Effects of Epichloë Endophyte and Transgenerational Effects on Physiology of Achnatherum inebrians under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Experimental Design
2.3. Measurement Protocols
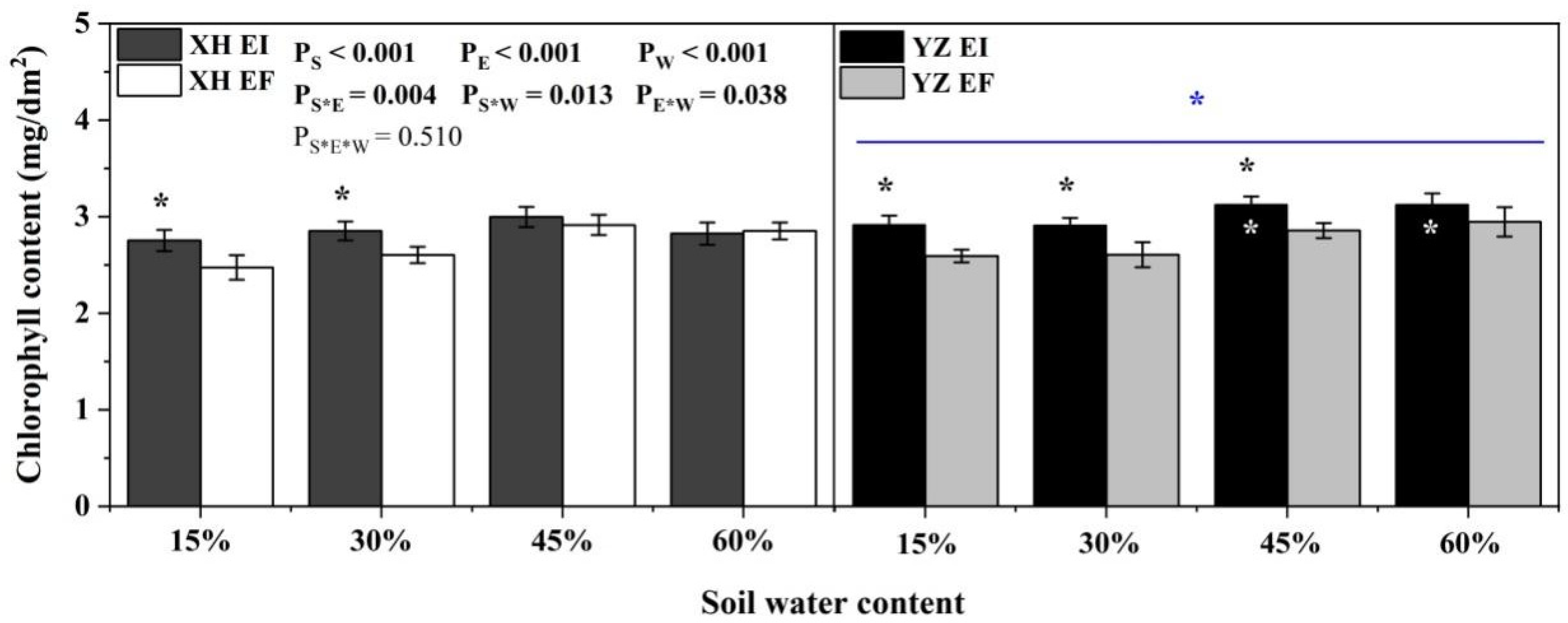
2.3.1. Chlorophyll Content
2.3.2. Photosynthetic Indexes
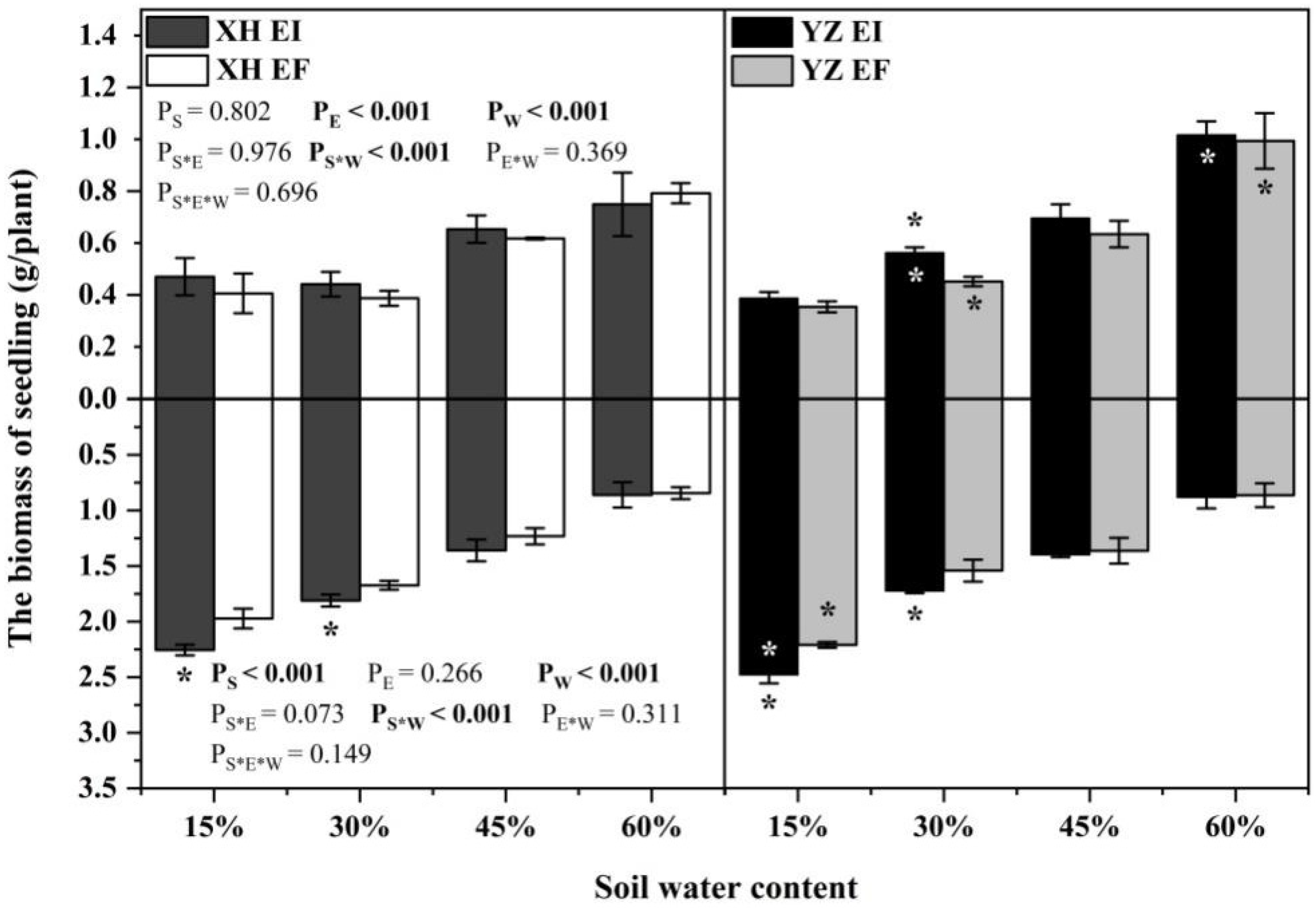
2.3.3. Biomass
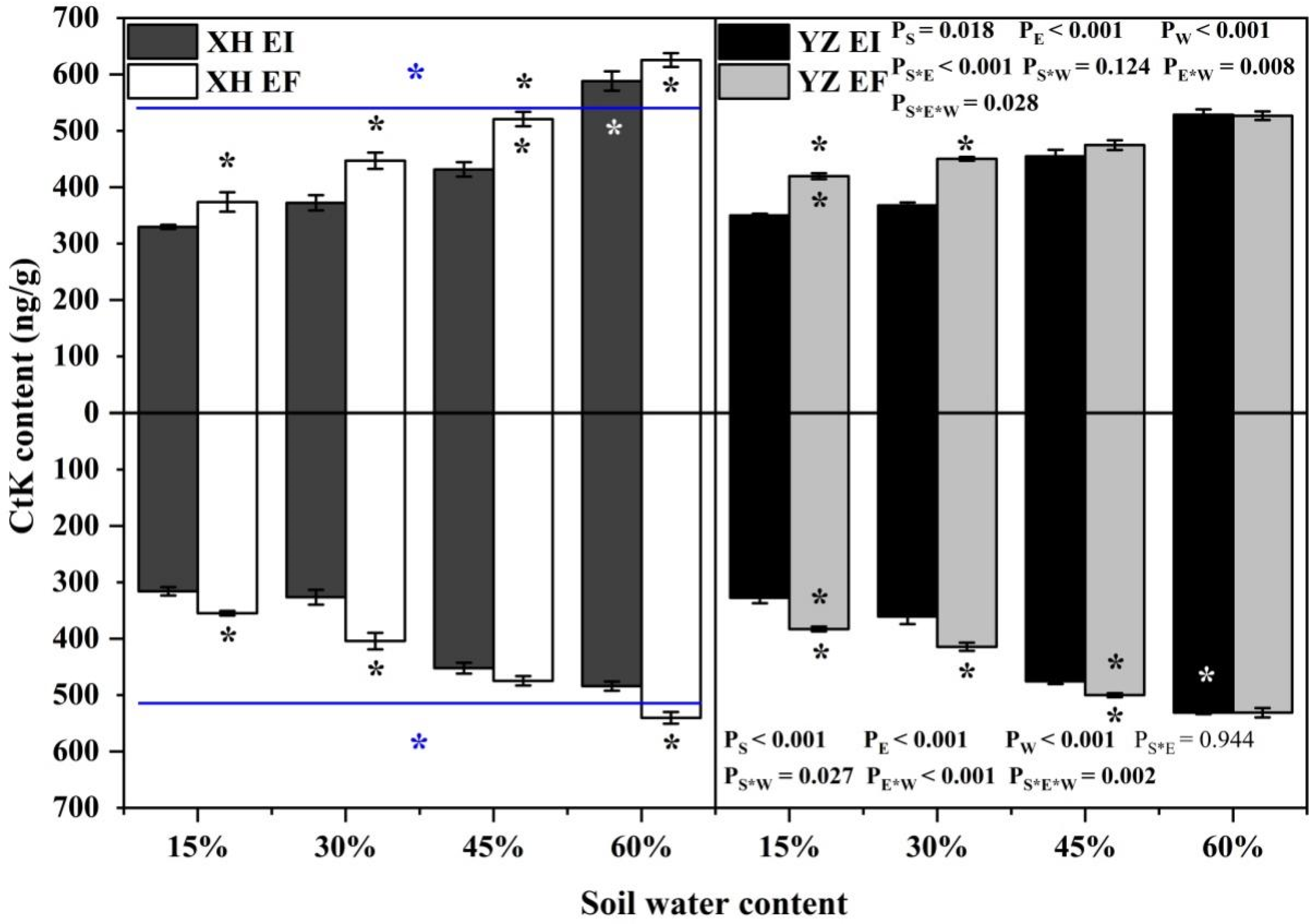
2.3.4. Abscisic Acid (ABA), Cytokinin (CtK), and IndolE−3-acetic Acid (IAA)
Content Determination
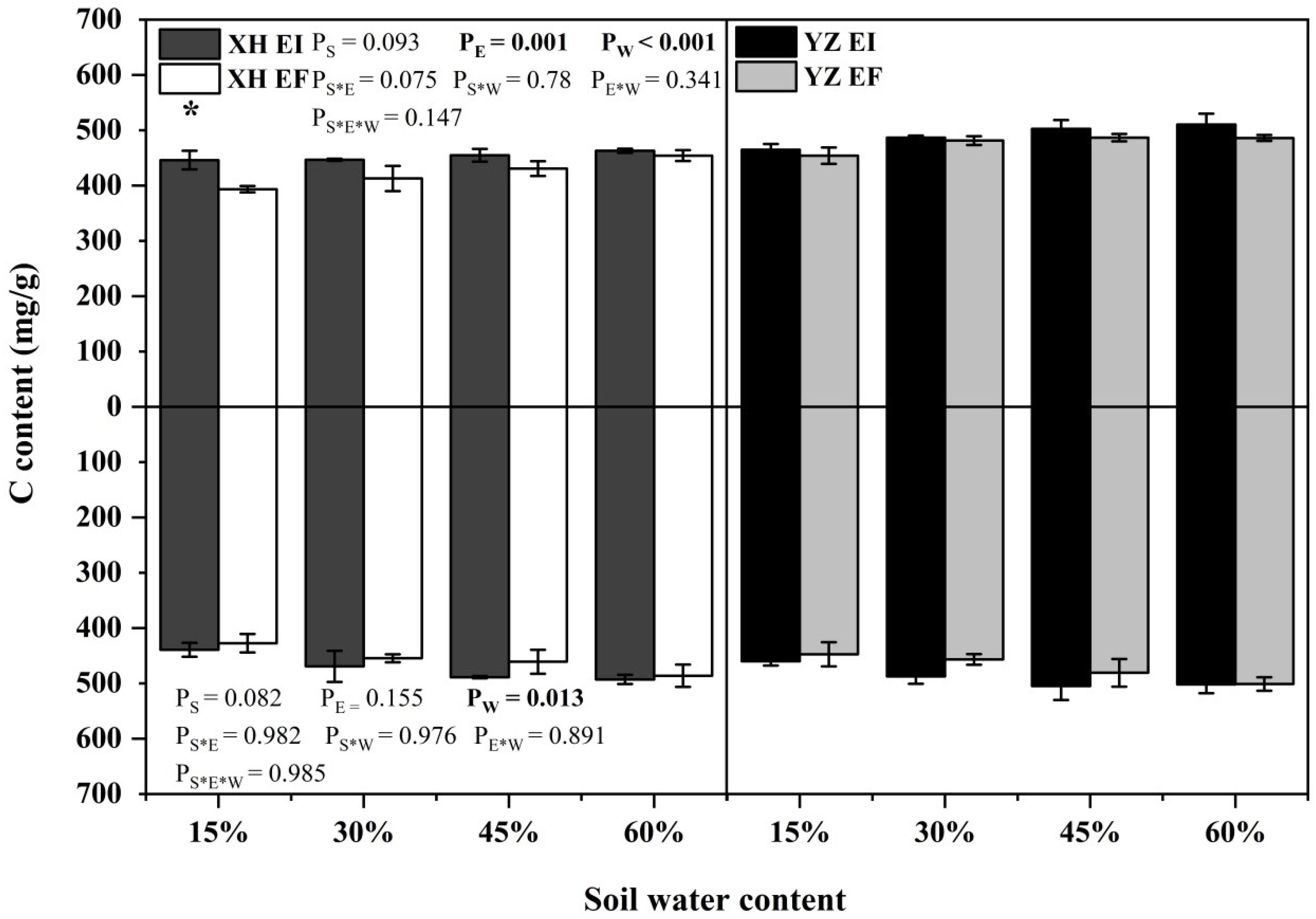
2.3.5. Elemental Analysis
2.4. Statistical Analysis
3. Results
3.1. Biomass
3.2. Phytohormones
3.3. Photosynthetic Indexes
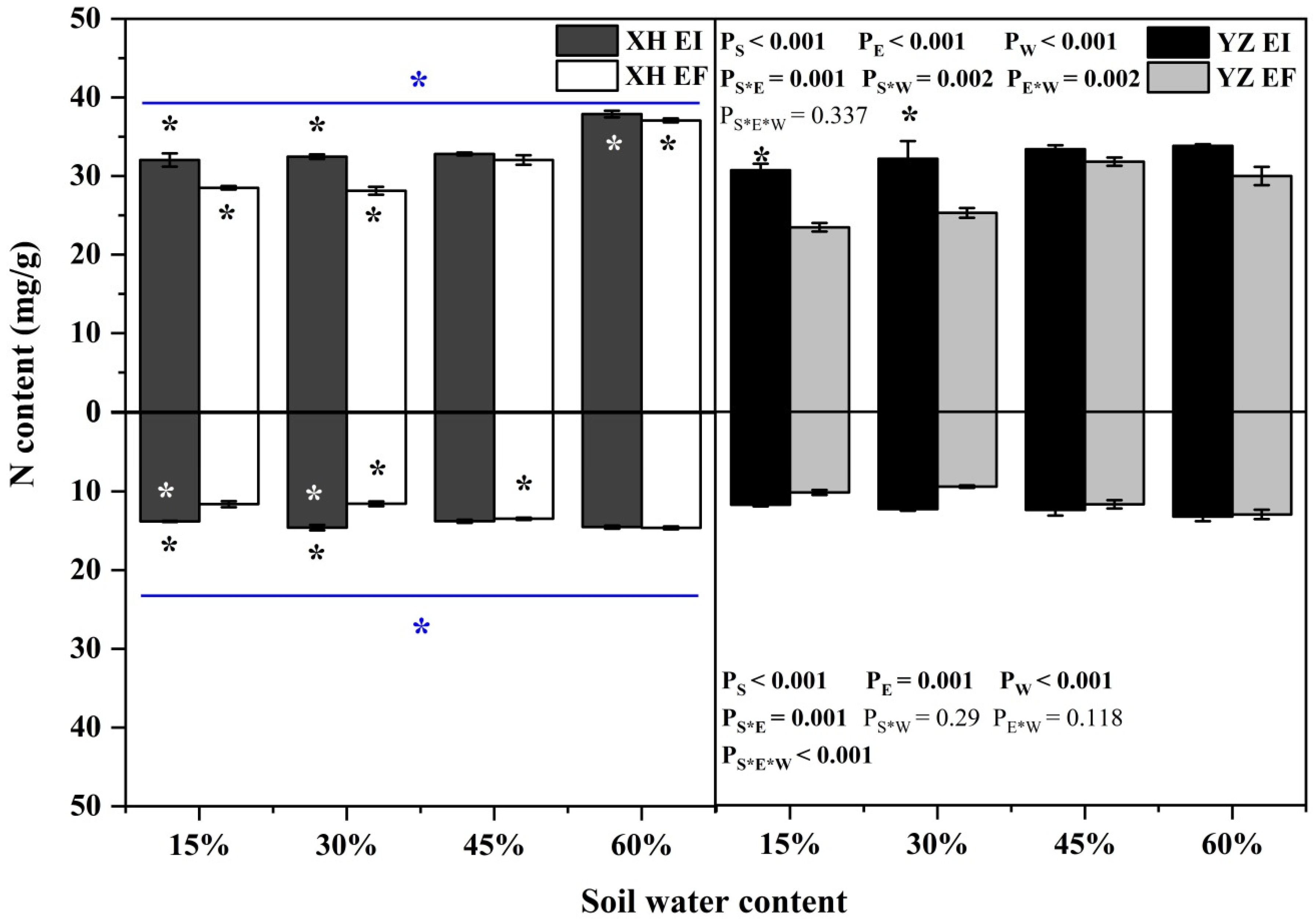
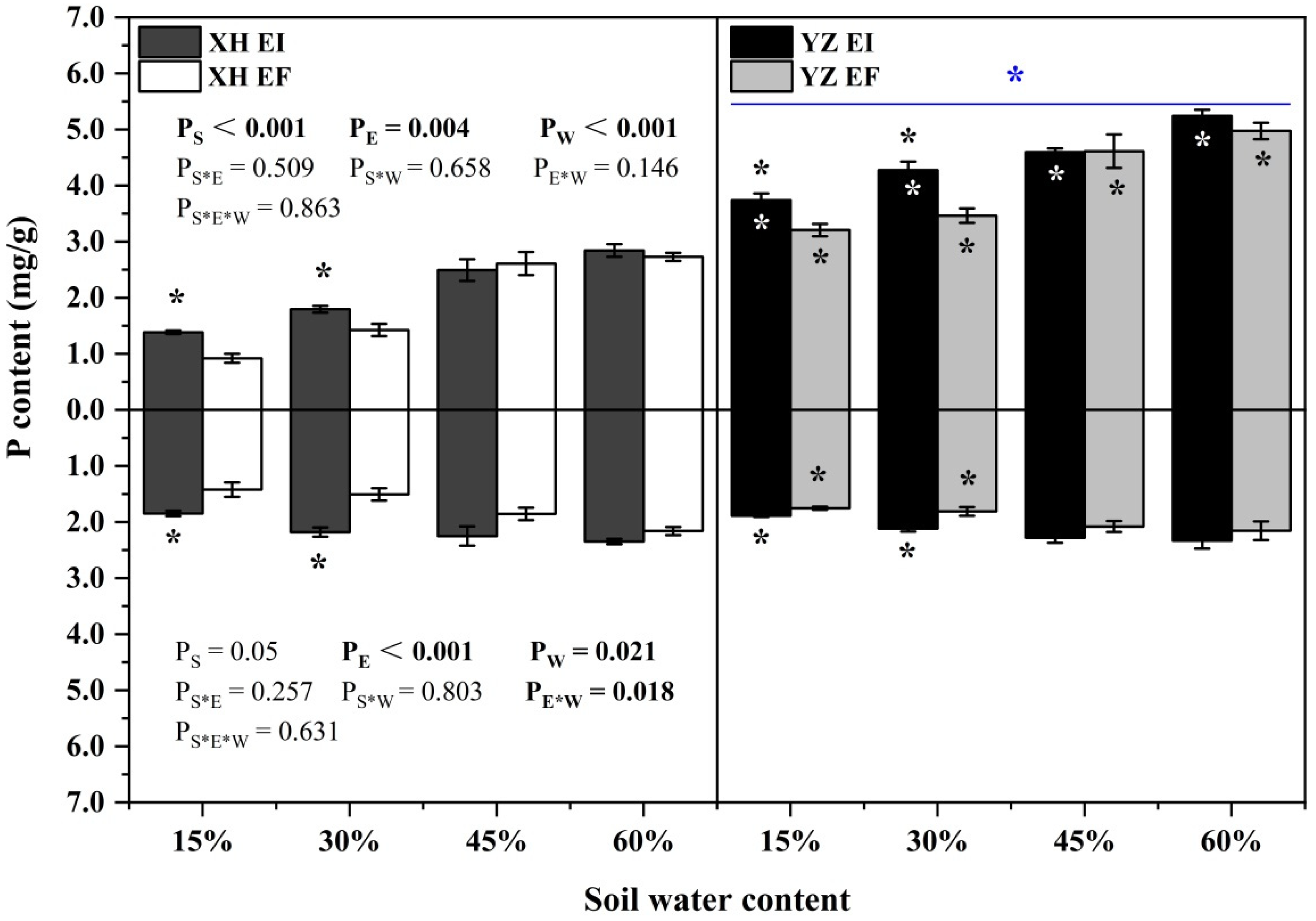
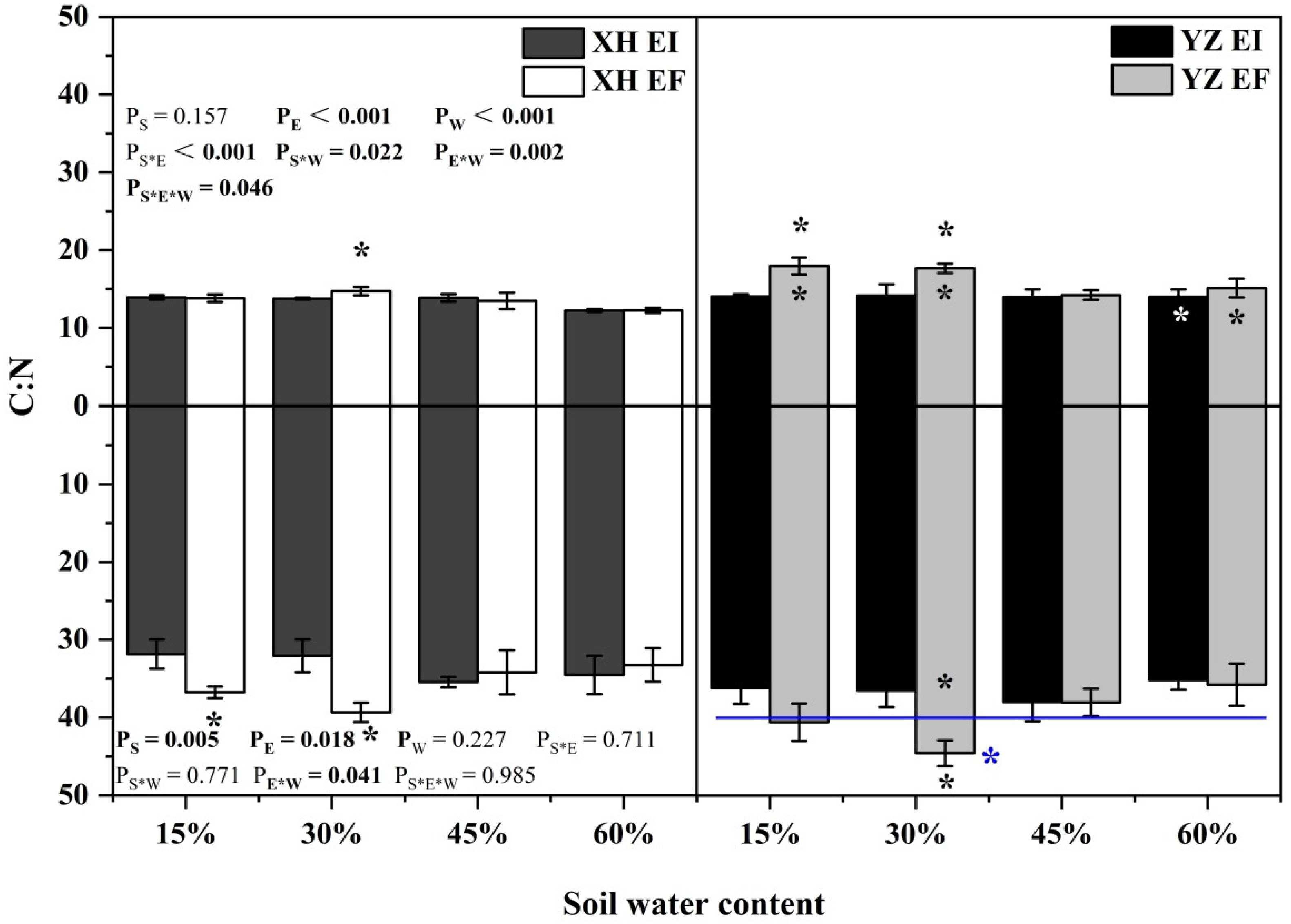
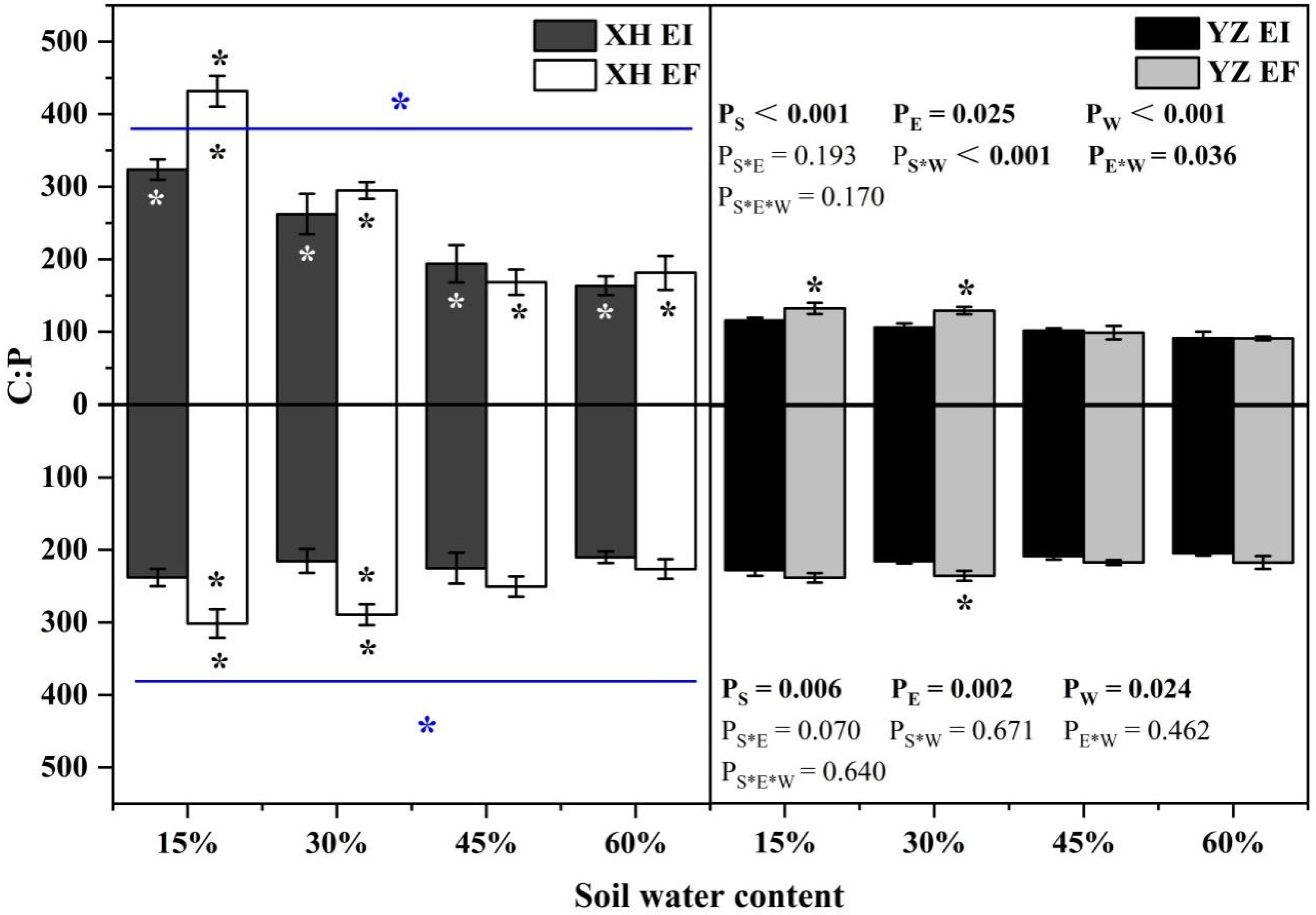
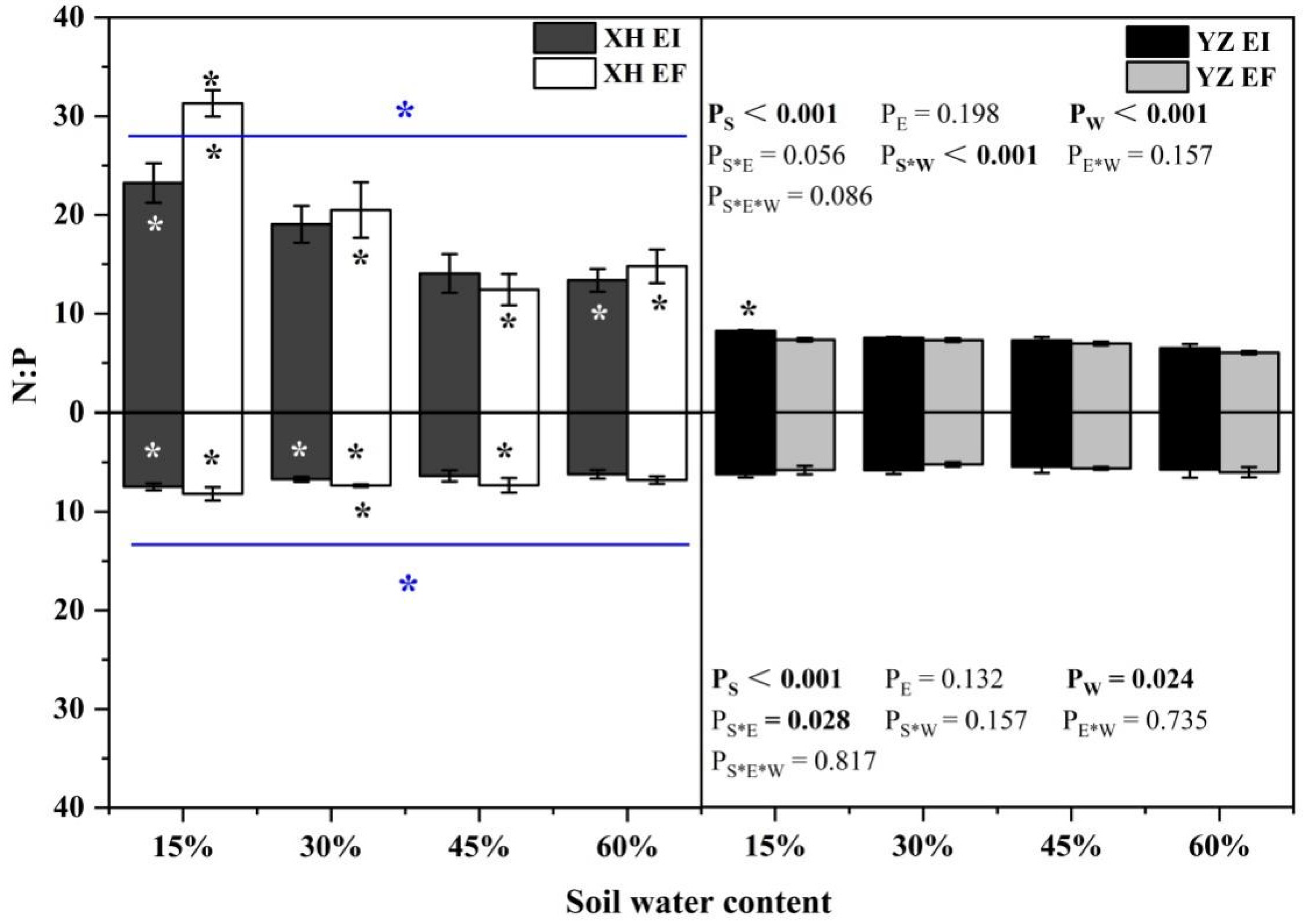
3.4. Elements and Stoichiometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Lowman, S.; Kim-Dura, S.; Mei, C.; Nowak, J. Strategies for enhancement of switchgrass (Panicum virgatum L.) performance under limited nitrogen supply based on utilization of N-fixing bacterial endophytes. Plant Soil 2016, 405, 47–63. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Epichloë (formerly Neotyphodium) fungal endophytes increase adaptation of cool-season perennial grasses to environmental stresses. Acta Agrobot. 2019, 72, 1767. [Google Scholar] [CrossRef]
- Hallasgo, A.M.; Spangl, B.; Steinkellner, S.; Hage-Ahmed, K. The fungal endophyte Serendipita williamsii does not affect phosphorus status but carbon and nitrogen dynamics in arbuscular mycorrhizal tomato plants. J. Fungi 2020, 6, 233. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium speices with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Simpson, W.R.; Koolaard, J.P.; Nickless, E.M.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Müller, C.B.; Krauss, J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005, 8, 450–456. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, L.M.; Popay, A.J.; Glare, T.R.; Finch, S.C.; Cave, V.M.; Rostás, M. Olfactory responses of Argentine stem weevil to herbivory and endophyte-colonisation in perennial ryegrass. J. Pest Sci. 2022, 95, 263–277. [Google Scholar] [CrossRef]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Li, X.Z.; Nan, Z.B. Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J. Agric. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.C.; Gundel, P.E.; Molina-Montenegro, M.A.; Ramos, P.; Ghersa, C.M. Getting ready for the ozone battle: Vertically transmitted fungal endophytes have transgenerational positive effects in plants. Plant Cell Environ. 2021, 44, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Saedi, T.; Mosaddeghi, M.R.; Sabzalian, M.R.; Zarebanadkouki, M. Effect of Epichloë fungal endophyte symbiosis on tall fescue to cope with flooding-derived oxygen-limited conditions depends on the host genotype. Plant Soil 2021, 468, 353–373. [Google Scholar] [CrossRef]
- Johnson, L.J.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; Tapper, B.A. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Becker, M.; Becker, Y.; Green, K.; Scott, B. The endophytic symbiont Epichloë festucae establishes an epiphyllous net on the surface of Lolium perenne leaves by development of an expressorium, an appressoriumdophytic syexit structure. New Phytol. 2016, 211, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Soto-Barajas, M.C.; Zabalgogeazcoa, I.; Gómez-Fuertes, J.; GonzálezBlanco, V.; Vázquez-De-Aldana, B.R. Epichloë endophytes affect the nutrient and fiber content of Lolium perenne, regardless of plant genotype. Plant Soil. 2016, 405, 65–277. [Google Scholar] [CrossRef]
- Nan, Z.B.; Li, C.J. Neotyphodium in native grasses in China and observations on endophyte/host interactions. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000. [Google Scholar]
- Li, C.J.; Nan, Z.B.; Liu, Y. Methodology of endophyte detection of drunken horse grass. Edible Fungi China 2008, 27, 16–19. [Google Scholar]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.X.; Nan, Z.B.; Li, C.J.; Gao, K. Cytotoxic effect of ergot alkaloids in Achnatherum inebrians infected by the Neotyphodium gansuense endophyte. J. Agric. Food Chem. 2014, 62, 7419–7422. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.C.; Li, C.J.; Nan, Z.B.; Li, F.D. Effects of feeding drunken horse grass infected with Epichloë gansuensis endophyte on animal performance, clinical symptoms and physiological parameters in sheep. BMC Vet. Res. 2017, 13, 223. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Zhang, X.X.; Christensen, M.J.; Nan, Z.B.; Li, C.J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Kou, M.Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.B.; Zhang, X.X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Li, X.Z.; White, J.F.; Wei, X.K.; Li, C.J. Epichloë endophyte improves ergot disease resistance of host (Achnatherum inebrians) by regulating leaf senescence and photosynthetic capacity. J. Plant Growth Regul. 2021, 41, 808–817. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B.; Matthew, C. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res. 2012, 52, 70–78. [Google Scholar] [CrossRef]
- He, Y.L.; Chen, T.X.; Zhang, H.J.; White, J.F.; Li, C.J. Fungal endophytes help grasses to tolerate sap-sucking herbivores through a hormone-signaling system. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, R.L.; Chai, Q.; Li, C.J.; Nan, Z.B. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.F.; Hou, W.P.; Malik, K.; Zhao, C.Z.; Niu, X.L.; Liu, Y.L.; Huang, R.; Li, C.J.; Nan, Z.B. Elucidating the molecular mechanisms by which seed-borne endophytic fungi, Epichloë gansuensis, increases the tolerance of Achnatherum inebrians to NaCl stress. Int. J. Mol. Sci. 2021, 22, 13191. [Google Scholar] [CrossRef]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, X.X.; Nan, Z.B. The fungal endophyte Epichloë gansuensis increases NaCl-tolerance in Achnatherum inebrians through enhancing the activity of plasma membrane H+-ATPase and glucose-6-phosphate dehydrogenase. Sci. China Life Sci. 2021, 64, 452–465. [Google Scholar] [CrossRef]
- Xia, C.; Christensen, M.J.; Zhang, X.X.; Nan, Z.B. Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Lee, K.; Missaoui, A.; Mahmud, K.; Presley, H.; Lonnee, M. Interaction between grasses and Epichloë endophytes and its significance to biotic and abiotic stress tolerance and the rhizosphere. Microorganisms 2021, 9, 2186. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Yu, J.J.; Han, L.B.; Huang, B.R. Photosynthetic enzyme activities and gene expression associated with drought tolerance and post-drought recovery in Kentucky bluegrass. Environ. Exp. Bot. 2013, 89, 28–35. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Tsuda, K.; Affiliations, A.I. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef] [Green Version]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Xu, W.B.; Li, M.M.; Lin, W.H.; Nan, Z.B.; Tian, P. Effects of Epichloë sinensis endophyte and host ecotype on physiology of festuca sinensis under different soil moisture conditions. Plants 2021, 10, 1649. [Google Scholar] [CrossRef]
- Zhao, Z.R.; Kou, M.Z.; Zhong, R.; Xia, C.; Christensen, M.J.; Zhang, X.X. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by Epichloë gansuensis in Achnatherum inebrians under different soil moisture availability. J. Fungi 2021, 7, 640. [Google Scholar] [CrossRef]
- Song, M.L.; Li, X.Z.; Saikkonen, K.; Li, C.J.; Nan, Z.B. An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.; Li, K.; Liu, Y.; Gong, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Gundel, P.E.; Rudgers, J.A.; Whitney, K.D. Vertically transmitted symbionts as mechanisms of transgenerational effects. Am. J. Bot. 2017, 104, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Zhou, M.; Lin, Z.; Li, Q.Q.; Zhang, Y.Y. Transgenerational effects benefit offspring across diverse environments: A meta-analysis in plants and animals. Ecol. Lett. 2019, 22, 1976–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, J.J.; Sultan, S.E. Adaptive transgenerational plasticity in plants: Case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef] [Green Version]
- Gundel, P.E.; Rudgers, J.A.; Ghersa, C.M. Incorporating the process of vertical transmission into understanding of host-symbiont dynamics. Oikos 2011, 120, 1121–1128. [Google Scholar] [CrossRef]
- Gundel, P.E.; Sorzoli, N.; Ueno, A.C.; Ghersa, C.M.; Seal, C.E.; Bastías, D.A.; Martínez-Ghersa, M.A. Impact of ozone on the viability and antioxidant content of grass seeds is affected by a vertically transmitted symbiotic fungus. Environ. Exp. Bot. 2015, 113, 40–46. [Google Scholar] [CrossRef]
- Ye, W.; Hu, S.; Wu, L.; Ge, C.; Cui, Y.; Chen, P.; Xu, J.; Dong, G.; Guo, L.; Qian, Q. Fine mapping a major QTL qFCC7L for chlorophyll content in rice (Oryza sativa L.) cv. PA64s. Plant Growth Regul. 2017, 81, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Tanveer, S.K.; Zhang, J.L.; Lu, X.L.; Wen, X.X.; Wei, W.; Yang, L.; Liao, Y.C. Effect of corn residue mulch and N fertilizer application on nitrous oxide (N2O) emission and wheat crop productivity under rain-fed condition of loess plateau China. Int. J. Agric. Biol. 2014, 16, 505–512. [Google Scholar]
- Gundel, P.E.; Irisarri, J.G.N.; Fazio, L.; Casas, C.; Pérez, L.I. Inferring field performance from drought experiments can be misleading: The case of symbiosis between grasses and Epichloë fungal endophytes. J. Arid Environ. 2016, 132, 60–62. [Google Scholar] [CrossRef]
- Ren, A.Z.; Gao, Y.B.; Wang, W.; Wang, J.L. Photosynthetic pigments and photosynthetic products of endophyte-infected and endophyte-free Lolium perenne L. under drought stress conditions. Front. Biol. China 2006, 1, 168–173. [Google Scholar] [CrossRef]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an auxin response factor in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- West, C.P.; Izekor, E.; Turner, K.E.; Elmi, A.A. Endophyte effects on growth and persistence of tall fescue along a water-supply gradient. Agron. J. 1993, 85, 264–270. [Google Scholar] [CrossRef]
- Elmi, A.A.; West, C.P. Endophyte infection affects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytol. 1995, 131, 61–67. [Google Scholar] [CrossRef]
- Belesky, D.P.; Stringer, W.C.; Plattner, R.D. Influence of endophyte and water regime upon tall fescue accessions. Ann. Bot. 1989, 64, 343–349. [Google Scholar] [CrossRef]
- Belesky, D.P.; Fedders, J.M. Tall fescue development in response to Acremonium coenophialum and soil acidity. Crop Sci. 1995, 35, 529–533. [Google Scholar] [CrossRef]
- Assuero, S.G.; Matthew, C.; Kemp, P.D.; Latch, G.C.M.; Barker, D.J.; Haslett, S.J. Morphological and physiological effects of water deficit and endophyte infection on contrasting tall fescue cultivars. N. Z. J. Agron. Res. 2000, 43, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Faeth, S.H.; Sullivan, T.J. Mutualistic asexual endophytes in a native grass are usually parasitic. Am. Nat. 2003, 161, 310–325. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing rhizobium strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pustovoitova, T.N.; Zhdanova, N.E.; Zholkevich, V.N. Changes in the Levels of IAA and ABA in Cucumber Leaves under Progressive Soil Drought. Russ. J. Plant Physiol. 2004, 51, 513–517. [Google Scholar] [CrossRef]
- Zholkevich, V.N.; Gusev, N.A.; Kaplya, A.V.; Pakhomova, T.I.; Pil’shikova, N.V.; Samuiliv, F.D.; Slavnii, P.S.; Shmat’ko, I.G. Water Metabolism in Plants; Tarchevskii, I.A., Zholkevich, V.N., Eds.; Nauka: Moscow, Russia, 1989. [Google Scholar]
- De Battista, J.P.; Bouton, J.H.; Bacon, C.W.; Siegel, M.R. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Agron. J. 1990, 82, 651–654. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Acharya, B.R.; Assmann, S.M. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Elbersen, W.W.; West, C.P. Growth and water relations of field-grown tall fescue as influenced by drought and endophytes. Grass Forage Sci. 1996, 51, 333–342. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Leuchtmann, A.; Schmidt, D.; Nösberger, J. Symbiosis with Neotyphodium uncinatum endophyte may increase the competitive ability of meadow fescue. Agron. J. 1997, 89, 833–839. [Google Scholar] [CrossRef]
- Manzur, M.E.; Garello, F.A.; Omacini, M.; Schnyder, H.; Sutka, M.R.; García-Parisi, P.A. Endophytic fungi and drought tolerance: Ecophysiological adjustment in shoot and root of an annual mesophytic host grass. Funct. Plant Biol. 2022, 49, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Davitt, A.J.; Chen, C.; Rudgers, J.A. Understanding context-dependency in plant–microbe symbiosis: The influence of abiotic and biotic contexts on host fitness and the rate of symbiont transmission. Environ. Exp. Bot. 2011, 71, 137–145. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Arrieta, A.M.; Iannone, L.J.; Scervino, J.M.; Vignale, M.V.; Novas, M.V. A foliar endophyte increases the diversity of phosphorus-solubilizing rhizospheric fungi and mycorrhizal colonization in the wild grass Bromus auleticus. Fungal. Ecol. 2015, 17, 146–154. [Google Scholar] [CrossRef]
- Hou, W.P.; Wang, J.F.; Nan, Z.B.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, X.X.; Niu, X.L. Epichloë gansuensis endophyte-infection alters soil enzymes activity and soil nutrients at different growth stages of Achnatherum inebrians. Plant Soil 2020, 455, 227–240. [Google Scholar] [CrossRef]
- Li, X.; Ren, A.Z.; Han, R.; Yin, L.J.; Wei, M.Y.; Gao, Y.B. Endophyte mediated effects on the growth and physiology of Achnatherum sibiricum are conditional on both N and P availability. PLoS ONE 2012, 7, e48010. [Google Scholar]
- Zaidi, A.; Khan, M.S. Stimulatory effects of dual inoculation with phosphate solubilising microorganisms and arbuscular mycorrhizal fungus on chickpea. Aust. J. Exp. Agric. 2007, 47, 1016–1022. [Google Scholar] [CrossRef]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommonmutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagabhyru, P.; Dinkins, R.D.; Wood, C.L.; Bacon, C.W.; Schardl, C.L. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol. 2013, 13, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-de-Aldana, B.R.; García-Ciudad, A.; García-Criado, B.; Vicente-Tavera, S.; Zabalgogeazcoa, I. Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS ONE 2013, 8, e84539. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of salt and drought stress on alkaloid production in endophyte-infected drunken horse grass (Achnatherum inebrians). Biochem. Syst. Ecol. 2011, 39, 471–476. [Google Scholar] [CrossRef]
- Schardl, C.; Young, C.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blankenship, J.D.; Houseknecht, J.B.; Pal, S.; Bush, L.P.; Grossman, R.B.; Schardl, C.L. Biosynthetic precursors of fungal pyrrolizidines, the loline alkaloids. ChemBioChem 2005, 6, 1016–1022. [Google Scholar] [CrossRef]
- Bacon, C.W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agric. Ecosyst. Environ. 1993, 44, 123–141. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smish, A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Sterner, R.; Gorokhova, E.; Fagan, W.; Markow, T.; Cotner, J.; Harrison, J.; Hobbie, S.; Odell, G.; Weider, L. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Hessen, D.O.; Jensen, T.C.; Kyle, M.; Elser, J.J. RNA responses to N- and P-limitation; reciprocal regulation of stoichiometry and growth rate in Brachionus. Funct. Ecol. 2007, 21, 956–962. [Google Scholar] [CrossRef]
- Elser, J.J.; Hamilton, A. Stoichiometry and the new biology: The future is now. PLoS Biol. 2007, 5, e181. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.X.; Richard, J.; Chen, S.H.; Lv, H.; Zhou, J.L.; Li, C.J. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Zhang, X.; Shi, L.; Christensen, M.J.; Nan, Z.; Xia, C. Effects of Epichloë Endophyte and Transgenerational Effects on Physiology of Achnatherum inebrians under Drought Stress. Agriculture 2022, 12, 761. https://doi.org/10.3390/agriculture12060761
Cui X, Zhang X, Shi L, Christensen MJ, Nan Z, Xia C. Effects of Epichloë Endophyte and Transgenerational Effects on Physiology of Achnatherum inebrians under Drought Stress. Agriculture. 2022; 12(6):761. https://doi.org/10.3390/agriculture12060761
Chicago/Turabian StyleCui, Xuelian, Xingxu Zhang, Lielie Shi, Michael John Christensen, Zhibiao Nan, and Chao Xia. 2022. "Effects of Epichloë Endophyte and Transgenerational Effects on Physiology of Achnatherum inebrians under Drought Stress" Agriculture 12, no. 6: 761. https://doi.org/10.3390/agriculture12060761
APA StyleCui, X., Zhang, X., Shi, L., Christensen, M. J., Nan, Z., & Xia, C. (2022). Effects of Epichloë Endophyte and Transgenerational Effects on Physiology of Achnatherum inebrians under Drought Stress. Agriculture, 12(6), 761. https://doi.org/10.3390/agriculture12060761








