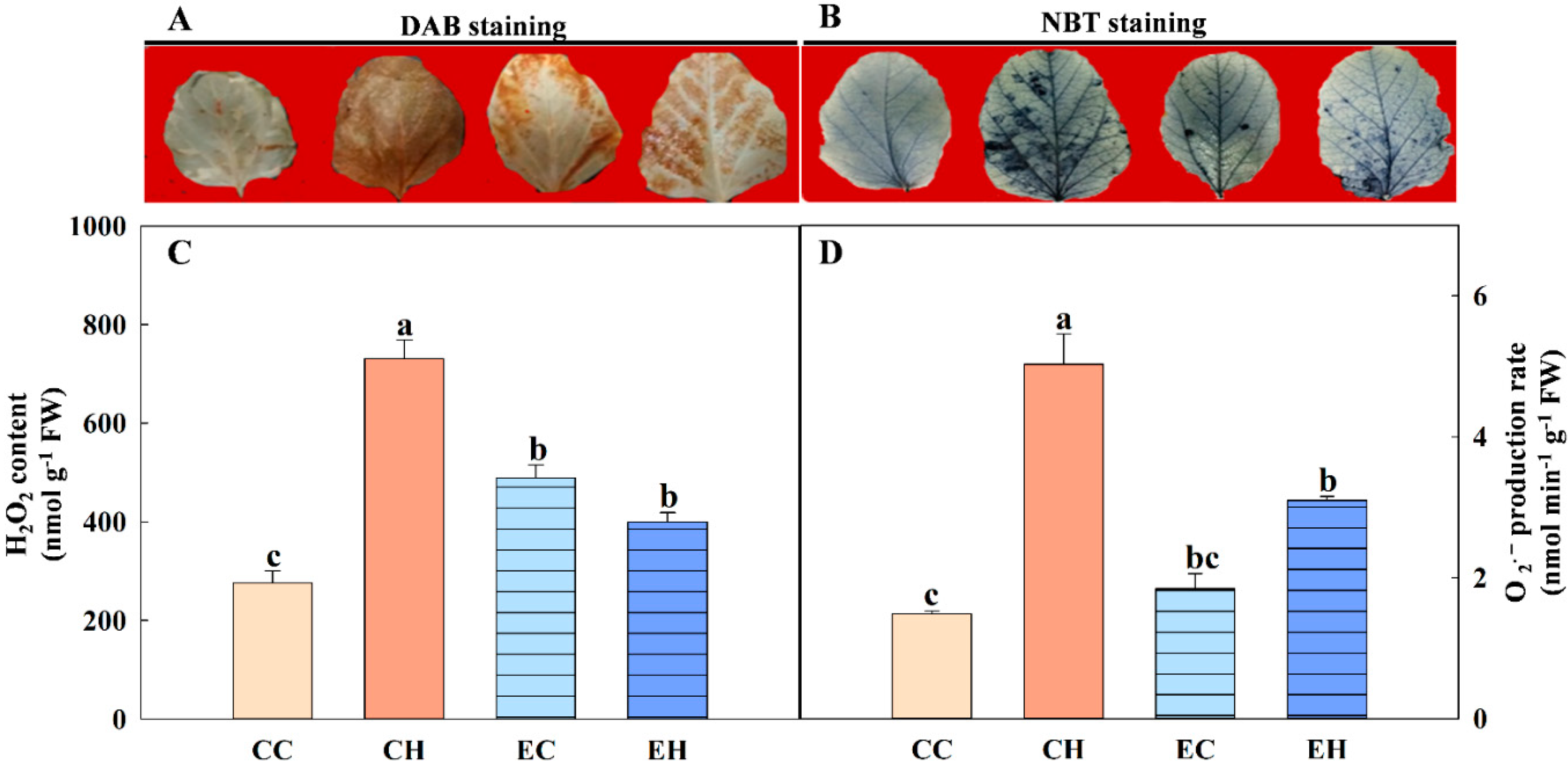
2.5. ROS Histochemical Detection and Quantification
2.6. Chlorophyll Fluorescence Analysis
2.7. Leaf Net Photosynthetic Rate
2.8. Plant Dry Mass
2.9. Statistical Analysis
3. Results
3.1. Effects of EBR Treatment on Soybean Heat Tolerance
3.2. Effects of EBR Treatment on ROS Level in Soybean Leaves
3.3. Effects of EBR Treatment on Antioxidant Capacity in Soybean Leaves
3.4. Effects of EBR Treatment on Osmolytes in Soybean Leaves
3.5. Effects of EBR Treatment on Chlorophyll Fluorescence in Soybean Leaves
3.6. Effects of EBR Treatment on the Photosynthesis and Biomass Accumulation of Soybean
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Liu, L.; Ma, Y.; Li, S.; Dong, S.; Zu, W. Transcriptome profilling analysis characterized the gene expression patterns responded to combined drought and heat stresses in soybean. Comput. Biol. Chem. 2018, 77, 413–429. [Google Scholar] [CrossRef]
- Lia, J.; Nadeema, M.; Chen, L.; Wang, M.; Wan, M.; Qiu, L.; Wang, X. Differential proteomic analysis of soybean anthers by iTRAQ under high-temperature stress. J. Proteom. 2020, 229, 103968. [Google Scholar] [CrossRef]
- Siebers, M.H.; Yendrek, C.R.; Drag, D.; Locke, A.M.; Acosta, L.R.; Leakey, A.; Ainsworth, E.A.; Bernacchi, C.J.; Ort, D.R. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Glob. Change Biol. 2015, 21, 3114–3125. [Google Scholar] [CrossRef]
- David, B.L.; Gregory, P.A. Climate and management contributions to recent trends in U.S. agricultural yelds. Science 2003, 299, 1032. [Google Scholar]
- Godoy, F.; Olivos, H.K.; Stange, C.; Handford, M. Abiotic stress in crop species: Improving tolerance by applying plant metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Arif, M.S.; Ahmad, R.; Hasanuzzaman, M.; Ali, B.; Hussain, A. Approaches in enhancing thermotolerance in plants: An updated review. J. Plant Growth Regul. 2020, 39, 456–480. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804–103813. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2013, 74, 185–192. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; He, N.; Guo, F. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Alleviation of field low-temperature stress in winter wheat by exogenous application of salicylic acid. J. Plant Growth Regul. 2021, 40, 811–823. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.; Zhou, Y.; Shi, K.; Chen, Z.; Yu, J. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhu, T.; Zou, L.; Han, X.; Zhou, X.; Xi, D.; Zhang, D.; Lin, H. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016, 85, 478–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogweno, J.O.; Song, X.; Shi, K.; Hu, W.; Mao, W.; Zhou, Y.; Yu, J.; Nogueś, S. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J. Plant Growth Regul. 2007, 27, 49–57. [Google Scholar] [CrossRef]
- Thussagunpanit, U.; Jutamanee, K.; Kaveeta, L.; Chai-arree, W.; Pankean, P.; Homvisasevongsa, S.; Suksamrarn, A. Comparative effects of brassinosteroid and brassinosteroid mimic on improving photosynthesis, lipid peroxidation, and rice seed set under heat stress. J. Plant Growth Regul. 2015, 34, 320–331. [Google Scholar] [CrossRef]
- Yue, J.; You, Y.; Zhang, L.; Fu, Z.; Guy, R.D. Exogenous 24-epibrassinolide alleviates effects of salt stress on chloroplasts and photosynthesis in Robinia pseudoacacia L. seedlings. J. Plant Growth Regul. 2019, 38, 669–682. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Sharma, V.; Goel, P.; Kumar, S.; Singh, A.K. An apple transcription factor, MdDREB76, confers salt and drought tolerance in transgenic tobacco by activating the expression of stress-responsive genes. Plant Cell Rep. 2019, 38, 221–241. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Min, Q.; Chen, M.; Li, D.; Chen, X.; Li, L.; Gao, D. 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persicae L.) leaves. Plant Physiol. Biochem. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2019, 147, 21–42. [Google Scholar] [CrossRef]
- Dong, C.; Li, L.; Shang, Q.; Liu, X.; Zhang, Z. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tian, S.; Zheng, X.; Zhou, Z.; Xu, Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol. Plant. 2007, 130, 112–121. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zhou, Y.; Tao, Y.; Mao, W.; Shi, K.; Asami, T.; Chen, Z.; Yu, J. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front. Plant Sci. 2018, 9, 1137. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, X.; Wang, Y.; Huo, Z.; Huang, M.; Cai, J.; Zhou, Q.; Jiang, D. Involvement of salicylic acid in cold priming-induced freezing tolerance in wheat plants. Plant Growth Regul. 2020, 93, 117–130. [Google Scholar] [CrossRef]
- Si, T.; Wang, X.; Wu, L.; Zhao, C.; Zhang, L.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Zhu, J. Nitric oxide and hydrogen peroxide mediate wounding-induced freezing tolerance through modifications in photosystem and antioxidant system in wheat. Front. Plant Sci. 2017, 8, 1284. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Dong, N.; Niu, M.; Zhang, X.; Li, L.; Liu, J.; Liu, B.; Tong, H. Brassinosteroid-regulated plant growth and development and gene expression in soybean. Crop J. 2019, 7, 411–418. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Salicylic acid and cold priming induce late-spring freezing tolerance by maintaining cellular redox homeostasis and protecting photosynthetic apparatus in wheat. Plant Growth Regul. 2021, 90, 109–121. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinases-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef]
- Jin, S.; Li, X.; Wang, G.G.; Zhu, X. Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 2015, 7, plv009. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Grossjohann, A.; Schneeberger, K.; Bürstenbinder, K.; Quint, M.; Klecker, M.; Martinez, C.; Lippmann, R.; Janitza, P.; James, G.V. Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 2018, 28, 303–310.e3. [Google Scholar]
- Su, Q.; Zheng, X.; Tian, Y.; Wang, C. Exogenous brassinolide alleviates salt stress in Malus hupehensis Rehd. by regulating the transcription of NHX-Type Na+(K+)/H+ antiporters. Front. Plant Sci. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Wrzaczek, M.; Brosche, M.; Kangasja, J. ROS signaling loops-production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.S.; Barbara, D.S.; Claire, R.; Xun, C.K.; Frank, V.B. Reactive oxygen species and organellar signaling. J. Exp. Bot. 2021, 72, 5807–5824. [Google Scholar]
- Sánchez-Moreiras, A.; Graa, E.; Reigosa, M.J.; Araniti, F. Imaging of chlorophyll a fluorescence in natural compound-induced stress detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef]
- Imran, M.; Khan, M.A.; Shahzad, R.; Bilal, S.; Lee, I.J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Thomey, M.L.; Slattery, R.A.; Köhler, I.H.; Bernacchi, C.J.; Ort, D.R. Yield response of field-grown soybean exposed to heat waves under current and elevated [CO2]. Glob. Change Biol. 2019, 25, 4352–4368. [Google Scholar] [CrossRef]
- Wang, C.; Gu, Q.; Zhao, L.; Li, C.; Ren, J.; Zhang, J. Photochemical efficiency of photosystem II in inverted leaves of soybean [Glycine max (L.) Merr.] affected by elevated temperature and high light. Front. Plant Sci. 2022, 12, 772644. [Google Scholar] [CrossRef]
- Ribeiro, D.G.D.S.; Silva, B.R.S.D.; Lobato, A.K.D.S. Brassinosteroids induce tolerance to water deficit in soybean seedlings: Contributions linked to root anatomy and antioxidant enzymes. Acta Physiol. Plant. 2019, 41, 82. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Y.; Ding, J. Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ. 2010, 34, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Gao, C.; Song, L.; Zhou, Y.; Shi, K.; Yu, J. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Qi, C.; Ren, H.; Huang, A.; Hei, S.; She, X. Ethylene mediates brassinosteroid-induced stomatal closure via Gα protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J. 2015, 82, 280–301. [Google Scholar] [CrossRef] [PubMed]


| Items | CC | CH | EC | EH |
|---|---|---|---|---|
| SOD (U mg−1 protein) | 98.59 ± 4.010 d | 128.1 ± 7.021 c | 144.9 ± 9.387 a | 175.3 ± 4.569 b |
| CAT (U mg−1 protein) | 1.566 ± 0.045 d | 1.951 ± 0.186 c | 3.257 ± 0.301 a | 2.650 ± 0.029 b |
| POD (U mg−1 protein) | 22.78 ± 1.729 d | 50.65 ± 2.582 b | 46.86 ± 3.298 c | 56.86 ± 3.272 a |
| ASA (μmol g−1 FW) | 1.057 ± 0.035 b | 0.674 ± 0.071 c | 1.657 ± 0.035 a | 1.092 ± 0.032 b |
| GSH (μmol g−1 FW) | 0.274 ± 0.005 b | 0.157 ± 0.007 d | 0.326 ± 0.012 a | 0.226 ± 0.012 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Xie, Y.; Liu, C.; Jiang, H. The Exogenous Application of Brassinosteroids Confers Tolerance to Heat Stress by Increasing Antioxidant Capacity in Soybeans. Agriculture 2022, 12, 1095. https://doi.org/10.3390/agriculture12081095
Wang W, Xie Y, Liu C, Jiang H. The Exogenous Application of Brassinosteroids Confers Tolerance to Heat Stress by Increasing Antioxidant Capacity in Soybeans. Agriculture. 2022; 12(8):1095. https://doi.org/10.3390/agriculture12081095
Chicago/Turabian StyleWang, Weiling, Yuncan Xie, Chang Liu, and Haidong Jiang. 2022. "The Exogenous Application of Brassinosteroids Confers Tolerance to Heat Stress by Increasing Antioxidant Capacity in Soybeans" Agriculture 12, no. 8: 1095. https://doi.org/10.3390/agriculture12081095





