Effects of Metal Oxide Nanoparticles on Nitrous Oxide Emissions in Agriculture Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Soil and Nanoparticles
2.2. Microcosm Setup
2.3. N2O Measurement
2.4. Soil NH4+, NO3−, and DOC Measurement
2.5. DNA Extraction and Real-Time PCR Quantification
2.6. Statistical Analysis
3. Results
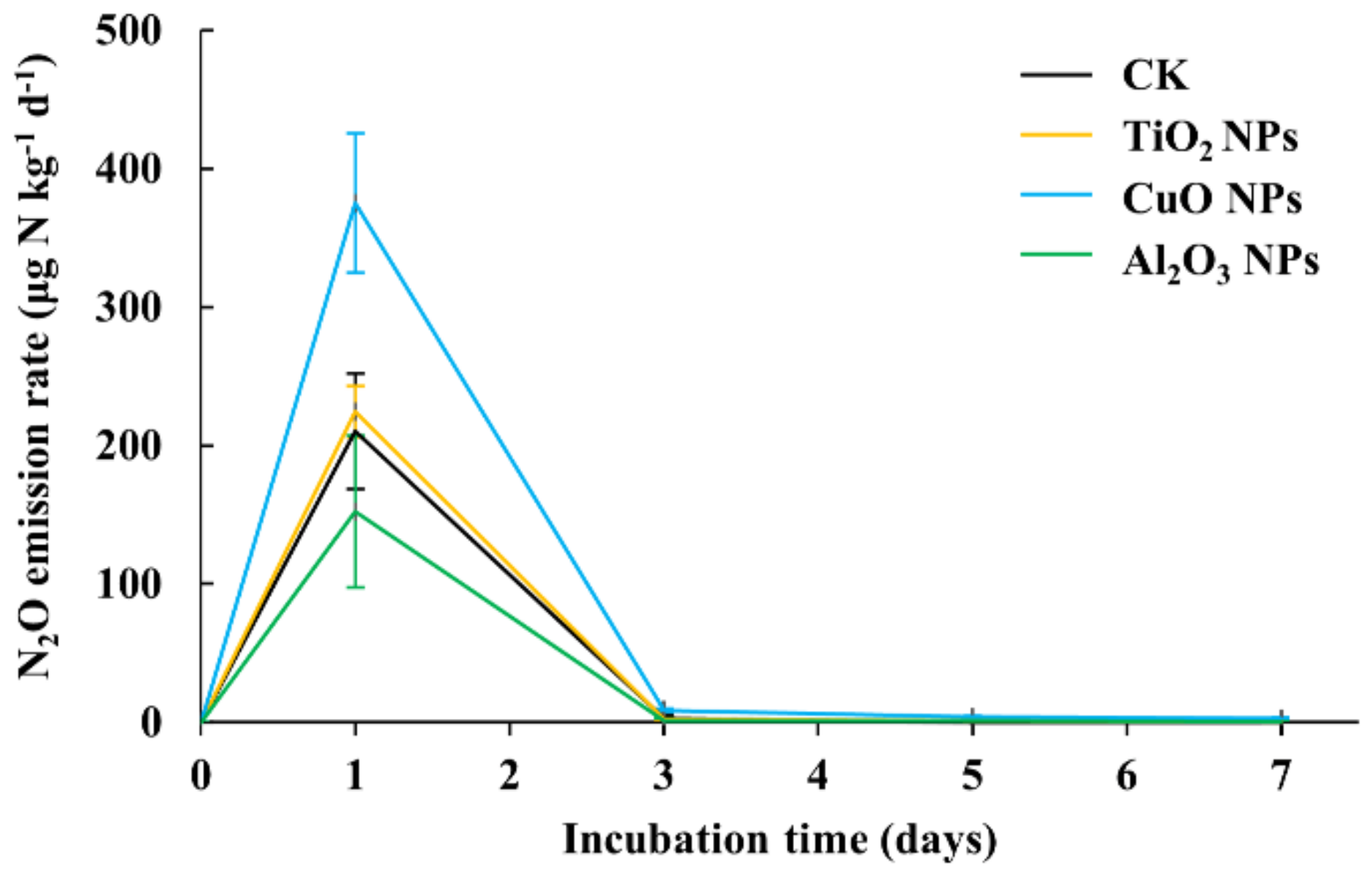
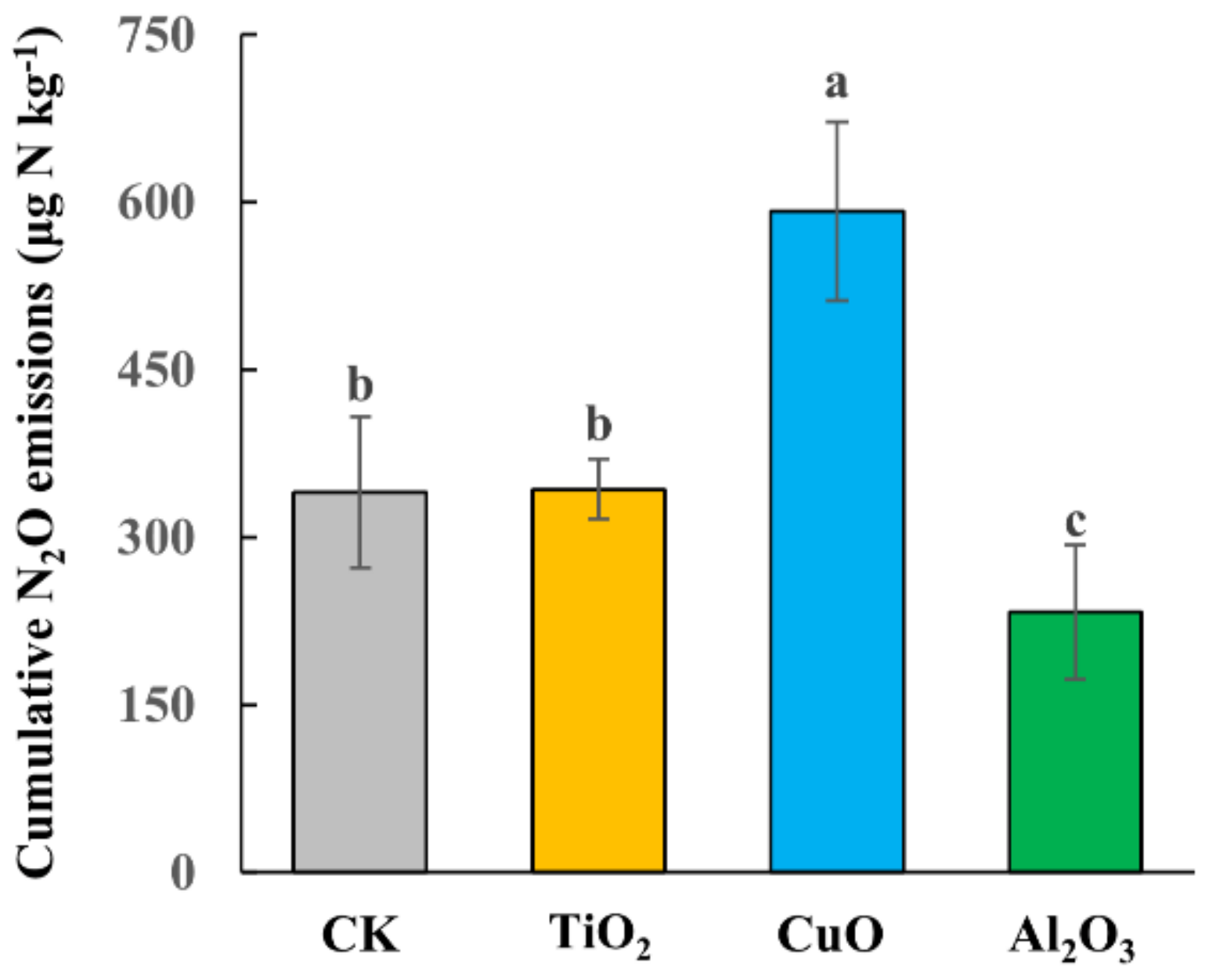
3.1. N2O Emissions Affected by Different Nanoparticles
3.2. Effects of Different Nanoparticles on Soil DOC, NH4+, and NO3− Contents
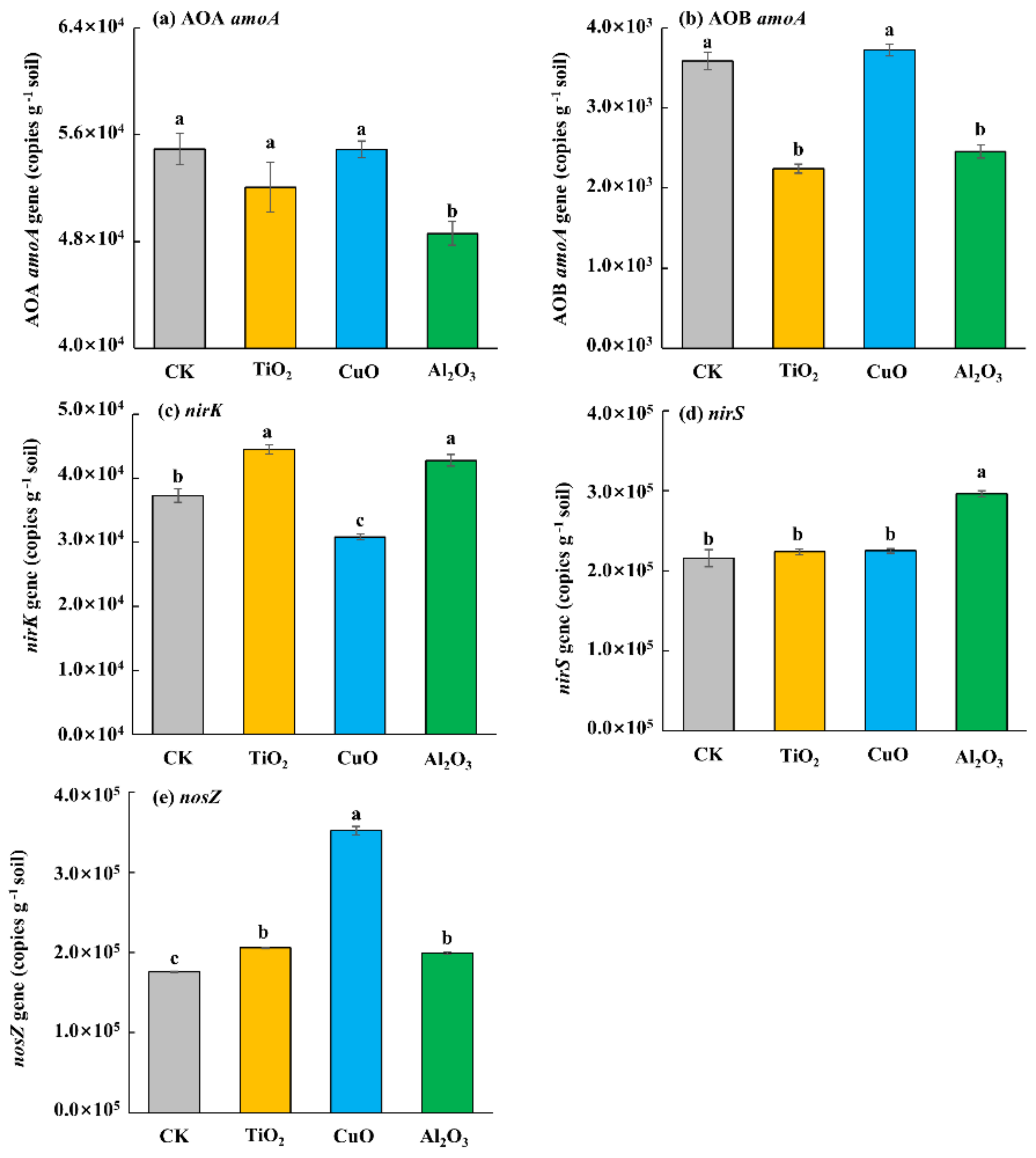
3.3. Effects of Nanoparticles on Soil Microbial Functional Gene Abundance
3.4. Correlation Analysis of Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial Production Quantities and Uses of Ten Engineered Nanomaterials in Europe and the World. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-Based Nanotoxicity and Detoxification Pathways in Higher Plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Metal Based Nanoparticles in Agricultural System: Behavior, Transport, and Interaction with Plants. Chem. Spec. Bioavailab. 2018, 30, 123–134. [Google Scholar] [CrossRef] [Green Version]
- D’Amora, M.; Schmidt, T.J.N.; Konstantinidou, S.; Raffa, V.; De Angelis, F.; Tantussi, F. Effects of Metal Oxide Nanoparticles in Zebrafish. Oxid. Med. Cell. Longev. 2022, 2022, 3313016. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mat. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for Negative Effects of TiO2 and ZnO Nanoparticles on Soil Bacterial Communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in Plant Protection and Fertilization: Current State, Foreseen Applications, and Research Priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Wang, L.; Giesy, J.P. Al2O3 Nanoparticles Promote Secretion of Antibiotics in Streptomyces Coelicolor by Regulating Gene Expression through the Nano Effect. Chemosphere 2019, 226, 687–695. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in Agriculture: Current Status, Challenges and Future Opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Bouguerra, S.; Gavina, A.; Natal-da-Luz, T.; Sousa, J.P.; Ksibi, M.; Pereira, R. The Use of Soil Enzymes Activity, Microbial Biomass, and Basal Respiration to Assess the Effects of Cobalt Oxide Nanomaterial in Soil Microbiota. Appl. Soil Ecol. 2022, 169, 104246. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Ke, X.; Wu, L.; Christie, P. Biological Transfer of Silver under Silver Nanoparticle Exposure and Nitrogen Transfer via a Collembolan-Predatory Mite Food-Chain and Ecotoxicity of Silver Sulfide. Soil Ecol. Lett. 2022, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Dang, D.; Zheng, L.; Wu, L.; Wu, Y.; Li, H.; Yu, Y. Effect of Ag Nanoparticles on Denitrification and Microbial Community in a Paddy Soil. Front. Microbiol. 2021, 12, 785439. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Ali, K.; Lee, J.; Zaidi, A.; Khan, M.S.; Musarrat, J. Nanoparticles in the Soil–Plant System: A Review. Environ. Chem. Lett. 2021, 19, 1545–1609. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Pan, B.; Zheng, X.; Yu, J.; Ding, H.; Zhang, Y. Pathways of Soil N2O Uptake, Consumption, and Its Driving Factors: A Review. Environ. Sci. Pollut. Res. 2022, 29, 30850–30864. [Google Scholar] [CrossRef]
- Ramzan, S.; Rasool, T.; Bhat, R.A.; Ahmad, P.; Ashraf, I.; Rashid, N.; ul Shafiq, M.; Mir, I.A. Agricultural Soils a Trigger to Nitrous Oxide: A Persuasive Greenhouse Gas and Its Management. Environ. Monit. Assess. 2020, 192, 436. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Feng, Z.; Xiao, M.; Ge, T.; Li, Y.; Yao, H. Polyethylene Microplastics Alter the Microbial Functional Gene Abundances and Increase Nitrous Oxide Emissions from Paddy Soils. J. Hazard. Mater. 2022, 432, 128721. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, G.; Ju, X.; Liu, R. How Nitrification-Related N2O Is Associated with Soil Ammonia Oxidizers in Two Contrasting Soils in China? Sci. Total Environ. 2021, 770, 143212. [Google Scholar] [CrossRef]
- Poh, L.S.; Jiang, X.; Zhang, Z.; Liu, Y.; Ng, W.J.; Zhou, Y. N2O Accumulation from Denitrification under Different Temperatures. Appl. Microbiol. Biotechnol. 2015, 99, 9215–9226. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, W. Effect of Metal and Metal Oxide Engineered Nano Particles on Nitrogen Bio-Conversion and Its Mechanism: A Review. Chemosphere 2022, 287, 132097. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ni, J.; Sun, Y.; Xue, L.; Feng, Y.; Yu, Y.; Lin, X.; Yang, L. Different Responses of Soil Microbial Metabolic Activity to Silver and Iron Oxide Nanoparticles. Chemosphere 2016, 147, 195–202. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, L.; Liu, M.; Newell, S.E.; Yin, G.; Yu, C.; Zhang, H.; Li, X.; Gao, D.; Gao, J.; et al. Effects of Silver Nanoparticles on Nitrification and Associated Nitrous Oxide Production in Aquatic Environments. Sci. Adv. 2017, 3, e1603229. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, D.; Zhu, X.; Zheng, X.; Feng, L. Long-Term Effects of Copper Nanoparticles on Wastewater Biological Nutrient Removal and N2O Generation in the Activated Sludge Process. Environ. Sci. Technol. 2012, 46, 12452–12458. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Yi, S.; Li, H.; Chen, X. Effects of Silver Nanoparticles on Denitrification and Associated N2O Release in Estuarine and Marine Sediments. J. Ocean Univ. China 2022, 21, 131–140. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, Y.; Yao, H.; Ge, C. Effect of Zinc Oxide Nanoparticles on Nitrous Oxide Emissions in Agricultural Soil. Agriculture 2021, 11, 730. [Google Scholar] [CrossRef]
- Baker, S.; Volova, T.; Prudnikova, S.V.; Satish, S.; Prasad, M.N.N. Nanoagroparticles Emerging Trends and Future Prospect in Modern Agriculture System. Environ. Toxicol. Pharmacol. 2017, 53, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wu, M.; Kang, G.; Zhu, B.; Li, H.; Hu, F.; Jiao, J. Soil Quality Response to Organic Amendments on Dryland Red Soil in Subtropical China. Geoderma 2020, 373, 114416. [Google Scholar] [CrossRef]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution Kinetics of Macronutrient Fertilizers Coated with Manufactured Zinc Oxide Nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef]
- Kamran, M.; Ali, H.; Saeed, M.F.; Bakhat, H.F.; Hassan, Z.; Tahir, M.; Abbas, G.; Naeem, M.A.; Rashid, M.I.; Shah, G.M. Unraveling the Toxic Effects of Iron Oxide Nanoparticles on Nitrogen Cycling through Manure-Soil-Plant Continuum. Ecotox. Environ. Saf. 2020, 205, 111099. [Google Scholar] [CrossRef]
- Simonin, M.; Guyonnet, J.P.; Martins, J.M.F.; Ginot, M.; Richaume, A. Influence of Soil Properties on the Toxicity of TiO2 Nanoparticles on Carbon Mineralization and Bacterial Abundance. J. Hazard. Mater. 2015, 283, 529–535. [Google Scholar] [CrossRef]
- Beddow, J.; Stolpe, B.; Cole, P.A.; Lead, J.R.; Sapp, M.; Lyons, B.P.; Colbeck, I.; Whitby, C. Nanosilver Inhibits Nitrification and Reduces Ammonia-Oxidising Bacterial but Not Archaeal AmoA Gene Abundance in Estuarine Sediments: Nanosilver Inhibits Estuarine Nitrification. Environ. Microbiol. 2017, 19, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Henry, S.; Baudoin, E.; López-Gutiérrez, J.C.; Martin-Laurent, F.; Brauman, A.; Philippot, L. Quantification of Denitrifying Bacteria in Soils by NirK Gene Targeted Real-Time PCR. J. Microbiol. Meth. 2004, 59, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, Ã.; Hallin, S. Reassessing PCR Primers Targeting NirS, NirK and NosZ Genes for Community Surveys of Denitrifying Bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, H.; Domingo-Félez, C.; Wu, J.; Zhan, M.; Yu, R.; Smets, B.F. Insights into Chronic Zinc Oxide Nanoparticle Stress Responses of Biological Nitrogen Removal System with Nitrous Oxide Emission and Its Recovery Potential. Bioresour. Technol. 2021, 327, 124797. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Richaume, A.; Guyonnet, J.P.; Dubost, A.; Martins, J.M.F.; Pommier, T. Titanium Dioxide Nanoparticles Strongly Impact Soil Microbial Function by Affecting Archaeal Nitrifiers. Sci. Rep. 2016, 6, 33643. [Google Scholar] [CrossRef]
- Zhao, S.; Su, X.; Wang, Y.; Yang, X.; Bi, M.; He, Q.; Chen, Y. Copper Oxide Nanoparticles Inhibited Denitrifying Enzymes and Electron Transport System Activities to Influence Soil Denitrification and N2O Emission. Chemosphere 2020, 245, 125394. [Google Scholar] [CrossRef]
- Guo, J.; Gao, S.-H.; Lu, J.; Bond, P.L.; Verstraete, W.; Yuan, Z. Copper Oxide Nanoparticles Induce Lysogenic Bacteriophage and Metal-Resistance Genes in Pseudomonas aeruginosa PAO1. ACS Appl. Mater. Interfaces 2017, 9, 22298–22307. [Google Scholar] [CrossRef]
- Chen, Y.; Su, Y.; Zheng, X.; Chen, H.; Yang, H. Alumina Nanoparticles-Induced Effects on Wastewater Nitrogen and Phosphorus Removal after Short-Term and Long-Term Exposure. Water Res. 2012, 46, 4379–4386. [Google Scholar] [CrossRef]
- Li, S.; Zhao, C.; Ma, B.; Wang, J.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Dong, J.; Gao, M. Effects of Aluminum Oxide Nanoparticles on the Performance, Extracellular Polymeric Substances, Microbial Community and Enzymatic Activity of Sequencing Batch Reactor. Environ. Technol. 2021, 42, 366–376. [Google Scholar] [CrossRef]
- Fan, F.; Zheng, R.; Liu, S.; Guo, X. Effects of TiO2 Nanoparticles on the Denitrification and N2O Emissions of Marsh Soil. Acta Ecol. Sin. 2021, 41, 6525–6532. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Li, T.; Chen, Q. Response of an Aerobic Denitrifier to Titanium Dioxide Nanoparticles Exposure. Environ. Technol. 2020, 41, 1446–1454. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, D.; Chen, W.; Gao, Y.; Zhou, G.; Zhang, Y.; Zhou, X.; Jin, M.-Q. Response of Aerobic Granular Sludge to the Long-Term Presence of CuO NPs in A/O/A SBRs: Nitrogen and Phosphorus Removal, Enzymatic Activity, and the Microbial Community. Environ. Sci. Technol. 2017, 51, 10503–10510. [Google Scholar] [CrossRef]
- Xu, C.; Peng, C.; Sun, L.; Zhang, S.; Huang, H.; Chen, Y.; Shi, J. Distinctive Effects of TiO2 and CuO Nanoparticles on Soil Microbes and Their Community Structures in Flooded Paddy Soil. Soil Biol. Biochem. 2015, 86, 24–33. [Google Scholar] [CrossRef]
- Avila-Arias, H.; Nies, L.F.; Gray, M.B.; Turco, R.F. Impacts of Molybdenum-, Nickel-, and Lithium- Oxide Nanomaterials on Soil Activity and Microbial Community Structure. Sci. Total Environ. 2019, 652, 202–211. [Google Scholar] [CrossRef]
- Ye, J.; Gao, H.; Wu, J.; Chang, Y.; Chen, Z.; Yu, R. Responses of Nitrogen Transformation Processes and N2O Emissions in Biological Nitrogen Removal System to Short-Term ZnO Nanoparticle Stress. Sci. Total Environ. 2020, 705, 135916. [Google Scholar] [CrossRef]
- Paredes, D.d.S.; Alves, B.J.R.; dos Santos, M.A.; Bolonhezi, D.; Sant’Anna, S.A.C.; Urquiaga, S.; Lima, M.A.; Boddey, R.M. Nitrous Oxide and Methane Fluxes Following Ammonium Sulfate and Vinasse Application on Sugar Cane Soil. Environ. Sci. Technol. 2015, 49, 11209–11217. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xu, C.; Li, Q.; Lin, W. Isotope Signatures of N2O Emitted from Vegetable Soil: Ammonia Oxidation Drives N2O Production in NH4+-Fertilized Soil of North China. Sci. Rep. 2016, 6, 29257. [Google Scholar] [CrossRef]
- Li, Y.; Clough, T.J.; Moinet, G.Y.K.; Whitehead, D. Emissions of Nitrous Oxide, Dinitrogen and Carbon Dioxide from Three Soils Amended with Carbon Substrates under Varying Soil Matric Potentials. Eur. J. Soil Sci. 2021, 72, 2261–2275. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Chen, Z.; Zhou, J.; Zhang, H.; Peng, Z.; Zhai, H.; Pang, Q.; Yang, J. Influence of CuO Nanoparticle with Different Particle Size on Nitrogen Removal and Microbial Community of Anammox Process. Environ. Eng. Sci. 2019, 36, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Rippner, D.A.; Margenot, A.J.; Fakra, S.C.; Aguilera, L.A.; Li, C.; Sohng, J.; Dynarski, K.A.; Waterhouse, H.; McElroy, N.; Wade, J.; et al. Microbial Response to Copper Oxide Nanoparticles in Soils Is Controlled by Land Use Rather than Copper Fate. Environ. Sci. Nano 2021, 8, 3560–3576. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Applications and Implications of Manufactured Nanoparticles in Soils: A Review. Eur. J. Soil Sci. 2012, 63, 437–456. [Google Scholar] [CrossRef]
- Wu, Q.; Shi, J.; Jiang, X.; Wu, H. Regulatory Mechanism of Copper Oxide Nanoparticles on Uptake of Different Species of Arsenic in Rice. Nanomaterials 2021, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Xu, Y.; Yin, Y.; Guo, H.; Du, W. Divergence in Response of Lettuce (Var. Ramosa Hort.) to Copper Oxide Nanoparticles/Microparticles as Potential Agricultural Fertilizer. Environ. Pollut. Bioavail. 2019, 31, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, S.A.; Roco, C.A.; Debenport, S.J.; Anderson, T.R.; Hofmeister, K.L.; Walter, M.T.; Shapleigh, J.P. Metagenomic Analysis Reveals Distinct Patterns of Denitrification Gene Abundance across Soil Moisture, Nitrate Gradients. Environ. Microbiol. 2019, 21, 1255–1266. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers | Pair Primer Sequences | Amplification Condition | Reference |
|---|---|---|---|---|
| AOA- amoA | CrenamoA23f CrenamoA616r | ATGGTCTGGCTWAGACG GCCATCCATCTGTATGTCCA | 95 °C–15 s, 53 °C–45 s, 72 °C–45 s, 83 °C–15 s, 45 cycles | [30] |
| AOB- amoA | amoA-1F amoA-2R | GGGGTTTCTACTGGTGGT CCCCTCKGSAAAGCCTTCTTC | 95 °C–15 s, 54 °C–40 s, 72 °C–45 s, 84 °C–15 s, 45 cycles | [30] |
| nirK | nirK-F1aCu nirK-R3Cu | ATCATGGTSCTGCCGCG GCCTCGATCAGRTTGTGGTT | 95 °C–10 s, 53 °C–45 s, 72 °C–45 s, 86 °C–15 s, 45 cycles | [31] |
| nirS | nirS-Cd3aF nirS-R3cd | GTSAACGTSAAGGARACSGG GASTTCGGRTGSGTCTTGA | 95 °C–15 s, 50 °C–45 s, 72 °C–45 s, 88 °C–15 s, 50 cycles | [32] |
| nosZ | nosZ-F nosZ-1662R | CGYTGTTCMTCGACAGCCAG CGSACCTTSTTGCCSTYGCG | 95 °C–15 s, 50 °C–30 s, 72 °C–30 s, 83 °C–15 s, 55 cycles | [32] |
| Nanoparticles | DOC (mg C kg−1) | NH4+ (mg N kg−1) | NO3− (mg N kg−1) |
|---|---|---|---|
| CK | 47.70 ± 7.47 a | 22.93 ± 0.38 b | 0.43 ± 0.06 a |
| TiO2 NPs | 52.10 ± 1.56 a | 23.90 ± 0.47 ab | 0.45 ± 0.07 a |
| CuO NPs | 47.30 ± 2.05 a | 21.03 ± 0.46 c | 0.45 ± 0.04 a |
| Al2O3 NPs | 30.40 ± 3.34 b | 23.25 ± 0.39 b | 0.25 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Feng, Z.; Yu, Y.; Yao, H. Effects of Metal Oxide Nanoparticles on Nitrous Oxide Emissions in Agriculture Soil. Agriculture 2022, 12, 770. https://doi.org/10.3390/agriculture12060770
Hu L, Feng Z, Yu Y, Yao H. Effects of Metal Oxide Nanoparticles on Nitrous Oxide Emissions in Agriculture Soil. Agriculture. 2022; 12(6):770. https://doi.org/10.3390/agriculture12060770
Chicago/Turabian StyleHu, Lanfang, Ziyi Feng, Yongxiang Yu, and Huaiying Yao. 2022. "Effects of Metal Oxide Nanoparticles on Nitrous Oxide Emissions in Agriculture Soil" Agriculture 12, no. 6: 770. https://doi.org/10.3390/agriculture12060770
APA StyleHu, L., Feng, Z., Yu, Y., & Yao, H. (2022). Effects of Metal Oxide Nanoparticles on Nitrous Oxide Emissions in Agriculture Soil. Agriculture, 12(6), 770. https://doi.org/10.3390/agriculture12060770






