Abstract
The present study explored the effects of an Epichloë endophyte on growth and physiology parameters of drunken horse grass (DHG, Achnatherum inebrians) under four different soil water content. The possible transgenerational effects (TGE) on the above-mentioned indicators were examined. DHG plants with (EI) and without (EF) this Epichloë endophyte, grown from seed of plants from the same seed line, were used. The seeds had originated in the relatively dry site at Yuzhong [YZ(D)], and also used were seed of plants from this original seed-line grown at the relatively wet site Xiahe [XH(W)]. The growth, photosynthesis, phytohormones, and elements were measured. This study showed that the endophyte increased the aboveground biomass and chlorophyll content, with the increasing of photosynthetic parameters. The presence of endophyte also significantly promoted abscisic acid and indolE−3-acetic acid content but decreased the cytokinin content. The nitrogen and phosphorus content of EI plants was significantly higher than that of EF plants, but the endophyte decreased ratios of C:N and C:P at drought condition. In addition, TGE were present, affecting host growth and the above-mentioned parameters, and which indicated that the plants grown from the seeds in YZ(D) site are more competitive than those in the XH(W) site under water deficiency conditions.
1. Introduction
In natural conditions, plants always form beneficial symbioses with various microorganisms, such as nitrogen-fixing bacteria, mycorrhizal fungi and endophytic fungi [1,2,3] that enhance growth and persistence, including under adverse environments. The establishment of symbiotic relationships between microorganism and plants is an important advancement of evolution [4]. Many cool-season grasses (subfamily Pooideae) are host of the Epichloë endophytes [5], which are nearly all asexual interspecific hybrids, and colonize intercellular spaces of host plant aboveground tissue, and are transmitted vertically via grass seeds [6,7]. The relationship between these fungi and grasses is considered to be mutualistic [8]; the Epichloë endophyte utilizes photosynthates from its host to carry out metabolic processes necessary for its survival and growth. In return, their presence can enhance plants tolerance to various biotic [9,10] and abiotic [11,12,13] stresses via the production and induction of fungal alkaloids and other secondary metabolites.
Most studies on Epichloë endophytes and grass symbiotic associations involved grasses of the genera Festuca and Lolium, as these endophytes improve the fitness and productivity of the symbiont under a variety of biotic and abiotic stresses in agricultural and grassland ecosystems [14,15,16], but there are fewer studies related to Achnatherum inebrians. A. inebrians is commonly known as drunken horse grass (DHG) because of its narcotic impacts on grazing livestock; it usually grows in the arid and semi-arid grassland in north and northwest China with annual precipitation from 129 to 444 mm [17]. It is the host of seed-borne Epichloë gansuensis [18] or E. inebrians [19] endophytes, and the infection rate can reach 100% in natural conditions [17]. The Epichloë endophytes make DHG toxic to grazing livestock as they produce abundant ergot alkaloids, including ergine and ergonovine [20,21] to grazing livestock. In addition, the presence of endophyte increases tolerance to biotic and abiotic stresses, such as fungal pathogens [22,23,24], insect pests [25,26], heavy metals [27], low temperature [28], salt [29,30], and drought [31].
Drought is one of the most detrimental abiotic stresses for plants. The beneficial effect of Epichloë endophytes on the host grass is often achieved through improving growth rates and allocating more resources to reproduction under drought stress [32]. Photosynthesis is crucial in this process, which converts atmospheric carbon dioxide (CO2) into organic compounds in the leaves of green plants using solar energy [33]. The Epichloë endophyte enhanced the ability of the symbiont to use available resources for photosynthesis, further improving growth [34]. Phytohormone balance largely governed plant physiological responses to biotic and abiotic stresses [35,36]. Phytohormones play central roles in the ability of plants to adapt to changing environments, by mediating growth, nutrient allocation, and source/sink transitions [37]. Epichloë infection increases the accumulation of phytohormones [38] and activates certain signaling pathways to enhance host tolerance [26,39]. The ability of plant growth and development, and the balance of multiple key elements, can be reflected through the ratios of C:N, C:P, and N:P [40,41]. Epichloë endophyte infection affects mineral uptake, nutrient elements assimilation, and resource allocation abilities of the host [42]. However, previous studies have indicated that the positive effects of Epichloë to drought resistance of host grass depended on genotypes of both host plant and endophyte, and environmental conditions [43].
A transgenerational effect (TGE) is the source of phenotypic variation expressed in the progeny from plants experiencing different environmental conditions. It occurs when an abiotic or biotic environmental factor acts on a parental individual and thereby affects the phenotype of offspring [44]. On account of the importance of TGE for understanding plant evolution and ecology, and as one of the mechanisms for plants to respond to biotic and abiotic factors of stress [45], their underlying mechanisms are of general interest. A TGE can be part of a suite of fine-tuned mechanisms, which was selected over evolutionary time. It allows parental organisms to plastically match the traits of the next generation to the prevailing local ecological conditions by transferring resources and/or information to offspring [46]. Maternal plants are major contributors of TGE, as they are directly influenced by the environment where seed production occurs. Under these circumstances, the phenotype of progeny will be affected by parental characteristics to adjust to the local environmental conditions by transferring resources and/or information to offspring [46]. The Epichloë endophytes are an ideal system to study TGE since they are vertically transmitted through the seed. Therefore, these endophytes have high potential as carriers to transfer information from parental individual to progeny [44,47]. Some studies have suggested that Epichloë endophytes have transgenerational positive effects when plants undergo ozone [12,48] and drought stress [31]. However, the role of Epichloë fungal endophytes in mediating drought induced TGE on effect of DHG physiology has not been studied.
The aims of the present study are to determine (1) how the Epichloë endophyte affects photosynthesis, phytohormones and/or nutrient elements of DHG under drought stress, and (2) whether vertically transmitted Epichloë mediates TGEs by affecting above-mentioned indicators under limited water conditions. A pot-based study was employed to test whether the Epichloë endophyte improved the above-mentioned indicators in DHG to enhance the drought resistance, and if the adjustment of the parental DHG taken place at two environmentally different sites also affected these indicators.
2. Materials and Methods
2.1. Biological Material
Endophyte-free (EF) and endophyte-infected (EI) DHG seeds were prepared as described in the previously reported study [31]. In May 2014, EF and EI seeds with similar genetic background were sown at Xiahe county of Gansu province, where it is more moist (wet site, XH(W)) and at Yuzhong campus of Lanzhou University, where is drier (dry site, YZ(D)). Seeds from the two hundred of EF and from the two hundred of EI DHG at both the XH(W) and YZ(D) sites were harvested in September 2015, bulked as EF or EI, and stored at a constant temperature of 4 °C for the current study. Ten seeds were selected randomly from both EF and EI seed samples of the XH(W) and YZ(D) site to confirm the infection statues before planting.
2.2. Experimental Design
On 24 August 2016, two hundred plastic pots with 140 mm diameter and 170 mm height were prepared, and 600 g vermiculite was put into the pots. The water-holding capacity of the vermiculite, from now on referred to as soil, was measured, and when saturated, 600 g medium is able to hold 1134 g available water on average. Five corresponding seeds of YZ(D)EF, YZ(D)EI, XH(W)EF, and XH(W)EI were planted into fifty pots, respectively. After germination, only one seedling was retained in each pot, and these were watered as needed. All pots were assigned randomly to the greenhouse of Yuzhong campus of Lanzhou University. The greenhouse conditions were maintained at a temperature of 24 ± 2 °C and a photoperiod of 16/8 h (light/dark) with a moisture of 76 ± 2%. After three weeks, for DHG of each seed type XH(W) and YZ(D), eighty pots with similar growth state DHG were selected, forty with EF and forty with EI plants. A total of 160 pots were employed in the present experiment. On 9 October, according to the measured saturated water content of the soil, the water gradient was established by reducing the water content of soil. The soil water-holding capacity (SWC) of each of the 40 pots of each of the four soil treatments was established: 15% SWC (strong drought), 30% SWC (light drought), 45% SWC (normal moisture) and 60% SWC (abundant moisture) conditions. Each SWC condition was assigned to 10 pots of the YZ(D)EF, YZ(D)EI, XH(W)EF and XH(W)EI seedlings, and every afternoon of the experiment at 6 o’clock, each pot was weighed, and water was added to maintain the required SWC. After that, the position of each pot was transposed at random.
2.3. Measurement Protocols
2.3.1. Chlorophyll Content
On 8 December, the leaf in the top of each DHG plant in each different soil water condition was selected to measure its chorophyll content using a SPAD-502Plus (Konica Minolta Sensing, Inc., Tokyo, Japan), according to the method of Ye et al. [49].
2.3.2. Photosynthetic Indexes
The same day as the chlorophyll content measurement, photosynthesis in the top leaf, that is, the same leaf that chlorophyll content was measured, of each DHG in each treatment was measured according to the method of Xia et al. [31]. An LI-6400 portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA) was used to measure the photosynthetic indexes between 9 o’clock and 11 o’clock in the morning. Five measurements were conducted at different points as 5 repetitions along the top leaf of each plant.
2.3.3. Biomass
After the measurement of chlorophyll content and photosynthetic, all plants were harvested and separated into shoots and roots and washed with distilled water. To determine the dry weight and elemental analysis, some of the samples were dried at 80 °C to a constant weight. The dry weight of shoot and root samples was measured.
2.3.4. Abscisic Acid (ABA), Cytokinin (CtK), and IndolE−3-acetic Acid (IAA)
Content Determination
The other subsamples were wrapped with aluminum foil and immediately frozen in liquid nitrogen on 8 December. These samples were stored in a refrigerator at −80 °C for subsequent ABA, CtK, and IAA content determination by enzyme-linked immunosorbent assay (ELISA) according to the methods of Xu et al. [38].
2.3.5. Elemental Analysis
All shoot and root dried tissues were ground in an MM400 ball mill (Retsch, Germany) to obtain homogenous samples. The plant organic carbon (C) content was measured using a K2CrO7-H2SO4 oxidation method [50], and total nitrogen (N) and total phosphorus (P) content were determined by flow injection analysis (FIAstar 5000 Analyzer, FOSS Analytical, Hillerød, Denmark) [31]. The proportion of C, N, and P including C:N, C:P, and N:P was calculated.
2.4. Statistical Analysis
Data analyses were conducted using SPSS v20.0 (SPSS, Inc., Chicago, IL, USA). The effects of origin of seed, water content, and endophyte on biomass, nutrient content, photosynthetic indexes, and phytohormone content parameters were analyzed with a three-way ANOVA. An independent-sample t-test was used to determine the differences in all indictors in the present study. These included the differences between EI and EF under the same soil water content, or XH(W) and YZ(D) of EF/EI DHG, or XH(W) and YZ(D) maternal habitat type. Statistical significance was considered at p value < 0.05. Data shown in figures are means with their standard errors. Mapping was with Origin 9.0 (Origin Lab, Northampton, MA, USA).
3. Results
3.1. Biomass
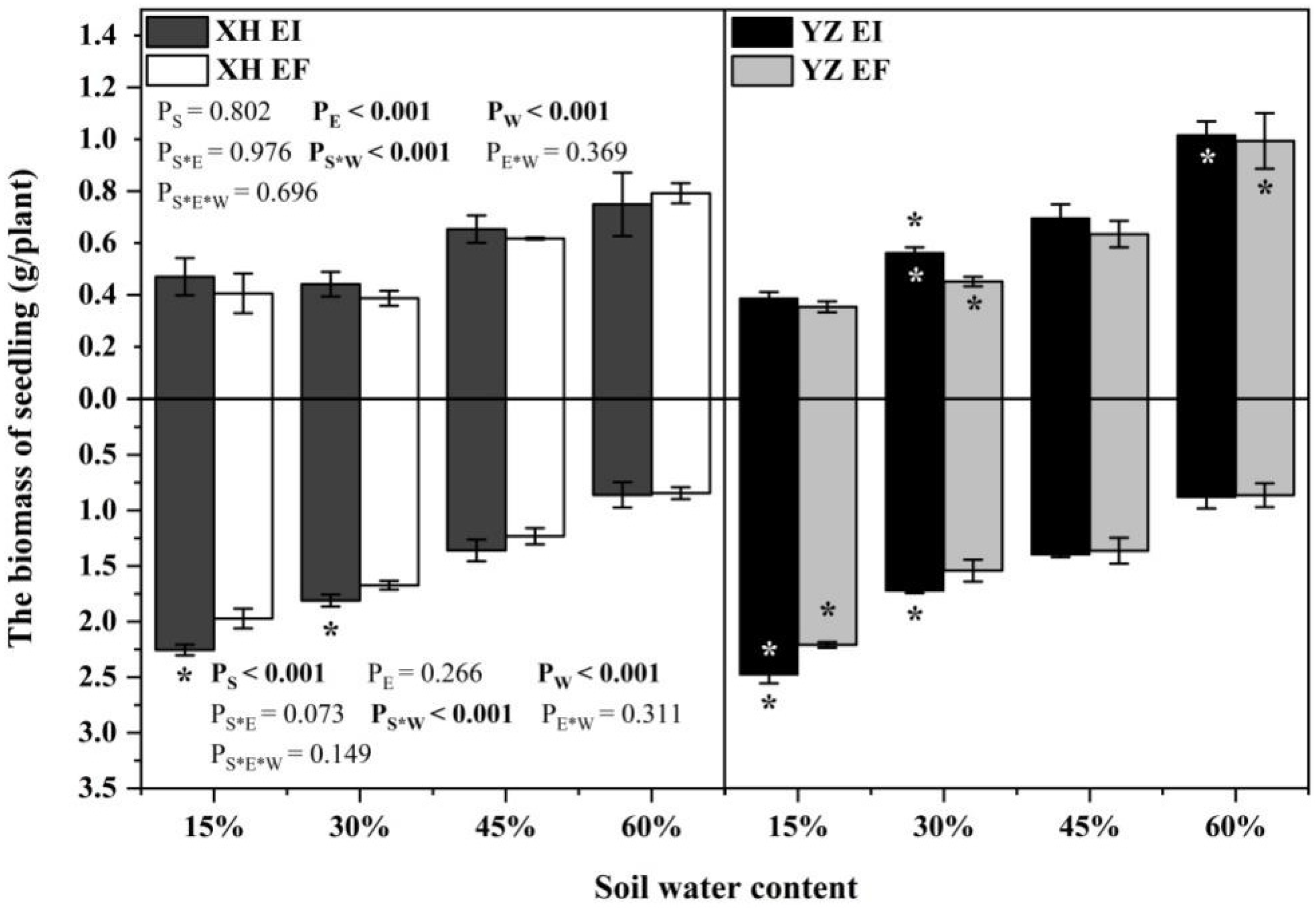
Endophyte (p < 0.001), water content (p < 0.001), and the interaction between seed origin and water content (p < 0.001) significantly affected the shoot biomass (Table S1). At the 30% SWC, endophyte increased the shoot biomass of DHG from the YZ(D) site significantly (p < 0.05), with a difference of 23.09%. Importantly, the shoots biomass of YZ(D)EI plants was significantly higher than XH(W)EI (p < 0.05) at 30% and 60% SWC, with an increase of 29.21% and 37.65%, respectively. The shoots biomass of YZ(D)EF plants was also higher than XH(W)EF (p < 0.05) at the same SWC, with a relative increase of 20.53% and 26.17%, respectively. The differences of shoot biomass between the EI plants from the two sites are higher than the difference between the EF plants of the two sites at each same SWC (Figure 1).
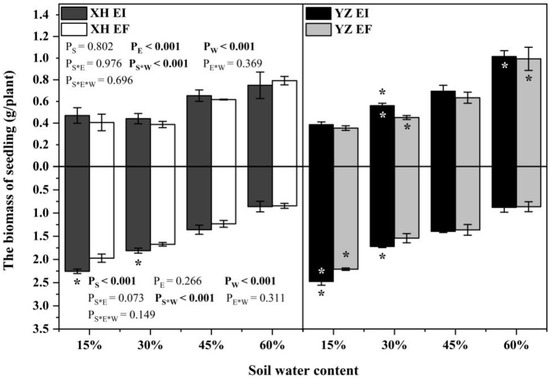
Figure 1.
The biomass of A.inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content (g/plant). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the black/gray bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses.
The root biomass of seedlings was significantly affected by the seed origin (p < 0.001), water content (p < 0.001) and the interaction between seed origin and water content (p < 0.001) (Table S1). Endophyte increased the root biomass of DHG from XH(W) sites at 15% and 30% SWC significantly (p < 0.05), with a difference of 15.35% and 8.09%. Endophyte also increased the root biomass of YZ(D) DHG at 15% and 30% SWC significantly (p < 0.05), with a relative difference of 11.89% to 11.95%. Additionally, the root biomass of YZ(D)EI plants was higher than XH(W)EI at 15% SWC significantly (p < 0.05), with an increase of 9.01%, while the root biomass of YZ(D)EF plants was also higher than XH(W)EF at 15% SWC significantly, with a relative increase of 12.38% (Figure 1).
3.2. Phytohormones
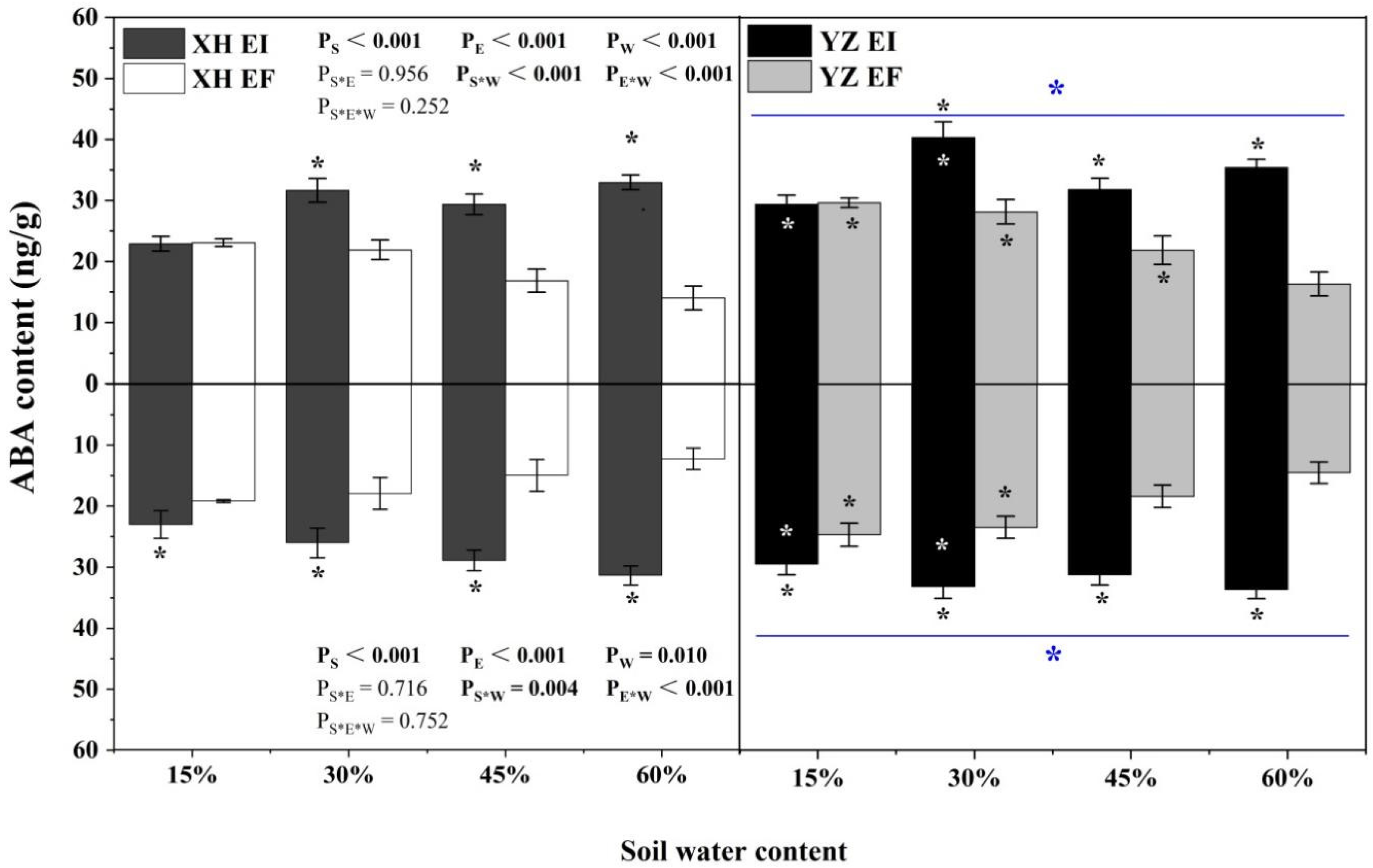
ABA content of shoots was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p < 0.001), seed origin × water content (p < 0.001), and endophyte × water content (p < 0.001) (Table S1). Endophyte increased the ABA content of shoots of both XH(W) and YZ(D) DHG at 30% to 60% SWC significantly (p < 0.05), with a difference of 43.23% to 135.01%. The shoot ABA content of YZ(D) DHG was higher than XH(W) DHG significantly (p < 0.05), with a relative difference of 20.54%. Importantly, the shoot ABA content of YZ(D)EI DHG was higher than XH(W)EI DHG significantly at 15% and 30% SWC (p < 0.05), with a difference of 27.99% and 27.08%. The shoot ABA content of YZ(D)EF plants was also higher than XH(W)EF DHG (p < 0.05) at 15%, 30%, and 45% SWC, with an increase of 27.96%, 28.19%, and 29.53%, respectively (Figure 2). The root ABA content was significantly affected by endophyte (p < 0.001), water content (p = 0.001), seed origin (p < 0.001), seed origin × water content (p = 0.001), and endophyte × water content (p < 0.001) (Table S1). Endophyte increased the ABA content of roots of DHG from the XH(W) and YZ(D) sites significantly at 15% to 60% SWC (p < 0.05), with a difference of 19.33% to 156.12%. The root ABA content of YZ(D) DHG was higher than XH(W) significantly (p < 0.05), with a relative difference of 20.18%. Importantly, the root ABA content of YZ(D)EI DHG was higher than XH(W)EI DHG significantly (p < 0.05) at 15% and 30% SWC, with a difference of 27.97% and 27.49%. The root ABA content of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at each same SWC, with a relative increase of 28.84% and 30.99%, respectively (Figure 2).
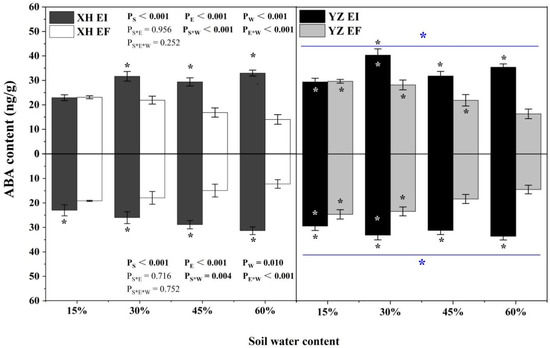
Figure 2.
The ABA content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content (ng/g). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the black/gray bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
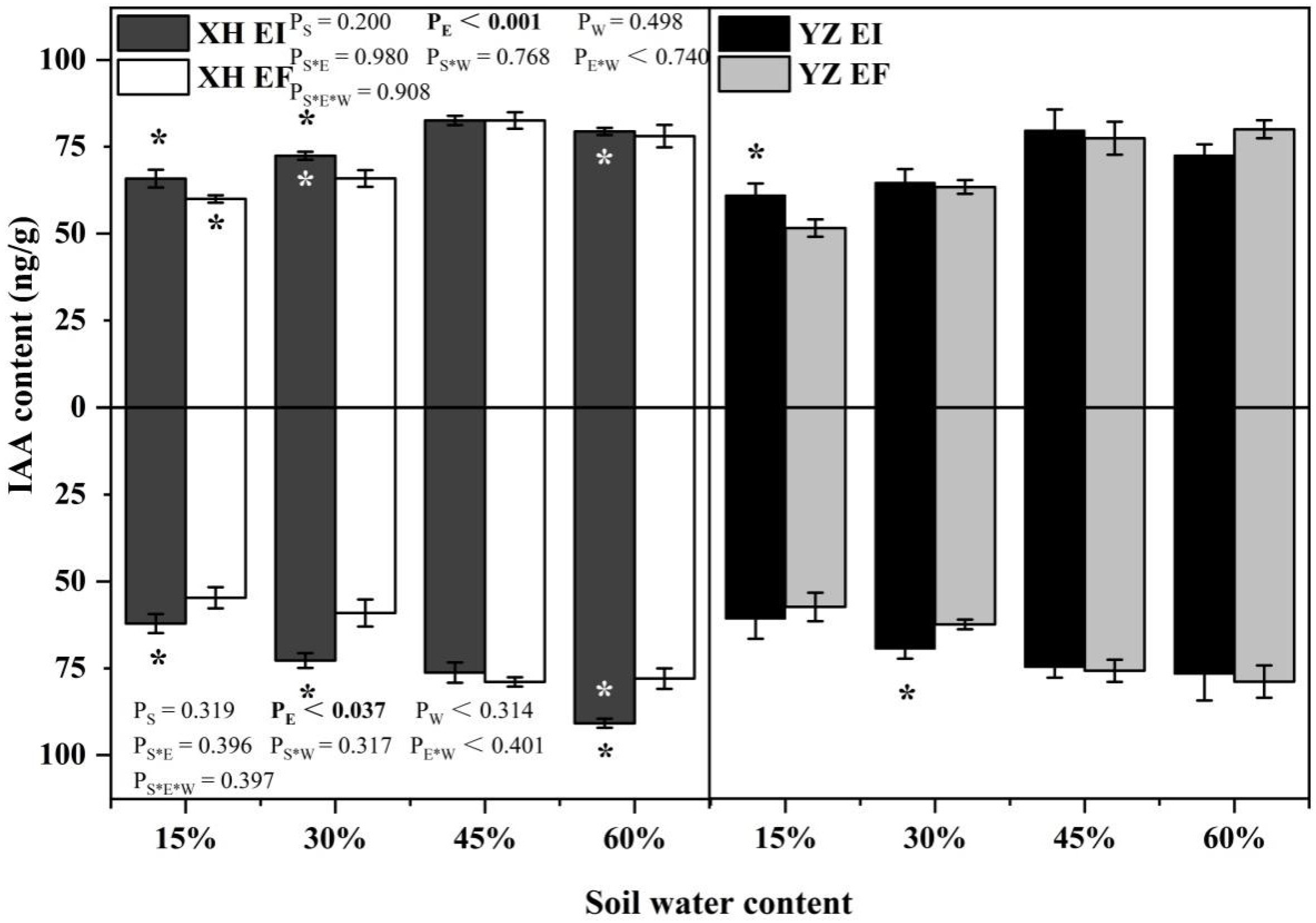
IAA content of shoots was only significantly affected by endophyte (p < 0.001) (Table S1). The shoots IAA content of XH(W)EI DHG were higher than XH(W)EF DHG significantly at 15% and 30% SWC, with a difference of 9.75% and 9.91%, respectively. Endophyte also increased the shoot IAA content of YZ(D) DHG at 15% SWC significantly (p < 0.05), with a difference of 18.27%. The shoots IAA content of YZ(D)EI DHG was lower than XH(W)EI significantly at 30% and 60% SWC (p < 0.05), with a decrease of 10.66% and 8.68%. The shoot IAA content of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 15% SWC, with a relative decrease of 13.99% (Figure 3). Endophyte (p = 0.037) significantly affected the root IAA content (Table S1). Endophyte increased the root IAA content of XH(W) DHG significantly (p < 0.05) at 15%, 30%, and 60% SWC, with a difference of 13.55%, 23.22%, and 16.48%, respectively. The root IAA content of YZ(D)EI DHG plants were higher than YZ(D)EF DHG at 30% SWC significantly, with a relative difference of 11.33%. The root IAA content of YZ(D)EI DHG was also lower than XH(W)EI DHG (p < 0.05) at 60% SWC, with a decrease of 15.61% (Figure 3).
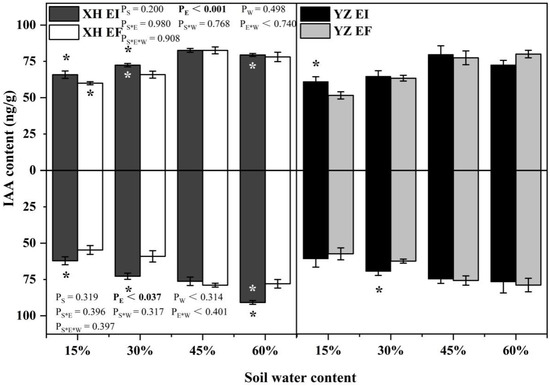
Figure 3.
The IAA content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content (ng/g). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the dark gray/white bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses.
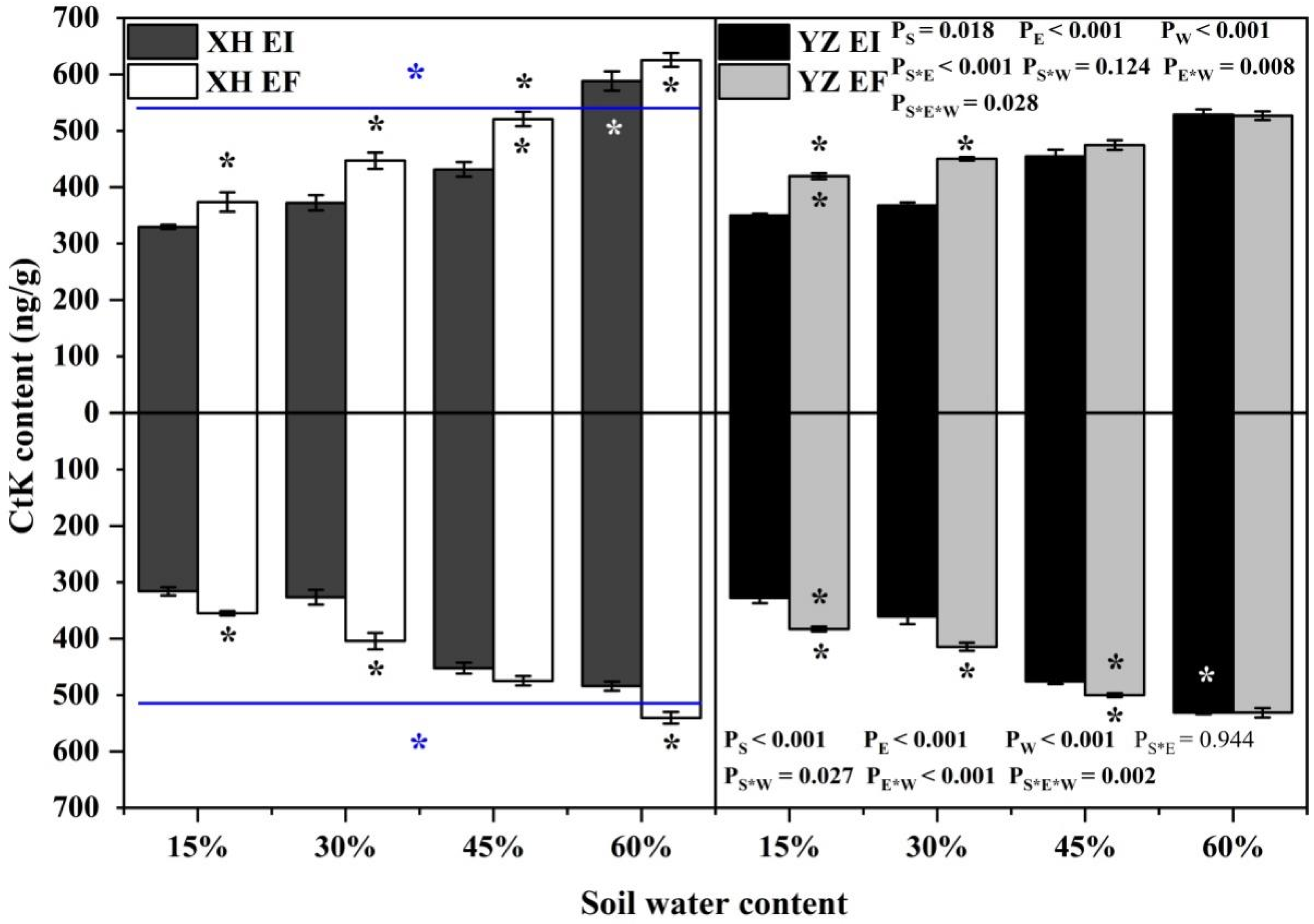
The shoot CtK content of DHG was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p = 0.018), endophyte × water content (p = 0.008), endophyte × seed origin (p < 0.001), and endophyte × water content × seed origin (p = 0.028) (Table S1). The shoot CtK content of XH(W)EI DHG was lower than XH(W)EF at significantly 15% to 45% SWC, with a difference of 11.47%, 16.60%, and 17.08%, respectively. Endophyte also decreased the shoot CtK content of YZ(D) DHG significantly (p < 0.05) at 15% and 30% SWC, with a relative decrease of 16.33% and 17.64%. Additionally, YZ(D) DHG had a lower CtK content of shoots than XH(W) significantly (p < 0.05), with a difference of 2.71%. The shoot CtK content of YZ(D)EI DHG was lower than XH(W)EI DHG significantly (p < 0.05) at 60% SWC, with a relative decrease of 10.04% (Figure 4). The root CtK content of DHG was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p < 0.001), seed origin × water content (p = 0.027), endophyte × water content (p < 0.001) and endophyte × water content × seed origin (p = 0.002) (Table S1). Endophyte decreased the root CtK content of XH(W) DHG (p < 0.05) at 15%, 30%, and 60% SWC significantly, with a decrease of 10.13%, 18.48%, and 10.66%, respectively. It also decreased the root CtK content of YZ(D) DHG significantly (p < 0.05) at 15%, 30%, and 45% SWC, with a relative decrease of 14.42%, 11.67%, and 4.50%, respectively. Additionally, YZ(D) DHG had higher root CtK content than XH(W) DHG significantly (p < 0.05). Importantly, the root CtK content of YZ(D)EI DHG was higher than XH(W)EI DHG significantly (p < 0.05) at 60% SWC, with a relative increase of 10.72%, respectively. The root CtK content of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at 15% and 45% SWC, with an increase of 9.93% and 5.87%, respectively (Figure 4).
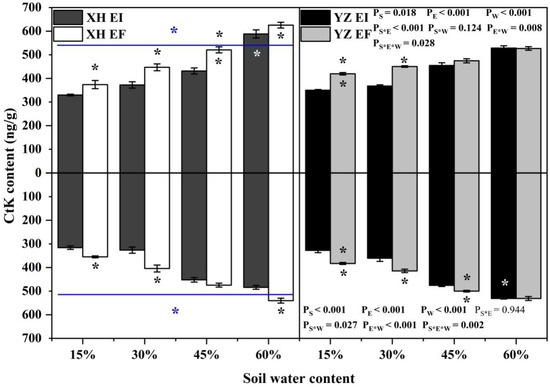
Figure 4.
The CtK content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content (ng/g). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the white, black and gray bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
3.3. Photosynthetic Indexes
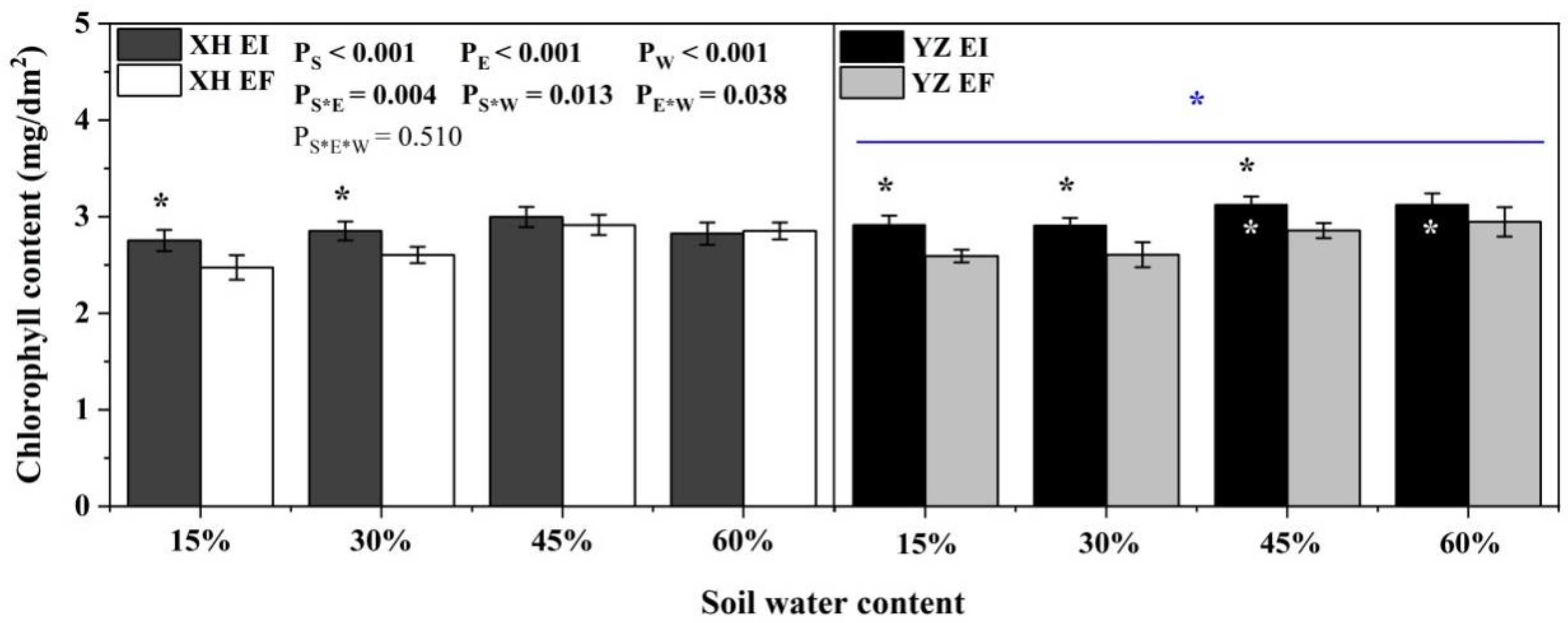
Chlorophyll content was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p < 0.001), endophyte × water content (p = 0.004), endophyte × seed origin (p = 0.013), water content × seed origin (p = 0.038) (Table S2). Endophyte increased the chlorophyll content of YZ(D) DHG at the 15% to 45% SWC significantly (p < 0.05), with a relative difference of 12.56%, 13.12%, and 9.60%, respectively. At the 15% and 30% SWC, endophyte increased the chlorophyll content of XH(W) DHG significantly (p < 0.05). YZ(D) DHG had a significantly higher chlorophyll content than XH(W) DHG (p < 0.05). Importantly, the chlorophyll content of YZ(D)EI DHG was higher than XH(W)EI DHG significantly at 45% and 60% SWC (p < 0.05), with an increase of 4.84% and 10.71%, respectively (Figure 5).
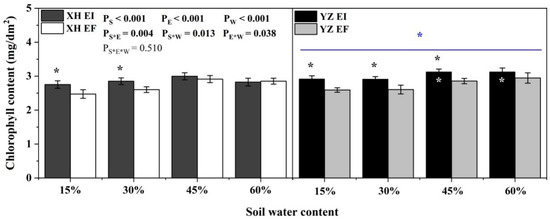
Figure 5.
The chlorophyll content of A. inebrians seedlings shoots from XH(W) and YZ(D) seeds under various soil water content (mg/dm2). The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White * in the black bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
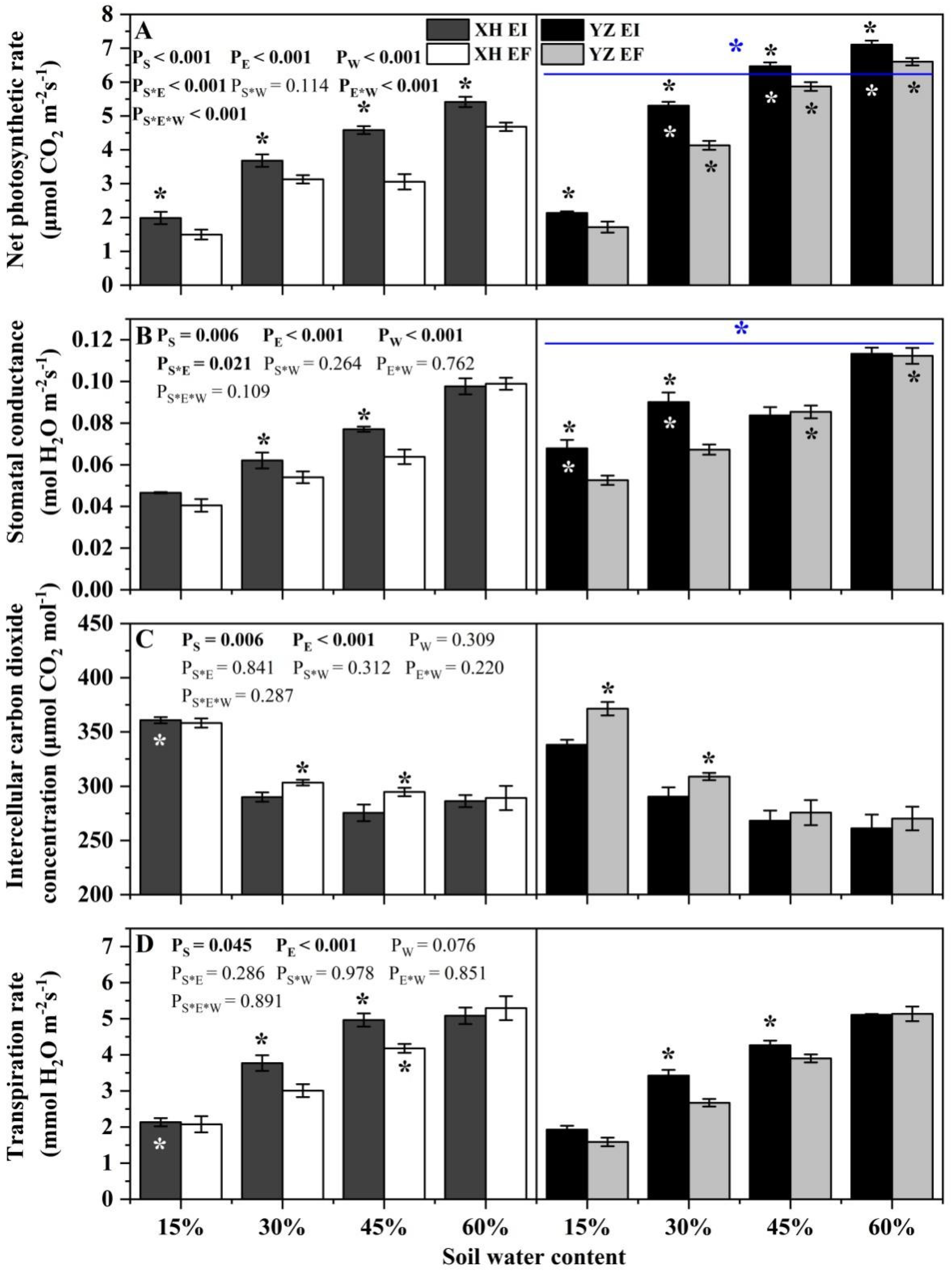
Net photosynthetic rate of DHG was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p < 0.001), endophyte × water content (p < 0.001), water content × seed origin (p < 0.001) and endophyte × water content × seed origin (p < 0.001) (Table S2). The photosynthetic rate of EI DHG was higher than EF DHG from both the XH(W) and YZ(D) site under each SWC significantly (p < 0.05). Importantly, YZ(D) DHG had a higher photosynthetic rate than XH(W) DHG significantly (p < 0.05), with an increase of 39.53%. Additionally, the photosynthetic rate of YZ(D)EI DHG was higher than those from the XH(W)EI DHG significantly (p < 0.05) at 30% to 60% SWC, with a relative increase of 43.16%, 40.04%, and 30.22%, respectively. The net photosynthetic rate of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at each same SWC, with an increase of 31.20%, 94.50%, and 41.13%, respectively. The difference of photosynthetic rate between the EI DHG from the two sites is higher than the difference between the EF DHG of the two sites at a relative drought of 30% SWC (Figure 6A).
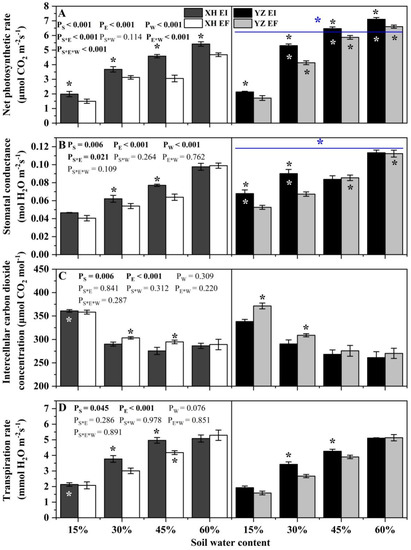
Figure 6.
The photosynthetic parameters of A. inebrians seedlings shoots from XH(W) and YZ(D) seeds under various soil water content. (A) Net photosynthetic rate (μmol CO2 m−2s−1), (B) Stomatal conductance (mol H2O m−2s−1), (C) Intercellular carbon dioxide concentration (μmol CO2 mol−1), (D) Transpiration rate (mmol H2O m−2s−1). The asterisk (*) above error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the dark gray, black and gray bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
The stomatal conductance of DHG was significantly affected by endophyte (p = 0.006), water content (p < 0.001), seed origin (p < 0.001) and endophyte × water content (p = 0.021) (Table S2). Endophyte increased the stomatal conductance of XH(W) DHG under 30% and 45% SWC significantly, with a difference of 15.67% and 21.53%, respectively. At the 15% and 30% SWC, endophyte also increased the stomatal conductance of YZ(D) DHG significantly (p < 0.05). YZ(D) DHG had higher stomatal conductance than XH(W) DHG significantly (p < 0.05), with an increase of 23.87%. Additionally, the stomatal conductance of YZ(D)EI DHG was higher than XH(W)EI DHG (p < 0.05) at 15% and 30% SWC significantly, with a relative increase of 44.48% and 43.68%, respectively. The stomatal conductance of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at 45% and 60% SWC, with an increase of 34.10% and 13.41%, respectively (Figure 6B).
Intercellular carbon dioxide concentration was significantly affected by endophyte (p = 0.006) and water content (p < 0.001) (Table S2). Endophyte decreased the intercellular carbon dioxide concentration of XH(W) DHG under 30% and 45% SWC significantly, with a difference of 4.13% and 6.32%, respectively. At the 15% and 30% SWC, endophyte also decreased the intercellular carbon dioxide concentration of YZ(D) DHG significantly (p < 0.05), with a difference of 9.17% and 5.80%, respectively. In addition, the intercellular carbon dioxide concentration of YZ(D)EI DHG was lower than XH(W)EI DHG (p < 0.05) at 15% SWC significantly, with a relative decrease of 6.02% (Figure 6C).
Transpiration rate was significantly affected by endophyte (p = 0.045) and water content (p < 0.001) (Table S2). Endophyte significantly increased the transpiration rate of XH(W) DHG at 30% and 45% SWC, with a difference of 27.72% and 18.53%, respectively. Endophyte also significantly increased the transpiration rate of YZ(D) DHG at the same SWC, with a relative difference of 29.67% and 9.17%, respectively. Additionally, the transpiration rate of YZ(D)EI DHG was significantly lower than XH(W)EI DHG (p < 0.05) at 15% SWC, with a relative decrease of 11.86%. The transpiration rate of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 45% SWC, with a decrease of 7.74% (Figure 6D).
3.4. Elements and Stoichiometry
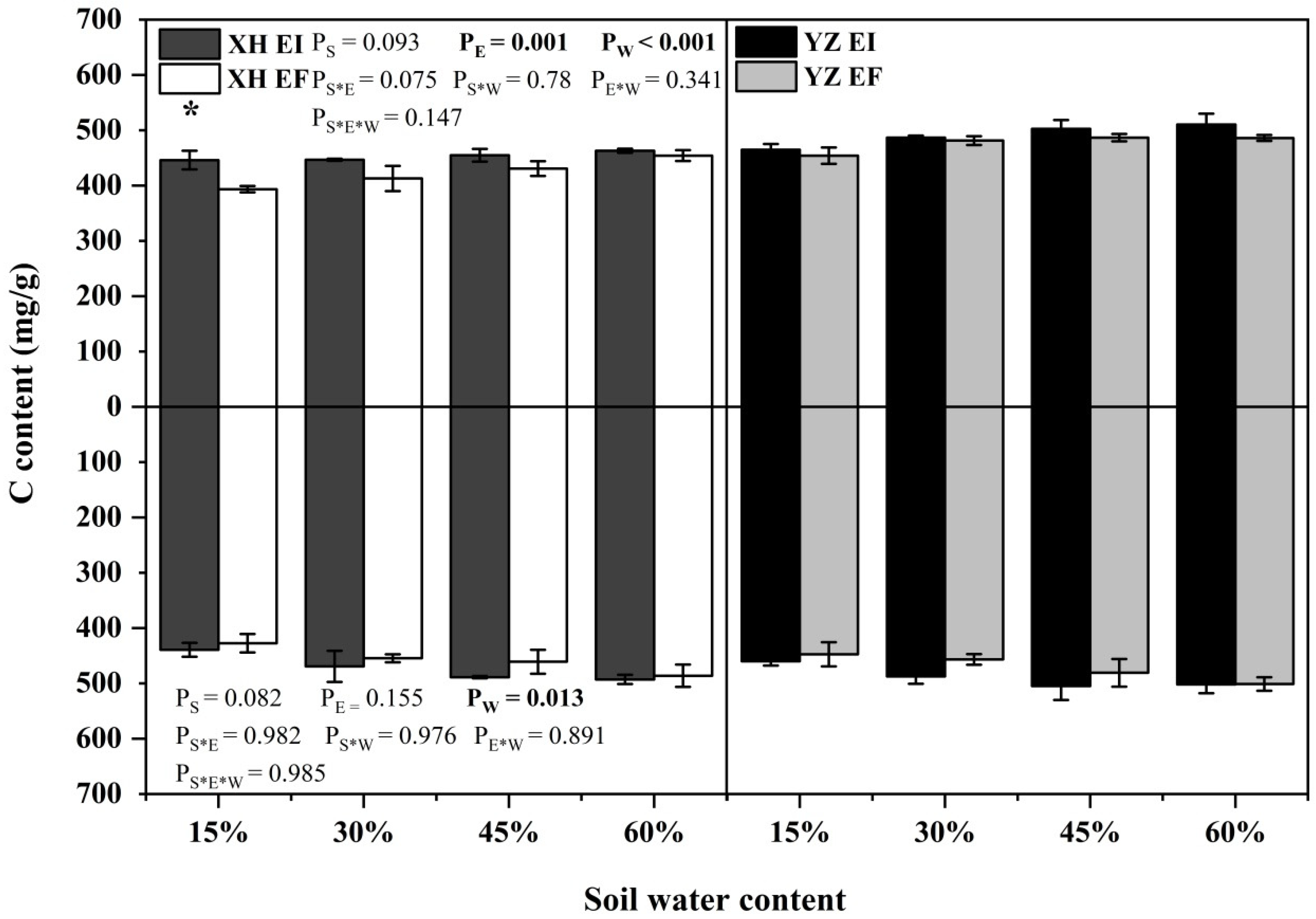
The C content of shoots was significantly affected by endophyte (p = 0.001) and water content (p < 0.001) (Table S3). The root C content was affected by water content significantly (p = 0.013) (Table S3). XH(W)EI DHG had higher shoot C content than XH(W)EF DHG with a relative increase of 13.35% at 15% SWC significantly (p < 0.05) (Figure 7).
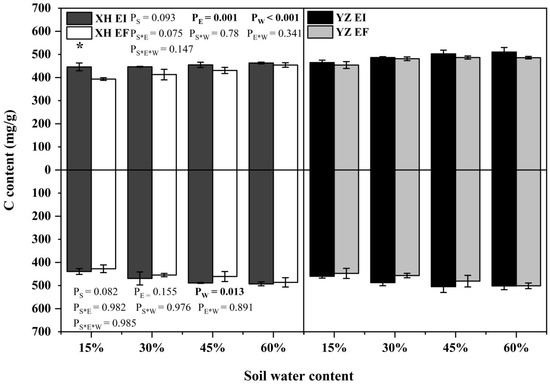
Figure 7.
The C content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content (mg/g). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above error bars means significant difference (p < 0.05) between EI and EF under the same soil water content.
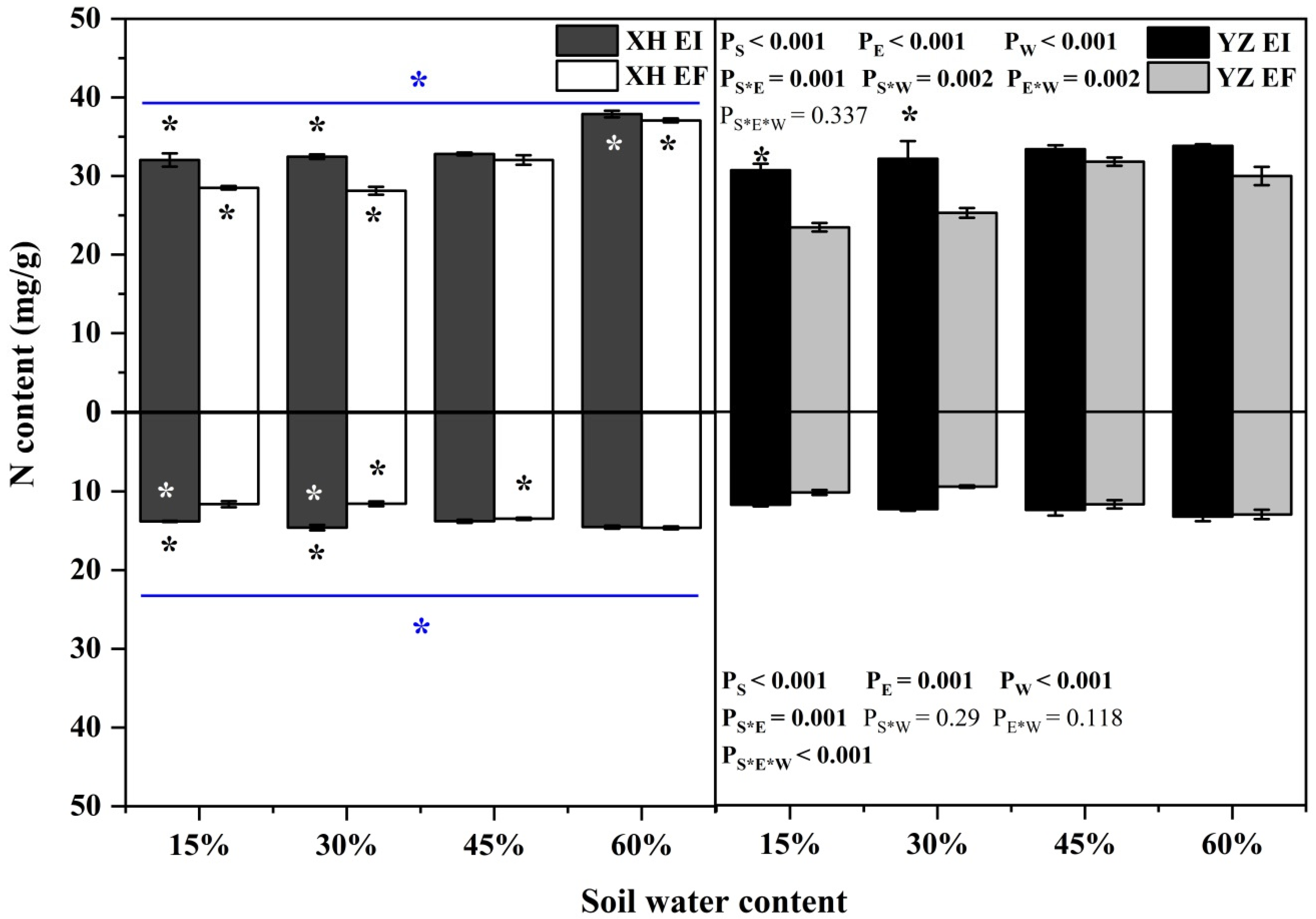
The shoot N content was affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p < 0.001), endophyte × water content (p = 0.002), water content × seed origin (p = 0.002) and endophyte × seed origin (p = 0.001) significantly (Table S3). Endophyte increased the N content of shoots of XH(W) DHG significantly (p < 0.05) by 12.42% and 15.47% at 15% and 30% SWC, respectively. Endophyte also increased the shoot N content of YZ(D) DHG significantly (p < 0.05) by 30.99% and 27.14% at 15% and 30% SWC, respectively. Further, YZ(D) DHG had significantly lower shoot N content than XH(W) DHG (p < 0.05) by 7.72%. The shoot N content of YZ(D)EI DHG was lower than XH(W)EI DHG (p < 0.05) at 60% SWC significantly, with a relative decrease of 10.71%. The shoot N content of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 15% to 60% SWC, with a decrease of 17.63% to 19.06% (Figure 8). The root N content was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin (p < 0.001), endophyte × seed origin (p = 0.001) and endophyte × water content × seed origin (p < 0.001) (Table S3). Endophyte increased the root N content of DHG significantly (p < 0.05) from the XH(W) site at 15% and 30% SWC, with a relative increase of 18.76% and 26.26%, respectively. Importantly, YZ(D) DHG had lower N root content than XH(W) site significantly (p < 0.05) by 12.40%. The root N content of YZ(D)EI DHG was significantly lower XH(W)EI DHG (p < 0.05) at 15% and 30% SWC, with a relative decrease of 14.37% and 15.18, respectively. The root N content of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 30% and 45% SWC, with a decrease of 17.70% and 12.64% (Figure 8).
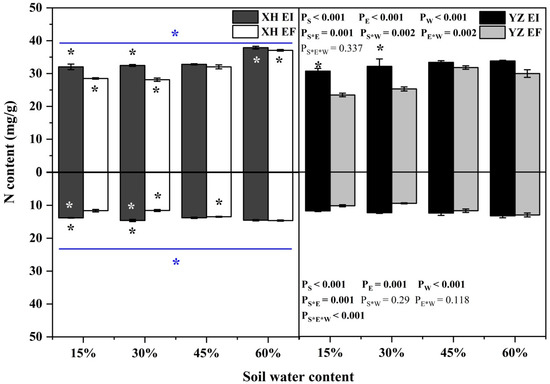
Figure 8.
The N content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content (mg/g). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the dark gray/white bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
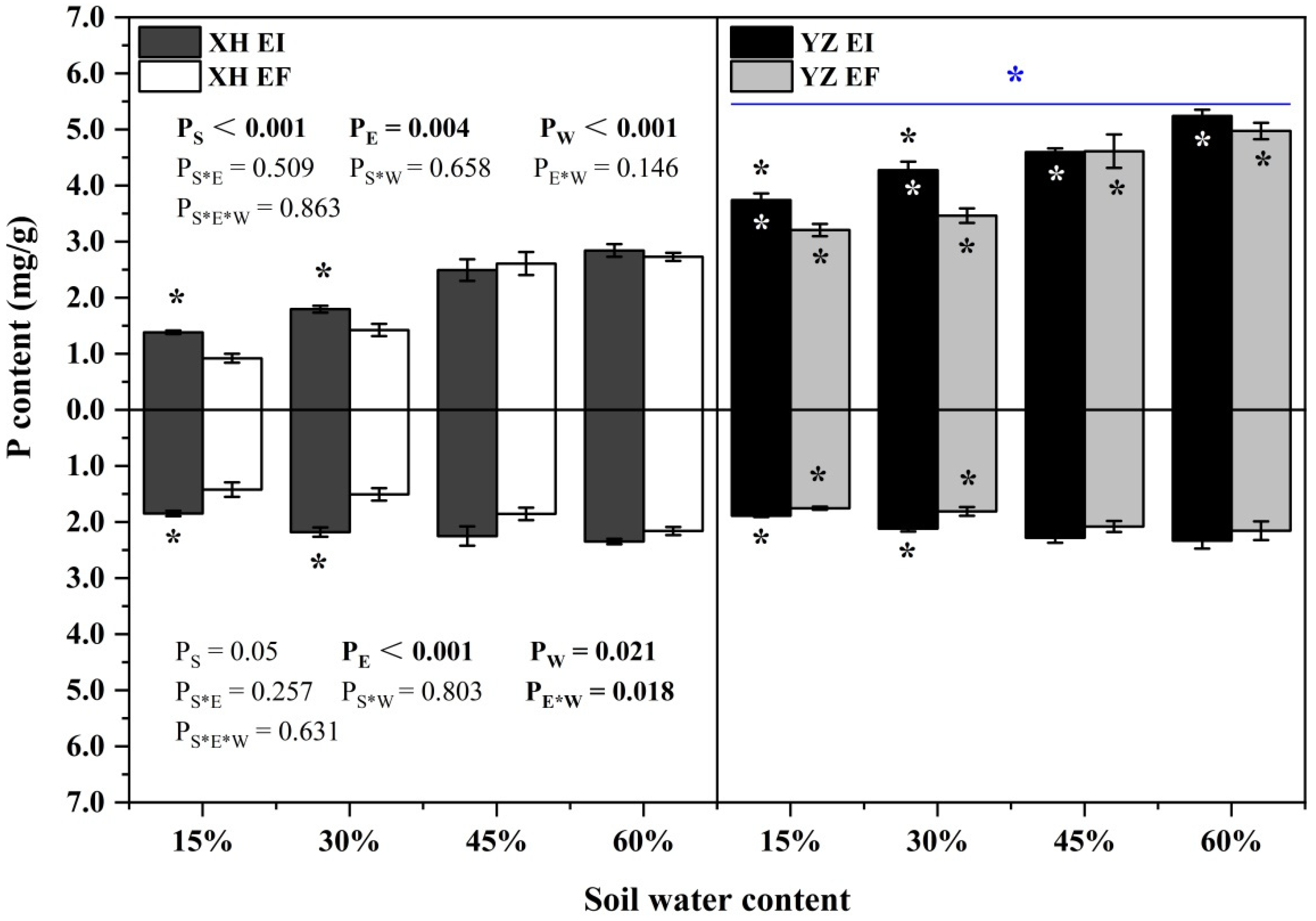
The shoot P content was significantly affected by endophyte (p = 0.004), seed origin (p < 0.001) and water content (p < 0.001) (Table S3). The shoot P content of both EI and EF DHG from the YZ(D) and XH(W) sites increased as the SWC increasing. Endophyte increased the shoot P content of XH(W) DHG significantly (p < 0.05) at 15% and 30% SWC, with an increase of 50.36% and 26.24%, respectively. The shoot P content of YZ(D)EI DHG was higher than YZ(D)EF DHG under 15% and 30% SWC significantly (p < 0.05) by 16.63% and 23.49%, respectively. Importantly, YZ(D) DHG had higher shoot P content than XH(W) DHG significantly (p < 0.05) by 90.08%. Additionally, YZ(D)EI DHG had a significantly higher shoot P content than XH(W)EI DHG at each SWC (p < 0.05), with a relative increase of 170.37%, 138.03%, 84.36%, and 84.29%, respectively. The shoot P content of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at each same SWC, with an increase of 248.55%, 143.33%, 76.75%, and 96.57%, respectively. The differences of shoot P content between the EI from the two sites are higher than the difference between the EF of the two sites at 45% SWC (Figure 9). The root P content was significantly affected by endophyte (p < 0.001), water content (p = 0.021), and endophyte × water content (p = 0.018) (Table S3). Endophyte significantly increased the root P content of XH(W) DHG at 15% and 30% SWC (p < 0.05), with a relative increase of 30.05% and 44.69%, respectively. The root P content of YZ(D)EI DHG was higher than YZ(D)EF DHG under 15% and 30% SWC significantly (p < 0.05), with an increase of 7.6% and 16.94%, respectively. In addition, the root P content of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at 15% and 30% SWC by 23.47% and 20.13% (Figure 9).
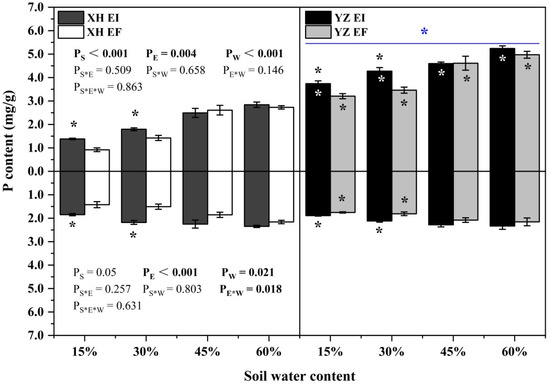
Figure 9.
The P content of A. inebrians seedlings growth shoots and roots from XH(W) and YZ(D) seeds under various soil water content (mg/g). Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the black/gray bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
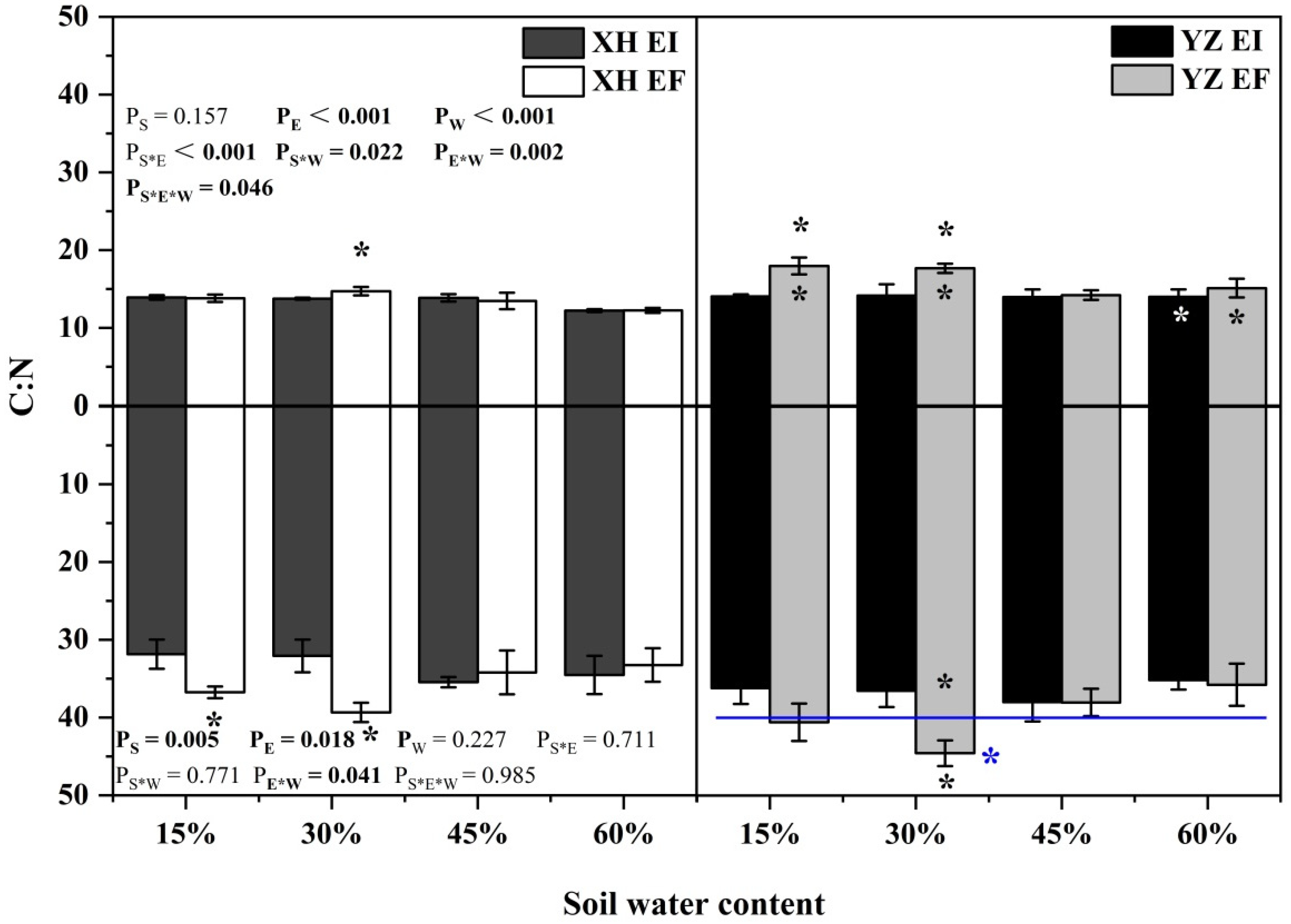
The shoot C:N ratio was significantly affected by endophyte (p < 0.001), water content (p < 0.001), seed origin × endophyte (p < 0.001), endophyte × water content (p = 0.002), seed origin × water content (p = 0.022) and endophyte × water content × seed origin (p = 0.046) (Table S4). The shoot C:N ratio of XH(W)EI DHG was lower than XH(W)EF DHG significantly (p < 0.05) at 30% SWC by 6.51%. Endophyte decreased the shoot C:N ratio from the YZ(D) site at 15% and 30% SWC significantly (p < 0.05), with a decrease of 21.83% and 19.85%, respectively. The shoot C:N ratio of YZ(D)EI DHG was higher than XH(W)EI DHG (p < 0.05) at 60% SWC significantly by 14.60%. The shoot C:N ratio of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at 15%, 30%, and 60% SWC, with a relative increase of 30.08%, 20.00%, and 23.43% (Figure 10). The root C:N ratio was significantly affected by endophyte (p = 0.018), seed origin (p = 0.005) and endophyte × water content (p = 0.041) (Table S4). Endophyte decreased the C:N ratio of roots significantly (p < 0.05) from the XH(W) site at 15% and 30% SWC, with a decrease of 13.34% and 18.47%, respectively. The root C:N ratio of YZ(D)EI DHG was lower than YZ(D)EF DHG significantly (p < 0.05) at 30% SWC by 18.02%. Importantly, YZ(D) DHG had a significantly higher root C:N ratio of roots than XH(W) DHG (p < 0.05), with an increase of 9.86%. The root C:N ratio of YZ(D)EF DHG was also higher than XH(W)EF DHG (p < 0.05) at 30% SWC by 13.25% (Figure 10).
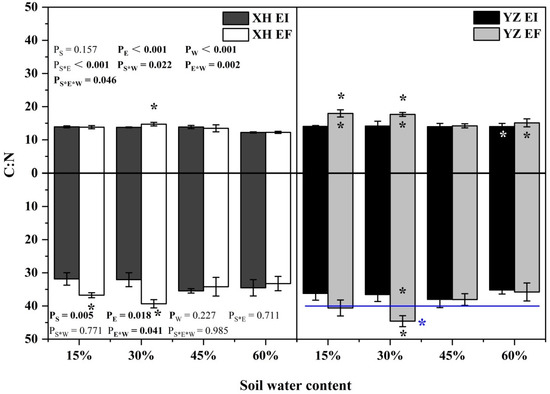
Figure 10.
The C:N content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content. Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the black/gray bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
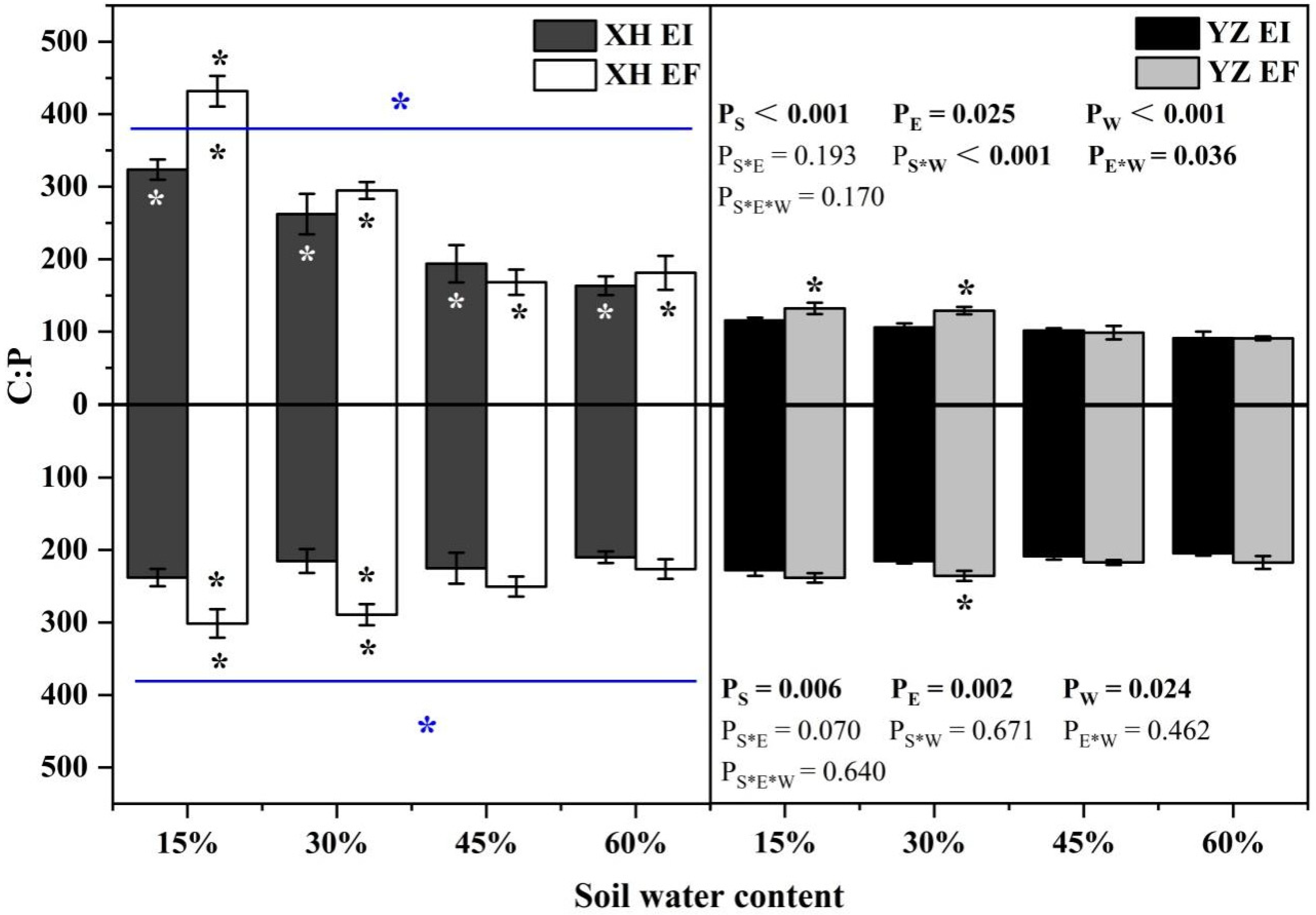
The shoot C:P ratio was significantly affected by seed origin (p < 0.001), endophyte (p = 0.025), water content (p < 0.001), endophyte × water content (p = 0.036), and seed origin × water content (p < 0.001) (Table S4). Endophyte decreased the shoot C:P ratio from the XH(W) site at 15% SWC significantly (p < 0.05) by 25.05%. The shoot C:P ratio of YZ(D)EI DHG was lower than YZ(D)EF DHG at 15% and 30% SWC significantly (p < 0.05). Importantly, YZ(D) DHG had a lower shoot C:P ratio than XH(W) DHG significantly (p < 0.05), with a decrease of 57.19%. Additionally, the shoot C:P ratio of YZ(D)EI DHG was significantly lower than XH(W)EI DHG (p < 0.05) at each SWC, with a relative decrease of 64.31%, 59.64%, 47.65%, and 44.19%, respectively. The shoot C:P ratio of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at each same SWC, with a decrease of 69.41%, 56.21%, 41.36%, and 49.91%, respectively (Figure 11). Endophyte (p = 0.002), seed origin (p = 0.006) and water content (p = 0.024) significantly affected the root C:P ratio (Table S4). The root C:P ratio decreased as SWC increasing. Endophyte decreased the root XH(W) DHG C:P ratio at 15% and 30% SWC significantly (p < 0.05) by 21.02% and 25.57%. The root C:P ratio of YZ(D)EI DHG was significantly lower than YZ(D)EF DHG at 30% SWC (p < 0.05). YZ(D) DHG had a lower root C:P ratio than XH(W) DHG significantly (p < 0.05), with a relative decrease of 10.22%. The root C:P ratio of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 15% and 30% SWC by 21.26% and 18.89% (Figure 11).
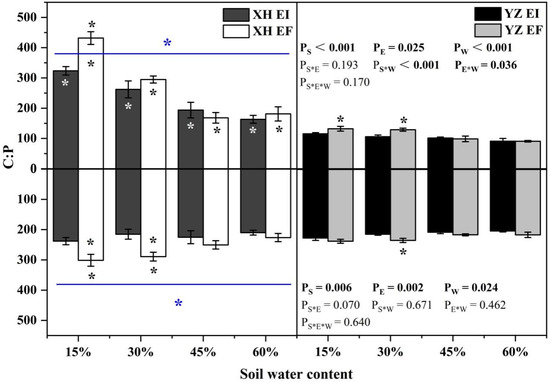
Figure 11.
The C:P content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content. Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the dark gray/white bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
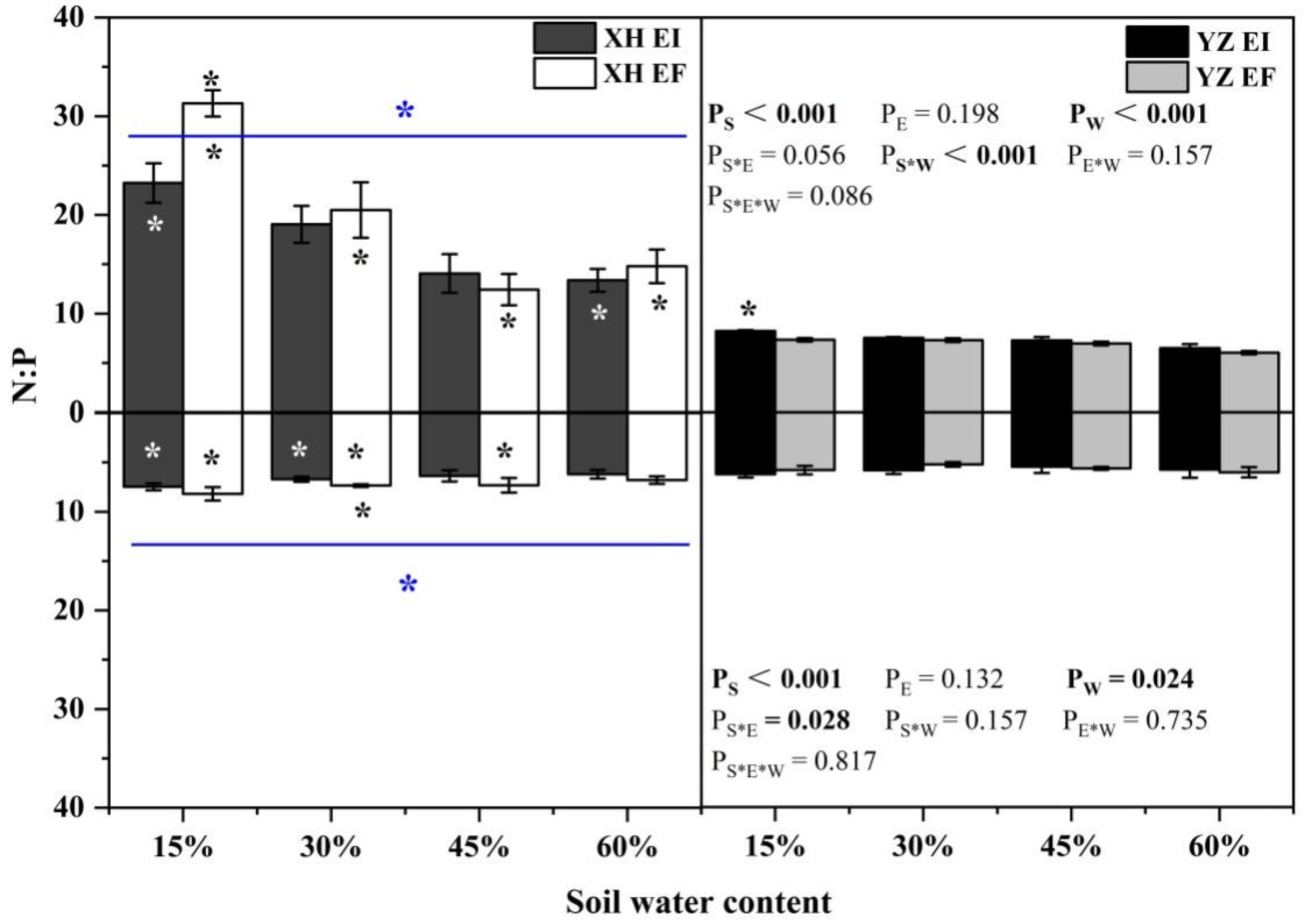
The N:P ratio of shoots was significantly affected by seed origin (p < 0.001), and water content (p < 0.001), and the interaction between seed origin and water content (p < 0.001) was significantly affected (Table S4). Endophyte decreased the XH(W) DHG N:P ratio of shoots at 15% SWC significantly (p < 0.05). However, the shoot N:P ratio of EI DHG was higher than EF DHG from the YZ(D) site significantly (p < 0.05) at 15% SWC, with an increase of 12.08%. In addition, YZ(D) DHG had a significantly lower shoot N:P ratio than XH(W) DHG (p < 0.05). Importantly, the N:P ratio of shoots of YZ(D)EI DHG was lower than XH(W)EI DHG at 15% and 60% SWC significantly (p < 0.05), with a decrease of 64.59% and 51.33%, respectively. The shoot N:P ratio of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 15% to 60% SWC, with a relative decrease of 76.55%, 64.32%, 43.94%, and 59.18%, respectively (Figure 12). Seed origin (p < 0.001), water content (p = 0.024) and seed origin × endophyte (p = 0.028) significantly affected the root N:P ratio (Table S4). The root N:P ratio of XH(W)EI DHG was lower than XH(W)EF DHG at 30% SWC significantly (p < 0.05) by 8.83%. In addition, YZ(D) DHG had a significantly lower root N:P ratio than XH(W) DHG (p < 0.05). Further, the root N:P ratio of YZ(D)EI DHG was lower than XH(W)EI DHG significantly (p < 0.05) at 15% and 30% SWC, with a decrease of 16.13% and 12.67%. The root N:P ratio of YZ(D)EF DHG was also lower than XH(W)EF DHG (p < 0.05) at 15% to 45% SWC, with a relative decrease of 28.59%, 28.37%, and 22.60%, respectively (Figure 12).
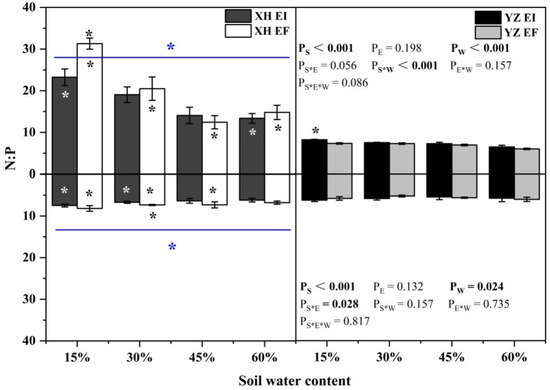
Figure 12.
The N:P content of A. inebrians seedlings shoots and roots from XH(W) and YZ(D) seeds under various soil water content. Upwards and downwards bars represent the value of shoot and root part, respectively. The asterisk (*) above/below error bars means significant difference (p < 0.05) between EI and EF under the same soil water content. White/black * in the dark gray/white bars indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type of EF/EI grasses. The blue * above/below full blue line indicates significant difference (p < 0.05) between Xiahe and Yuzhong maternal habitat type.
4. Discussion
The present study showed the Epichloë endophyte improved the drought stress tolerance of DHG. The growth, photosynthetic efficiency, phytohormones content and C, N and P content of EI DHG was higher than the EF DHG under water deficiency conditions. Moreover, DHG from the seeds of the YZ(D) site is more competitive than from those of the XH(W) site under drought conditions. The differences of the shoots biomass, photosynthetic rate and P content of shoots between the EI plants from the seed of the two sites is higher than the differences between the EF plants of the two sites. On the contrary, the differences of the CtK content of shoots between the EI plants from the two sites are lower than the differences between the EF plants of the two sites. A positive stimulus of maternal drought tolerance on progeny plant growth at certain conditions through the symbiotic association with Epichloë endophyte was found in the present study.
Previous studies have illustrated that Epichloë endophytes can improve the drought resistance of host plant, as revealed through the improved growth and biomass of host [51]. In the present study, EI plants had more shoot dry biomass than EF plants at drought stress, which means more photosynthetic products accumulated in plants to enhance survival through the adverse growth conditions [52]. Root mass and efficiency, as the most vital water-absorbing organ, is extremely important for maintaining plant growth, especially in arid environments [53]. Root dry biomass of EI plants was also higher than EF plants, and this is similar to several early studies that have reported that the Epichloë endophyte could increase root dry matter, improving water uptake from the soil to enhance drought resistance [54]. However, the results of previous studies of the effects of Epichloë endophytes about growth indicators were equivocal. Some studies suggest that these endophytes can promote the growth of symbionts under drought stress [55,56], while other results indicate that endophytes have no effect upon host growth [57,58], and even have a negative effect, inhibiting the growth of symbionts [59,60].
Phytohormones play central roles in integrating various environmental cues with endogenous growth programs, which can regulate various aspects of plant growth and development, as well as the responses of plants to abiotic and biotic stresses [37]. ABA is the most studied phytohormone; its synthesis is one of the fastest responses of plants to abiotic stress [61]. ABA is involved in the synthesis of stress-induced proteins to help in maintaining cellular water status and to protect other proteins, enzymes and cellular organelles from collapsing under water stress [62]. In the present experiment, the Epichloë endophyte significantly increased the ABA content in leaves and roots at different water contents. Therefore, it is speculated that these endophytes can improve the drought tolerance of the host by promoting the synthesis and accumulation of ABA in host grasses [38,39]. IAA participates in drought stress tolerance in various physiological activities to regulate plant growth and development [39,63]. Its content increased in various plant organs subjected to water deficit [64], which promoted water uptake into the protoplasts [65]. A previous study has found that an Epichloë endophyte can increase IAA content in symbionts to enhance the growth and development of host grasses under drought stress conditions [66]. In the present study, similar results were obtained, which were based on the results of growth indicators of host grasses. The upregulation of IAA content may be one of the methods that Epichloë endophytes improve the drought resistance of host grasses. However, these adjustments were not accomplished independently by one or two hormones but by crosstalk between the different phytohormones that resulted in synergetic or antagonistic interactions [35]. CtK could down-regulate ABA content [67,68]. The elevated drought resistance of plants may be attributed to the expression of the majority of CtK genes being down-regulated and a reduction in CtK levels when the plants were exposed to water-limiting conditions [69]. Early research has shown that Epichloë endophytes decreased the CtK content under drought stress [38]. This is similar to the present results.
In addition to the above-mentioned effects, phytohormones also significantly affect photosynthesis through regulating stomata, such as ABA involvement with the closure of stomata [70]. The CtK triggers responses to delay stomatal closure or stimulate stomatal opening [37,71]. Photosynthesis is a fundamental process of converting atmospheric carbon dioxide (CO2) into organic compounds in the leaves of green plants. This process is mainly affected by light intensity, water, carbon dioxide concentration and photosynthetic pigments, which is limited by various factors such as carboxylation capacity and stomatal conductance [34]. Previous studies on the drought resistance of tall fescue (Festuca arundinacea) and meadow fescue (F. pratensis) found that endophyte can promote stomatal closure of host grasses and effectively maintain plant water. Lower stomatal conductance enables the plants to have a strong competitiveness in the process of drought tolerance [72,73]. However, stomatal closure can also negatively affect plant growth rate since it reduces carbon dioxide (CO2) uptake, which is closely related to the efficiency of photosynthesis [74]. The present research indicated the Epichloë endophytes significantly increased the stomatal conductance of DHG. Higher stomatal conductance is beneficial to the exchange of CO2 and increases photosynthetic efficiency, a key determinant of plant growth under drought stress [75]. It was a possible function fulfilled by Epichloë endophytes to change host plant metabolism process from defense to growth and reproduction by allowing the plant to use limited resources for photosynthesis and C assimilation. The shift from defense to growth results in increasing growth rates, early flowering, and the allocation of more resources to reproduction to maximize resource use while water is available, which enables plants to finish seed production in maximize fitness [76]. The C assimilation is primarily by photosynthesis [77]. The Epichloë endophyte increased the photosynthetic capacity of the host grass under different conditions [31,38], thereby increasing the C concentration of the host. Comparing EI and EF DHG in the present study, the significant difference of C concentration only appeared at severe drought stress condition (15% SWC), but EI DHG had a higher C concentration than EF DHG at the other SWC. Although the Epichloë endophyte did not significantly increase the C concentration, the EI DHG had a greater biomass than EF DHG. Therefore, EI plants generally contained more C content, a consequence of the increase in the photosynthetic capacity from the presence of the Epichloë endophyte. N and P assimilation occurs by nutrient uptake in soil, which is different from C. The ability of plants to absorb and transport nutrients through the root system was positive correlated with plant transpiration [40,78]. Plants reduce water loss after the sensing of soil water deficit through changed stomatal conductance by the control of stomatal behavior [70]; this occurrence then leads to a decrease in the absorption of N and P from soil. The present experimental results confirmed these inferences that drought stress significantly inhibited the N and P assimilation of DHG by significantly reducing the transpiration of DHG. The presence of the Epichloë endophyte increased the photosynthetic ability and transpiration rate under water deficit stress, which provided energy for the uptake and transportation of N and P [40,78]. Moreover, previous research has indicated that EI plants had more phosphate-solubilizing fungal (PSF) diversity in their rhizosphere soil [79] or secreted more acid phosphatase by roots than of EF plants [80]. The positive association between a higher PSF diversity and acid phosphatase secretion by roots, and P-solubilization activity in the soil would generate an increase in P available for plant assimilation [81]. Thus, the Epichloë endophyte also makes a contribution for host plant P absorption by improving the P pool in the rhizosphere indirectly, making P available to the host plant [82]. This could be another reason why EI DHG had higher P concentration than EF DHG in the present study.
The element N is an important component of alkaloids [83,84,85]. The increase in N concentration may be related to the alkaloid synthesis of the host. Epichloë endophytes confer protection for the host grass against various stresses from the production of alkaloids [86]. Higher ergonovine alkaloids and ergine alkaloids levels were recorded for DHG under drought stress [87]. Previous research has also indicated that proline in the symbionts can be converted into precursor compounds of ergot alkaloids or loline under drought stress [88,89]. Therefore, changes in the content of alkaloid can also be regarded as an effective way of osmotic regulation to protect plants under water deficit stress [90]. P is an essential element for plant growth [91]. As we know, rRNA is needed to satisfy the demand of protein synthesis for enhancing the growth ability or/and improving various stress resistances [92,93]. Varying P levels in organisms may be partly caused by the allocation of P to the rRNA. Thus, increased N and P content may be one of the causes why the Epichloë endophyte enhanced the growth ability of host plants under water deficient condition.
Plant growth rate has intrinsic linkage with tissue elemental stoichiometry [40]. The growth rate hypothesis (GRH) indicates that higher growth rates are related to lower C:N and C:P ratios [94]. The health and growth status of plants could be reflected by the ratio of C:N and C:P [93]. The present study indicated that the ratios of C:N and C:P of plants were significantly higher under drought stress, and EF DHG had higher ratios of C:N and C:P. These results could explain why drought stress significantly reduced the growth rate of plants, and the Epichloë endophyte effectively alleviated the effect of drought on the growth of the host plant. Additionally, it is possible that the lower C:N ratio was caused by the increased synthesis of N-containing metabolites under drought stress. It may be one of the adaptive strategies of host plants to the drought conditions [95]. The N:P ratio is a vital indicator to identify limiting nutrients during the growth process [94]. In the present study, N:P increased with the decrease in soil water content, which indicated that plant growth is more susceptible to P deficiency under drought stress, and the Epichloë endophyte alleviated the growth restriction caused by P deficiency. This is associated with the Epichloë endophyte indirectly contributing to plant nutrition absorption through increasing P available to plants by increasing the diversity of phosphate-solubilizing fungi [79].
Previous research had indicated that the effects of Epichloë endophytes upon the drought resistance of host plants were uncertain, sometimes dependent on the genotype of both host plant and endophyte and environmental conditions [43]. In the present study, although all the experimental DHG plants were originally from the same population, after their maternal plants were grown for two years in different environmental conditions, the drought resistance of their progeny started to be different. All of the growth and photosynthetic indexes of YZ(D) DHG were better under each treatment than XH(W) DHG. Additionally, a lower CtK content and higher ABA content of YZ(D) DHG indicated a faster response to water deficit and a stronger sensitivity level than XH(W) DHG [72]. TGE, as a mechanism for plants to cope with abiotic and biotic factors of stress, has been intensively reviewed [45]. The differences of the shoot biomass, photosynthetic rate and shoot P content between the EI plants from the two sites is higher than the differences between the EF plants of the two sites, while the CtK content is the opposite. All of these are evidence of a TGE in response to the different SWC of parental plants to stress resulting from the previous experience of water deficit [45]. These results indicated the Epichloë endophyte may be involved in host plant TGE by regulating the above-mentioned parameters due to its vertical transmission by seeds [47]. The Epichloë endophyte may be a carrier to transfer information and/or some substances from maternal plants to progeny to mediate the TGEs.
5. Conclusions
The present study found that the presence of an Epichloë endophyte improved the drought stress of host plants by maintaining the growth of the plant, improving photosynthesis, promoting nutrient absorption, and adjusting the metabolism of phytohormones. The results also indicated that the DHG grown from the seeds of plants in the relatively dry site are more competitive under water deficiency conditions than those from seeds of plants from the relatively wet site. Importantly, the difference of EI DHG from the two sites is higher than the differences between the EF plants of the two sites, which confirms the presence of TGEs in DHG, and the vertically transmitted endophyte mediates TGEs via above-mentioned indicators. Nonetheless, more studies are needed to totally identify and understand the mechanisms underlying the Epichloë endophyte mediated TGEs in plants, particularly in relation to whether they are widening the capacity of plants to deal with more than one factor of stress at once. All the present and prospective findings would help to understand the mechanisms why EI DHG has selective advantage in arid grassland ecosystems, and how to make more use of Epichloë endophyte attributes to breeding new drought-resistant forage germplasms for arid and semiarid regions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture12060761/s1, Table S1: Results of univariate analysis of variance for the interaction effects of habitat type of Parent (H), endophyte (E) and water stress (W) on Biomass, ABA, CtK and IAA content of A. inebrians. Table S2: Results of univariate analysis of variance for the interaction effects of habitat type of Parent (H), endophyte (E) and water stress (W) on Chlorophyll content, Net photosynthetic rate, Stomatal conductance, Intercellular carbon dioxide concentration and Transpiration rate of A. inebrians. Table S3: Results of univariate analysis of variance for the interaction effects of habitat type of Parent (H), endophyte (E) and water stress (W) on C, N and P content of A. inebrians. Table S4: Results of univariate analysis of variance for the interaction effects of habitat type of Parent (H), endophyte (E) and water stress (W) on C:N, C:P and N:P of A. inebrians.
Author Contributions
Conceptualization, X.Z., Z.N. and C.X.; Formal analysis, C.X.; Funding acquisition, X.Z., Z.N. and C.X.; Methodology, X.C., L.S. and C.X.; Project administration, Z.N.; Software, X.C.; Writing—original draft, X.C. and L.S.; Writing—review and editing, X.C., X.Z., M.J.C., Z.N. and C.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Nature Science Foundation of China (32001387 and 31772665), the National Basic Research Program of China (2014CB138702), Lanzhou University “Double First-Class” guiding special project-team construction fund-scientific research start-up fee standard (561119208).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Lowman, S.; Kim-Dura, S.; Mei, C.; Nowak, J. Strategies for enhancement of switchgrass (Panicum virgatum L.) performance under limited nitrogen supply based on utilization of N-fixing bacterial endophytes. Plant Soil 2016, 405, 47–63. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Epichloë (formerly Neotyphodium) fungal endophytes increase adaptation of cool-season perennial grasses to environmental stresses. Acta Agrobot. 2019, 72, 1767. [Google Scholar] [CrossRef]
- Hallasgo, A.M.; Spangl, B.; Steinkellner, S.; Hage-Ahmed, K. The fungal endophyte Serendipita williamsii does not affect phosphorus status but carbon and nitrogen dynamics in arbuscular mycorrhizal tomato plants. J. Fungi 2020, 6, 233. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium speices with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Simpson, W.R.; Koolaard, J.P.; Nickless, E.M.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Müller, C.B.; Krauss, J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005, 8, 450–456. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef]
- Hennessy, L.M.; Popay, A.J.; Glare, T.R.; Finch, S.C.; Cave, V.M.; Rostás, M. Olfactory responses of Argentine stem weevil to herbivory and endophyte-colonisation in perennial ryegrass. J. Pest Sci. 2022, 95, 263–277. [Google Scholar] [CrossRef]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Li, X.Z.; Nan, Z.B. Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J. Agric. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.C.; Gundel, P.E.; Molina-Montenegro, M.A.; Ramos, P.; Ghersa, C.M. Getting ready for the ozone battle: Vertically transmitted fungal endophytes have transgenerational positive effects in plants. Plant Cell Environ. 2021, 44, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Saedi, T.; Mosaddeghi, M.R.; Sabzalian, M.R.; Zarebanadkouki, M. Effect of Epichloë fungal endophyte symbiosis on tall fescue to cope with flooding-derived oxygen-limited conditions depends on the host genotype. Plant Soil 2021, 468, 353–373. [Google Scholar] [CrossRef]
- Johnson, L.J.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; Tapper, B.A. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Becker, M.; Becker, Y.; Green, K.; Scott, B. The endophytic symbiont Epichloë festucae establishes an epiphyllous net on the surface of Lolium perenne leaves by development of an expressorium, an appressoriumdophytic syexit structure. New Phytol. 2016, 211, 240–254. [Google Scholar] [CrossRef]
- Soto-Barajas, M.C.; Zabalgogeazcoa, I.; Gómez-Fuertes, J.; GonzálezBlanco, V.; Vázquez-De-Aldana, B.R. Epichloë endophytes affect the nutrient and fiber content of Lolium perenne, regardless of plant genotype. Plant Soil. 2016, 405, 65–277. [Google Scholar] [CrossRef]
- Nan, Z.B.; Li, C.J. Neotyphodium in native grasses in China and observations on endophyte/host interactions. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000. [Google Scholar]
- Li, C.J.; Nan, Z.B.; Liu, Y. Methodology of endophyte detection of drunken horse grass. Edible Fungi China 2008, 27, 16–19. [Google Scholar]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef]
- Zhang, X.X.; Nan, Z.B.; Li, C.J.; Gao, K. Cytotoxic effect of ergot alkaloids in Achnatherum inebrians infected by the Neotyphodium gansuense endophyte. J. Agric. Food Chem. 2014, 62, 7419–7422. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.C.; Li, C.J.; Nan, Z.B.; Li, F.D. Effects of feeding drunken horse grass infected with Epichloë gansuensis endophyte on animal performance, clinical symptoms and physiological parameters in sheep. BMC Vet. Res. 2017, 13, 223. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, X.X.; Christensen, M.J.; Nan, Z.B.; Li, C.J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Kou, M.Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.B.; Zhang, X.X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Li, X.Z.; White, J.F.; Wei, X.K.; Li, C.J. Epichloë endophyte improves ergot disease resistance of host (Achnatherum inebrians) by regulating leaf senescence and photosynthetic capacity. J. Plant Growth Regul. 2021, 41, 808–817. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B.; Matthew, C. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res. 2012, 52, 70–78. [Google Scholar] [CrossRef]
- He, Y.L.; Chen, T.X.; Zhang, H.J.; White, J.F.; Li, C.J. Fungal endophytes help grasses to tolerate sap-sucking herbivores through a hormone-signaling system. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, R.L.; Chai, Q.; Li, C.J.; Nan, Z.B. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.F.; Hou, W.P.; Malik, K.; Zhao, C.Z.; Niu, X.L.; Liu, Y.L.; Huang, R.; Li, C.J.; Nan, Z.B. Elucidating the molecular mechanisms by which seed-borne endophytic fungi, Epichloë gansuensis, increases the tolerance of Achnatherum inebrians to NaCl stress. Int. J. Mol. Sci. 2021, 22, 13191. [Google Scholar] [CrossRef]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, X.X.; Nan, Z.B. The fungal endophyte Epichloë gansuensis increases NaCl-tolerance in Achnatherum inebrians through enhancing the activity of plasma membrane H+-ATPase and glucose-6-phosphate dehydrogenase. Sci. China Life Sci. 2021, 64, 452–465. [Google Scholar] [CrossRef]
- Xia, C.; Christensen, M.J.; Zhang, X.X.; Nan, Z.B. Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Lee, K.; Missaoui, A.; Mahmud, K.; Presley, H.; Lonnee, M. Interaction between grasses and Epichloë endophytes and its significance to biotic and abiotic stress tolerance and the rhizosphere. Microorganisms 2021, 9, 2186. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Yu, J.J.; Han, L.B.; Huang, B.R. Photosynthetic enzyme activities and gene expression associated with drought tolerance and post-drought recovery in Kentucky bluegrass. Environ. Exp. Bot. 2013, 89, 28–35. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Tsuda, K.; Affiliations, A.I. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Xu, W.B.; Li, M.M.; Lin, W.H.; Nan, Z.B.; Tian, P. Effects of Epichloë sinensis endophyte and host ecotype on physiology of festuca sinensis under different soil moisture conditions. Plants 2021, 10, 1649. [Google Scholar] [CrossRef]
- Zhao, Z.R.; Kou, M.Z.; Zhong, R.; Xia, C.; Christensen, M.J.; Zhang, X.X. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by Epichloë gansuensis in Achnatherum inebrians under different soil moisture availability. J. Fungi 2021, 7, 640. [Google Scholar] [CrossRef]
- Song, M.L.; Li, X.Z.; Saikkonen, K.; Li, C.J.; Nan, Z.B. An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.; Li, K.; Liu, Y.; Gong, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Gundel, P.E.; Rudgers, J.A.; Whitney, K.D. Vertically transmitted symbionts as mechanisms of transgenerational effects. Am. J. Bot. 2017, 104, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhou, M.; Lin, Z.; Li, Q.Q.; Zhang, Y.Y. Transgenerational effects benefit offspring across diverse environments: A meta-analysis in plants and animals. Ecol. Lett. 2019, 22, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.J.; Sultan, S.E. Adaptive transgenerational plasticity in plants: Case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef]
- Gundel, P.E.; Rudgers, J.A.; Ghersa, C.M. Incorporating the process of vertical transmission into understanding of host-symbiont dynamics. Oikos 2011, 120, 1121–1128. [Google Scholar] [CrossRef]
- Gundel, P.E.; Sorzoli, N.; Ueno, A.C.; Ghersa, C.M.; Seal, C.E.; Bastías, D.A.; Martínez-Ghersa, M.A. Impact of ozone on the viability and antioxidant content of grass seeds is affected by a vertically transmitted symbiotic fungus. Environ. Exp. Bot. 2015, 113, 40–46. [Google Scholar] [CrossRef]
- Ye, W.; Hu, S.; Wu, L.; Ge, C.; Cui, Y.; Chen, P.; Xu, J.; Dong, G.; Guo, L.; Qian, Q. Fine mapping a major QTL qFCC7L for chlorophyll content in rice (Oryza sativa L.) cv. PA64s. Plant Growth Regul. 2017, 81, 81–90. [Google Scholar] [CrossRef]
- Tanveer, S.K.; Zhang, J.L.; Lu, X.L.; Wen, X.X.; Wei, W.; Yang, L.; Liao, Y.C. Effect of corn residue mulch and N fertilizer application on nitrous oxide (N2O) emission and wheat crop productivity under rain-fed condition of loess plateau China. Int. J. Agric. Biol. 2014, 16, 505–512. [Google Scholar]
- Gundel, P.E.; Irisarri, J.G.N.; Fazio, L.; Casas, C.; Pérez, L.I. Inferring field performance from drought experiments can be misleading: The case of symbiosis between grasses and Epichloë fungal endophytes. J. Arid Environ. 2016, 132, 60–62. [Google Scholar] [CrossRef]
- Ren, A.Z.; Gao, Y.B.; Wang, W.; Wang, J.L. Photosynthetic pigments and photosynthetic products of endophyte-infected and endophyte-free Lolium perenne L. under drought stress conditions. Front. Biol. China 2006, 1, 168–173. [Google Scholar] [CrossRef]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an auxin response factor in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- West, C.P.; Izekor, E.; Turner, K.E.; Elmi, A.A. Endophyte effects on growth and persistence of tall fescue along a water-supply gradient. Agron. J. 1993, 85, 264–270. [Google Scholar] [CrossRef]
- Elmi, A.A.; West, C.P. Endophyte infection affects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytol. 1995, 131, 61–67. [Google Scholar] [CrossRef]
- Belesky, D.P.; Stringer, W.C.; Plattner, R.D. Influence of endophyte and water regime upon tall fescue accessions. Ann. Bot. 1989, 64, 343–349. [Google Scholar] [CrossRef]
- Belesky, D.P.; Fedders, J.M. Tall fescue development in response to Acremonium coenophialum and soil acidity. Crop Sci. 1995, 35, 529–533. [Google Scholar] [CrossRef]
- Assuero, S.G.; Matthew, C.; Kemp, P.D.; Latch, G.C.M.; Barker, D.J.; Haslett, S.J. Morphological and physiological effects of water deficit and endophyte infection on contrasting tall fescue cultivars. N. Z. J. Agron. Res. 2000, 43, 49–61. [Google Scholar] [CrossRef][Green Version]
- Faeth, S.H.; Sullivan, T.J. Mutualistic asexual endophytes in a native grass are usually parasitic. Am. Nat. 2003, 161, 310–325. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing rhizobium strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef] [PubMed]
- Pustovoitova, T.N.; Zhdanova, N.E.; Zholkevich, V.N. Changes in the Levels of IAA and ABA in Cucumber Leaves under Progressive Soil Drought. Russ. J. Plant Physiol. 2004, 51, 513–517. [Google Scholar] [CrossRef]
- Zholkevich, V.N.; Gusev, N.A.; Kaplya, A.V.; Pakhomova, T.I.; Pil’shikova, N.V.; Samuiliv, F.D.; Slavnii, P.S.; Shmat’ko, I.G. Water Metabolism in Plants; Tarchevskii, I.A., Zholkevich, V.N., Eds.; Nauka: Moscow, Russia, 1989. [Google Scholar]
- De Battista, J.P.; Bouton, J.H.; Bacon, C.W.; Siegel, M.R. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Agron. J. 1990, 82, 651–654. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Acharya, B.R.; Assmann, S.M. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Elbersen, W.W.; West, C.P. Growth and water relations of field-grown tall fescue as influenced by drought and endophytes. Grass Forage Sci. 1996, 51, 333–342. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Leuchtmann, A.; Schmidt, D.; Nösberger, J. Symbiosis with Neotyphodium uncinatum endophyte may increase the competitive ability of meadow fescue. Agron. J. 1997, 89, 833–839. [Google Scholar] [CrossRef]
- Manzur, M.E.; Garello, F.A.; Omacini, M.; Schnyder, H.; Sutka, M.R.; García-Parisi, P.A. Endophytic fungi and drought tolerance: Ecophysiological adjustment in shoot and root of an annual mesophytic host grass. Funct. Plant Biol. 2022, 49, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Davitt, A.J.; Chen, C.; Rudgers, J.A. Understanding context-dependency in plant–microbe symbiosis: The influence of abiotic and biotic contexts on host fitness and the rate of symbiont transmission. Environ. Exp. Bot. 2011, 71, 137–145. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Arrieta, A.M.; Iannone, L.J.; Scervino, J.M.; Vignale, M.V.; Novas, M.V. A foliar endophyte increases the diversity of phosphorus-solubilizing rhizospheric fungi and mycorrhizal colonization in the wild grass Bromus auleticus. Fungal. Ecol. 2015, 17, 146–154. [Google Scholar] [CrossRef]
- Hou, W.P.; Wang, J.F.; Nan, Z.B.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, X.X.; Niu, X.L. Epichloë gansuensis endophyte-infection alters soil enzymes activity and soil nutrients at different growth stages of Achnatherum inebrians. Plant Soil 2020, 455, 227–240. [Google Scholar] [CrossRef]
- Li, X.; Ren, A.Z.; Han, R.; Yin, L.J.; Wei, M.Y.; Gao, Y.B. Endophyte mediated effects on the growth and physiology of Achnatherum sibiricum are conditional on both N and P availability. PLoS ONE 2012, 7, e48010. [Google Scholar]
- Zaidi, A.; Khan, M.S. Stimulatory effects of dual inoculation with phosphate solubilising microorganisms and arbuscular mycorrhizal fungus on chickpea. Aust. J. Exp. Agric. 2007, 47, 1016–1022. [Google Scholar] [CrossRef]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommonmutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Nagabhyru, P.; Dinkins, R.D.; Wood, C.L.; Bacon, C.W.; Schardl, C.L. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol. 2013, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-de-Aldana, B.R.; García-Ciudad, A.; García-Criado, B.; Vicente-Tavera, S.; Zabalgogeazcoa, I. Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS ONE 2013, 8, e84539. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of salt and drought stress on alkaloid production in endophyte-infected drunken horse grass (Achnatherum inebrians). Biochem. Syst. Ecol. 2011, 39, 471–476. [Google Scholar] [CrossRef]
- Schardl, C.; Young, C.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, J.D.; Houseknecht, J.B.; Pal, S.; Bush, L.P.; Grossman, R.B.; Schardl, C.L. Biosynthetic precursors of fungal pyrrolizidines, the loline alkaloids. ChemBioChem 2005, 6, 1016–1022. [Google Scholar] [CrossRef]
- Bacon, C.W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agric. Ecosyst. Environ. 1993, 44, 123–141. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smish, A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Elser, J.J.; Sterner, R.; Gorokhova, E.; Fagan, W.; Markow, T.; Cotner, J.; Harrison, J.; Hobbie, S.; Odell, G.; Weider, L. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Hessen, D.O.; Jensen, T.C.; Kyle, M.; Elser, J.J. RNA responses to N- and P-limitation; reciprocal regulation of stoichiometry and growth rate in Brachionus. Funct. Ecol. 2007, 21, 956–962. [Google Scholar] [CrossRef]
- Elser, J.J.; Hamilton, A. Stoichiometry and the new biology: The future is now. PLoS Biol. 2007, 5, e181. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.X.; Richard, J.; Chen, S.H.; Lv, H.; Zhou, J.L.; Li, C.J. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).