A Comparative Study of Healthy and American Foulbrood-Infected Bee Brood (Apis mellifera L.) through the Investigation of Volatile Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Sampling of Healthy and Infected Brood
2.2. Apparatus
2.3. Pre-Treatment Methodology
2.3.1. Number of Larvae
2.3.2. Presence-Absence of Water
2.3.3. Larvae-Pupae
2.4. Determination of Volatile Compounds
2.4.1. Sample Preparation
2.4.2. Sample Extraction
- Warm-up: the sample was heated to low temperature (40 °C) for 2 min, without passing gas.
- Extraction: the volatiles were isolated by passing He of flow 40 mL min−1 through a glass vial for 40 min, maintaining at the same time the sample temperature at 40 °C. At this stage, the volatile and semi-volatile components of the analyte were collected in the Purge & Trap.
- Removal of moisture from the trap by heating to 100 °C for 2 min.
- Thermal desorption: the trapped components were released by heating the trap at 180 °C for 6 min and simultaneously passing He (40 mL min−1) and transferring on a thermostable (100 °C) transfer line to the gas chromatograph.
- Trap cleaning by heating for 7 min at 200 °C and prepare it for the next sample.
2.4.3. Gas Chromatography (GC) Analysis
2.5. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ash, C.; Priest, F.G.; Collins, M.D. Molecular identification of rRNA group bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Antonie Van Leeuwenhoek 1993, 64, 253–260. [Google Scholar] [CrossRef]
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus Larvae. J. Invetebr. Pathol. 2010, 103, S10–S19. [Google Scholar] [CrossRef]
- Okamoto, M.; Kumagai, M.; Kanamori, H.; Takamatsu, D. Antimicrobial Resistance Genes in Bacteria Isolated From Japanese Honey, and Their Potential for Conferring Macrolide and Lincosamide Resistance in the American Foulbrood Pathogen Paenibacillus Larvae. Front. Microbiol. 2021, 12, 667096. [Google Scholar] [CrossRef]
- Genersch, E. Paenibacillus larvae and American foulbrood–long since known and still surprising. J. Verbr. Lebensm. 2008, 3, 429–434. [Google Scholar] [CrossRef]
- Shimanuki, H.; Knox, D.A.; Furgala, B.; Caron, D.M.; Williams, J.L. Diseases and pest of honeybees. In The Hive and the Honey Be, 5th ed.; Graham, M.J., Ed.; Dadant and Sons: Hamilton, IL, USA, 1992; pp. 1083–1151. [Google Scholar]
- Holst, E.C. A simple field test for American foulbrood. Am. Bee J. 1946, 86, 14–34. [Google Scholar]
- De Graaf, D.C.; Alippi, A.M.; Brown, M.; Evans, J.D.; Feldlaufer, M.; Gregorc, A.; Hornitzky, M.; Pernal, S.F.; Schuch, D.M.T.; Titĕra, D.; et al. Diagnosis of American foulbrood in honey bees: A synthesis and proposed analytical protocols. Lett. Appl. Microbiol. 2006, 43, 583–590. [Google Scholar] [CrossRef]
- Schuch, D.M.T.; Madden, R.H.; Sattler, A. An improved method for the detection and presumptive identification of Paenibacillus larvae subsp larvae spores in honey. J. Apic. Res. 2001, 40, 59–64. [Google Scholar] [CrossRef]
- Ryba, S.; Titera, D.; Haklova, M.; Stopka, P. A PCR method of detecting American Foulbrood (Paenibacillus larvae) in winter beehive wax debris. Vet. Microbiol. 2009, 139, 193–205. [Google Scholar] [CrossRef]
- Hoage, T.R.; Rothenbuchler, W.C. Larval Honey Bee Response to Various Doses of Bacillus larvae Spores. J. Econ. Entomol. 1966, 59, 42–45. [Google Scholar] [CrossRef]
- Hornitzky, M.A.Z.; Wilson, S.C. A system for the diagnosis of the major bacterial brood diseases of honeybees. J. Apic. Res. 1989, 28, 191–195. [Google Scholar] [CrossRef]
- Bargańska, Z.; Namiesńik, J.; Ślebioda, M. Determination of antibiotic residues in honey. Trends Analyt. Chem. 2011, 30, 1035–1041. [Google Scholar] [CrossRef]
- Kivrak, Í.; Kivrak, S.; Harmandar, M. Development of a rapid method for the determination of antibiotic residues in honey using UPLC-ESI-MS/MS. Food Sci. Technol. 2016, 36, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Rothenbuhler, W.C. Behavior Genetics of Nest Cleaning in Honey Bees. IV. Responses of F1 and Backcross Generations to Disease-Killed Brood. Am. Zool. 1964, 4, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Arathi, H.S.; Burns, I.; Spivak, M. Ethology of Hygienic Behaviour in the Honey Bee Apis mellifera L. (Hymenoptera: Apidae): Behavioural repertoire of Hygienic bees. Ethology 2000, 106, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2011, 30, 1035–1041. [Google Scholar] [CrossRef]
- Nazzi, F.; Vedona, G.D.; D’Agaro, M. A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 2004, 35, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Chakroborty, N.K.; Bienefeld, K.; Menzel, R. Odor learning and odor discrimination of bees selected for enhanced hygienic behavior. Apidologie 2015, 46, 499–514. [Google Scholar] [CrossRef] [Green Version]
- McAfee, A.; Collins, T.F.; Madilao, L.L.; Foster, L.J. Odorant cues linked to social immunity induce lateralized antenna stimulation in honey bees (Apis mellifera L.). Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lim, S.; Choi, Y.S.; Lee, M.L.; Kwon, H.W. Volatile disease markers of American foulbrood-infected larvae in Apis mellifera. J. Insect Physiol. 2020, 122, 104040. [Google Scholar] [CrossRef]
- Mondet, F.; Kim, S.H.; de Miranda, J.R.; Beslay, D.; Le Conte, Y.; Mercer, A.R. Specific Cues Associated With Honey Bee Social Defence against Varroa destructor Infested Brood. Sci. Rep. 2016, 6, 25444. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, K.; Spivak, M.; Hefetz, A.; Reams, T.; Rueppell, O. Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Sci. Rep. 2019, 9, 8753. [Google Scholar] [CrossRef]
- Kathe, E.; Seidelmann, K.; Lewkowski, O.; Conte, Y.L.; Erler, S. Changes in chemical cues of Melissococcus plutonius infected honey bee larvae. Chemoecology 2021, 31, 189–200. [Google Scholar] [CrossRef]
- Swanson, J.A.I.; Torto, B.; Kells, S.A.; Mesce, K.A.; Tumlinson, J.H.; Spivak, M. Odorants that Induce Hygienic Behavior in Honeybees: Identification of Volatile Compounds in Chalkbrood-Infected Honeybee Larvae. J. Chem. Ecol. 2009, 35, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Gochnauer, T.A.; Shearer, D.A. Volatile acids from honeybee larvae infected with Bacillus larvae and from a culture of the organism. J. Apicul. Res. 1981, 20, 104–109. [Google Scholar] [CrossRef]
- Mărghitaş, L.; Dezmirean, D.; Chirilă, F.; Fiţ, N.; Bobiş, O. Antibacterial activity of different plant extracts and phenolic phytochemicals tested on Paenibacillus larvae bacteria. J. Anim. Sci. Biotechnol. 2011, 44, 94–99. [Google Scholar]
- Pellegrini, M.C.; Alonso-Salces, R.M.; Umpierrez, M.L.; Rossini, S.; Fusseli, S.R. Chemical Composition, Antimicrobial Activity, and Mode of Action of Essential Oils against Paenibacillus larvae, Etiological Agent of American Foulbrood on Apis mellifera. Chem. Biodivers. 2017, 14, e1600382. [Google Scholar] [CrossRef] [PubMed]
- Ratnieks, F.L.W. American Foulbrood: The Spread and Control of an Important Disease of the Honey Bee. Bee World 1992, 73, 177–191. [Google Scholar] [CrossRef]
- Mitroudi, C. Detection of Infestation of Bee Brood (Apis Mellifera L.) by the Bacterium Paenibacillus larvae Using Volatile Marker Compounds. Ph.D. Thesis, Department of Agriculture, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2015, unpublished work. [Google Scholar]
- Tananaki, C. The study of Factors Affective the Volatile Compounds from Honeydew Honeys. Ph.D. Thesis, Department of Agriculture, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2006, unpublished work. [Google Scholar]
- Tananaki, C.; Thrasyvoulou, A.; Giraudel, J.L.; Montury, M. Determination of volatile characteristics of Greek and Turkish pine honey samples and their classification by using Kohonen self organising maps. Food Chem. 2007, 101, 1687–1693. [Google Scholar] [CrossRef]
- Filipiak, W.; Sponring, A.; Filipiak, A.; Baur, M.; Ager, C.; Wiesenhofer, H.; Margesin, R.; Nagl, M.; Troppmair, J.; Amann, A. Microorganisms in vitro: Potential Breath Biomarkers for Early-Stage Diagnosis of Disease. In Volatile Biomarkers Non-Invasive Diagnosis in Physiology and Medicine, 1st ed.; Amann, A., Smith, D., Eds.; The Boulevard: Kidlington, UK, 2013; pp. 463–512. [Google Scholar]
- Carroll, M.J.; Duehl, A.J. Collection of volatiles from honeybee larvae and adults enclosed on brood frames. Apidologie 2012, 43, 715–730. [Google Scholar] [CrossRef] [Green Version]
- He, X.J.; Zhang, X.C.; Jiang, W.J.; Barron, A.B.; Zhang, J.H.; Zeng, Z.J. Starving honey bee (Apis mellifera) larvae signal pheromonally to worker bees. Sci. Rep. 2016, 6, 22359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisonnasse, A.; Lenoir, J.C.; Beslay, D.; Crauser, D.; Conte, Y. E-β-Ocimene, a Volatile Brood Pheromone Involved in Social Regulation in the Honey Bee Colony (Apis mellifera). PLoS ONE 2010, 5, e13531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabagias, I.K.; Badeka, A.; Kontominas, M.G. A decisive strategy for monofloral honey authentication using analysis of volatile compounds and pattern recognition techniques. Microchem. J. 2020, 152, 104263. [Google Scholar] [CrossRef]
- McAfee, A.; Chapman, A.; Iovinella, I.; Galagher-Kurtzke, Y.; Collins, T.F.; Higo, H.; Madilao, L.L.; Pelosi, P.; Foster, L.G. A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.). Sci. Rep 2018, 8, 5719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almenar, E.; Hernádez-Muñoz, P.; Gavara, R. Evolution of Selected Volatiles in Chitosan-Coated Strawberries (Fragaria × ananassa) during Refrigerated Storage. J. Agric. Food Chem. 2009, 57, 974–980. [Google Scholar] [CrossRef]
- Bissinger, B.W.; Apperson, C.S.; Watson, D.W.; Arellano, C.; Sonenshine, D.E.; Roe, R.M. Novel field assays and the comparative repellency of BioUD®, DEET and permethrin against Amblyomma americanum. Med. Vet. Entomol. 2011, 25, 217–226. [Google Scholar] [CrossRef]
- Bouseta, A.; Scheirman, V.; Collin, S. Flavor and free amino acid composition of Lavender and Eucalyptus Honeys. J. Food Sci. 1996, 61, 683–687. [Google Scholar] [CrossRef]
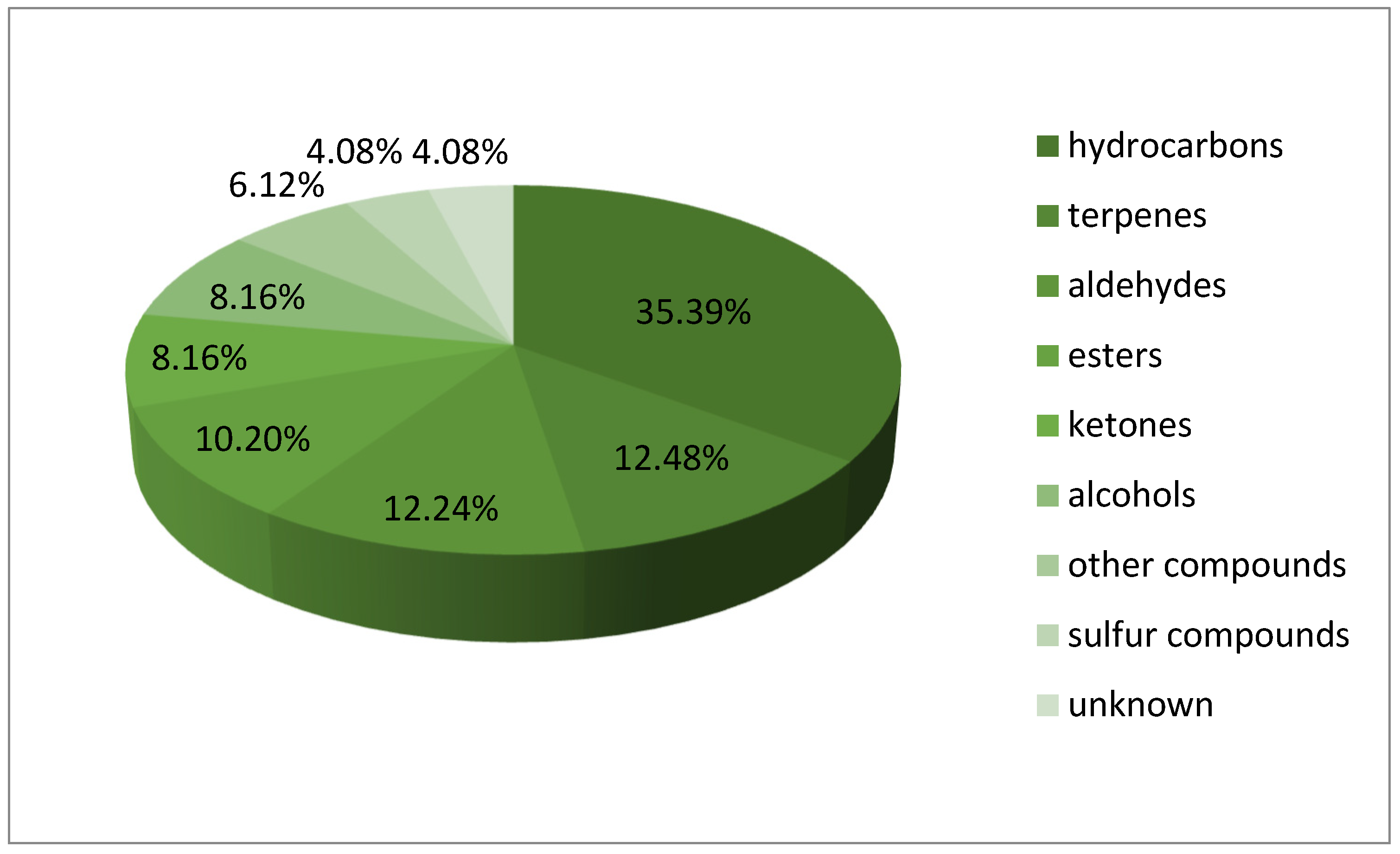

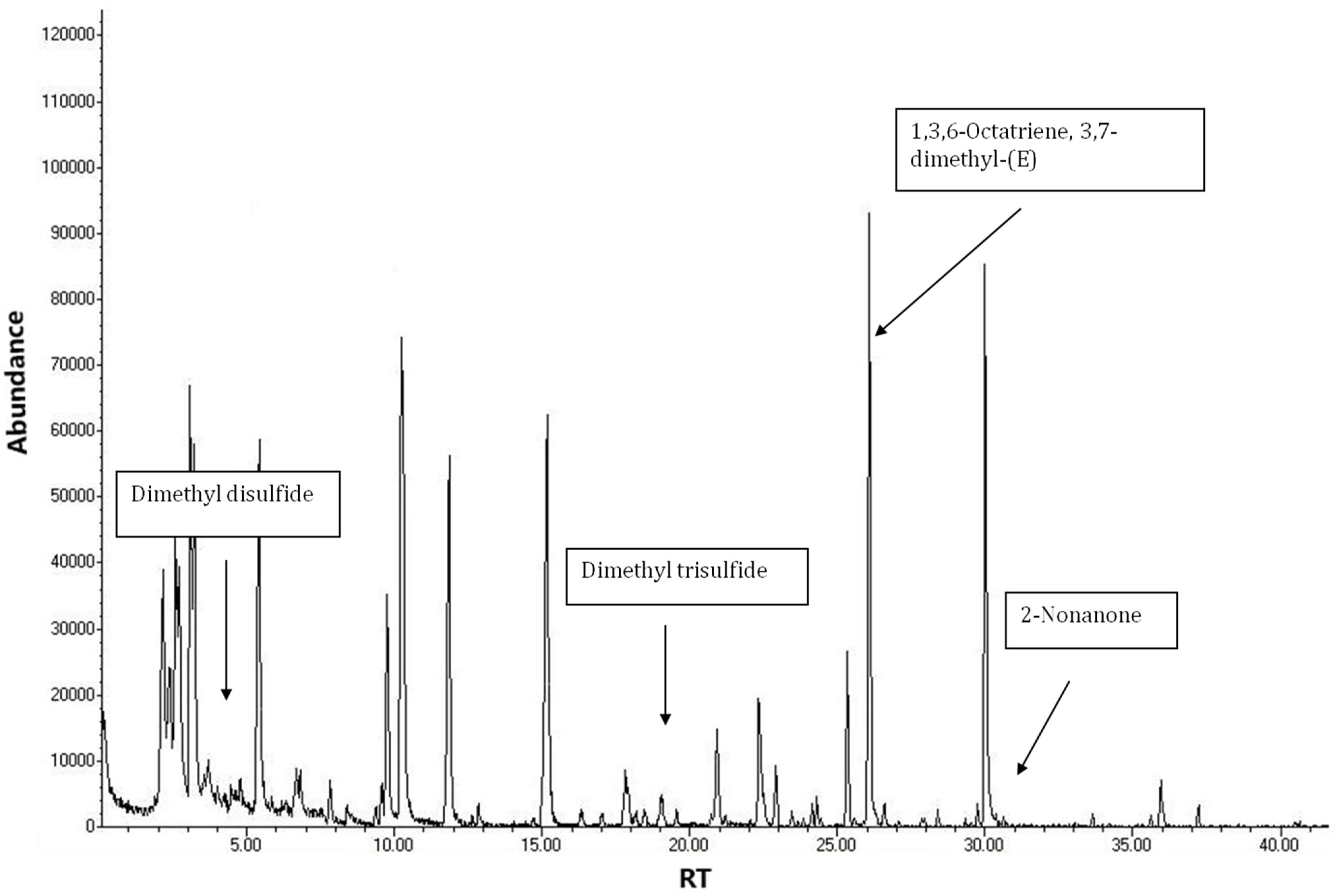
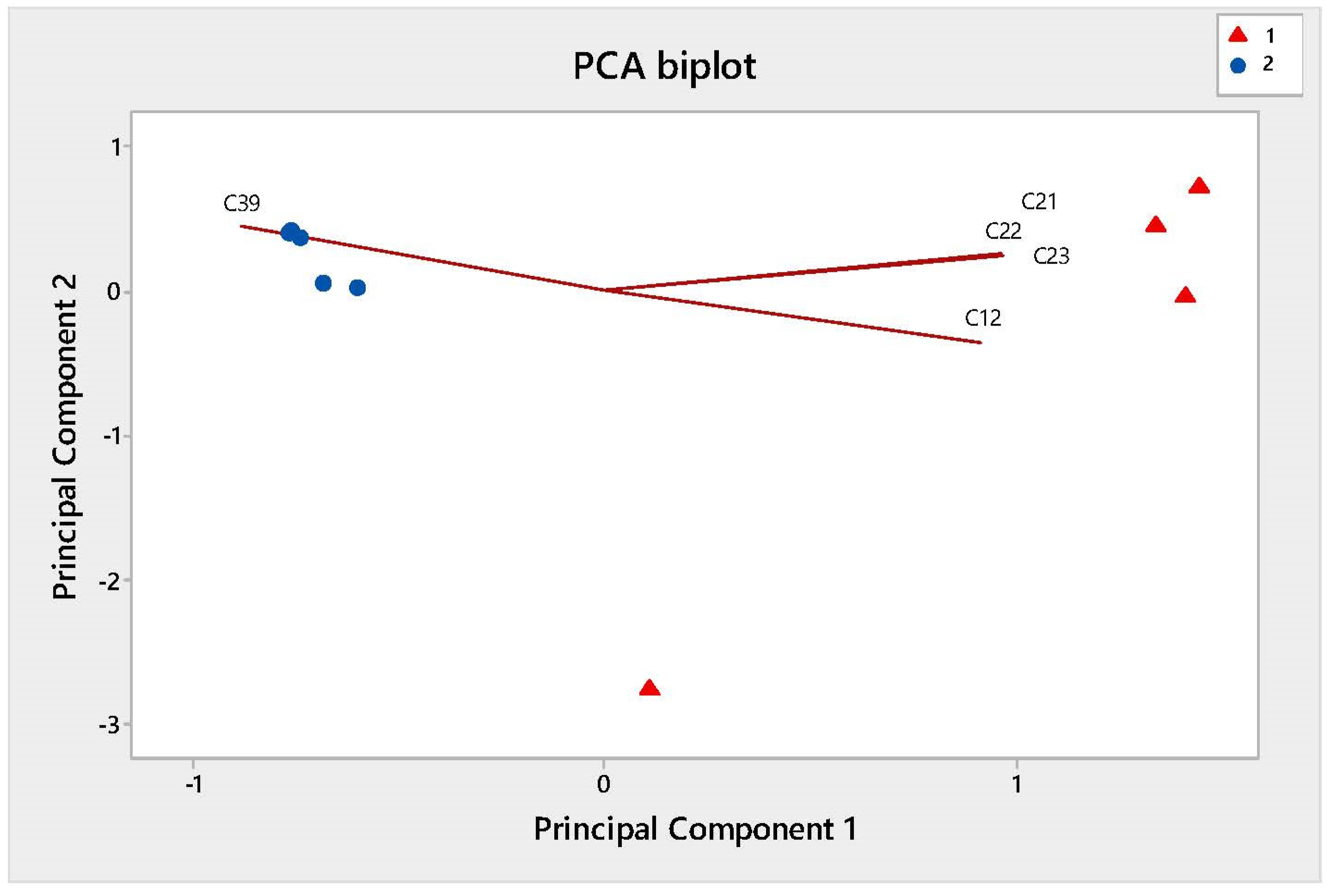
| Number of Samples | Degree of Infection (%) | Diagnosis with Test Kit VITA |
|---|---|---|
| 1 | 94 | positive |
| 2 | 96 | positive |
| 3 | 62 | positive |
| 4 | 77 | positive |
| 5 | 89 | positive |
| Code | R.T. (min) | R.I. Exp * | CAS Number | Volatile Compound (Mass Fractions) | Healthy Brood (%) (n = 5) | Infected Brood (%) (n = 5) |
|---|---|---|---|---|---|---|
| C1 | 3.09 | 624 | 590-86-3 | Butanal, 3-methyl-(53, 57, 58, 60, 71, 86) | n.d. ** | 0.81 ± 0.73 (60%) *** |
| C2 | 3.23 | 640 | 96-17-3 | Butanal, 2-methyl-(55, 57, 60, 71, 86) | n.d. | 0.50 ± 0.38 (60%) |
| C3 | 3.55 | 679 | 107-89-9 | 2-Pentanone (55, 58, 71, 86) | n.d. | 0.08 ± 0.08 (60%) |
| C4 | 3.73 | 700 | 142-82-5 | Heptane (55, 57, 60, 71) | n.d. | 0.48 ± 0.11 (80%) |
| C5 | 4.05 | 711 | - | unknown (55, 73, 88) | n.d. | 4.53 ± 3.67 (60%) |
| C6 | 4.45 | 725 | 763-32-6 | 3-Buten-1-ol, 3-methyl-(56, 68, 86) | n.d. | 0.09 ± 0.06 (60%) |
| C7 | 4.53 | 727 | 71-41-0 | 1-Pentanol (55,70) | n.d. | 2.35 ± 2.12 (60%) |
| C8 | 4.61 | 730 | 137-32-6 | 1-Butanol,2-methyl-(55, 57,70) | n.d. | 0.55 ± 0.15 (60%) |
| C9 | 4.70 | 733 | 96-54-8 | 1H-Pyrrole, 1-methyl-(55, 78, 80, 81) | nd | 0.58 ± 0.38 (60%) |
| C10 | 4.75 | 735 | 624-92-0 | Disulfide, dimethyl (61,79, 94) | nd | 0.58 ± 0.33 (100%) |
| C11 | 5.24 | 752 | 97-62-1 | Propanoic acid, 2-methyl-, ethyl ester (55, 71, 88, 116) | n.d. | 0.04 ± 0.04 (60%) |
| C12 | 5.40 | 757 | 108-88-3 | Benzene, methyl-(Toluene) (65, 77, 91) | 0.23 ± 0.05 (100%) | 9.82 ± 2.31 (100%) |
| C13 | 5.84 | 773 | 556-24-1 | Butanoic acid, 3-methyl-, methyl ester (57, 74, 88, 101) | n.d. | 0.08 ±0.08 (60%) |
| C14 | 6.25 | 787 | 120-92-3 | Cyclopentanone (55, 84) | n.d. | 0.24 ± 0.24 (60%) |
| C15 | 6.63 | 800 | 111-65-9 | Octane (57, 71, 85, 114) | 0.19 ± 0.09 (100%) | 0.34 ± 0.18 (80%) |
| C16 | 6.79 | 802 | 105-54-4 | Butanoic acid, ethyl ester (55, 60, 71, 88) | n.d. | 0.06 ± 0.06 (60%) |
| C17 | 8.39 | 829 | 98-01-1 | 2-Furancarboxaldehyde (Furfural) (60, 67, 96, 207) | 2.49 ± 2.47 (60%) | 0.03 ± 0.03 (60%) |
| C18 | 9.07 | 840 | - | unknown (55, 59, 73, 88) | n.d. | 6.45 ± 6.42 (60%) |
| C19 | 9.35 | 844 | 7452-79-1 | Butanoic acid, 2-methyl-, ethyl ester (57, 74, 85, 102) | n.d. | 0.32 ± 0.32 (60%) |
| C20 | 9.57 | 848 | 108-64-5 | Butanoic acid, 3-methyl-, ethyl ester (57, 60, 70, 73, 85, 88) | n.d. | 0.12 ± 0.10 (60%) |
| C21 | 9.75 | 851 | 100-41-4 | Ethylbenzene (91,106) | 0.02 ± 0.01 (80%) | 2.52 ± 0.97 (80%) |
| C22 | 10.27 | 859 | 95-47-6 | Benzene, 1,2-dimethyl-(o-xylene) (91,106) | 0.14 ± 0.03 (100%) | 11.21 ± 4.35 (100%) |
| C23 | 11.86 | 885 | 108-38-3 | Benzene, 1,3-dimethyl-(91,106) | 0.05 ± 0.02 (80%) | 4.43 ± 1.71 (100%) |
| C24 | 12.03 | 888 | 110-43-0 | 2-Heptanone (58, 71, 91) | n.d. | 0.71 ± 0.63 (80%) |
| C25 | 12.64 | 898 | 111-84-2 | Nonane (57, 71, 85, 95) | 0.09 ± 0.03 (100%) | 0.68 ± 0.51 (80%) |
| C26 | 15.13 | 925 | 80-56-8 | Bicyclo [3.1.1]hept-2-ene, 2,6,6-trimethyl- (α-Pinene) (67, 79, 93, 105) | 0.09 ± 0.03 (80%) | 8.23 ± 4.87 (80%) |
| C27 | 17.71 | 952 | 100-52-7 | Benzaldehyde (51, 77, 106) | 0.17 ± 0.16 (60%) | n.d. |
| C28 | 17.81 | 953 | 611-14-3 | Benzene, 1-ethyl-2-methyl-(78, 91, 105, 120) | n.d. | 0.39 ± 0.27 (60%) |
| C29 | 18.17 | 957 | 3658-80-8 | Dimethyl trisulfide (64, 79, 94, 111, 126) | n.d. | 0.16 ± 0.02 (100%) |
| C30 | 19.54 | 972 | 620-14-4 | Benzene, 1-ethyl-3-methyl- (91, 105, 120) | n.d. | 0.04 ± 0.04 (60%) |
| C31 | 20.75 | 985 | 13475-82-6 | Heptane, 2,2,4,6,6-pentamethyl-(57, 71, 85) | n.d. | 0.08 ± 0.08 (60%) |
| C32 | 20.92 | 986 | 622-96-8 | Benzene, 1-ethyl-4-methyl- (4-Ethyltoluene)(91, 105, 120) | n.d. | 0.47 ± 0.30 (60%) |
| C33 | 21.20 | 989 | 127-91-3 | Bicyclo [3.1.1]heptane, 6,6-dimethyl-2-methylene- (beta -Pinene) (56, 69, 93, 105) | 0.04 ± 0.03 (60%) | 0.02 ± 0.02 (60%) |
| C34 | 22.33 | 1002 | 124-13-0 | Octanal (56, 69, 84) | n.d. | 0.49 ± 0.49 (60%) |
| C35 | 22.50 | 1004 | 471-84-1 | Bicyclo(2.2.1)heptane, 7,7-dimethyl-2-methylene-(α-Fenchene) (56, 77, 85, 93, 105, 121, 136) | n.d. | 0.03 ± 0.03 (60%) |
| C36 | 24.16 | 1026 | 138-86-3 | Cyclohexene, 1-methyl-4-(1-methylethenyl)-(Limonene) (53, 67, 77, 79, 93, 107) | n.d. | 0.08 ± 0.08 (60%) |
| C37 | 24.29 | 1027 | 470-82-6 | 1,3,3-Trimethyl-2-oxabicyclo [2.2.2]octane (Eucalyptol) (55, 69, 81, 84, 93, 96, 108, 111) | n.d. | 0.06 ± 0.06 (60%) |
| C38 | 25.33 | 1041 | 3779-61-1 | 1,3,6-Octatriene, 3,7-dimethyl-(E) ((Ε)-β-ocimene)(53, 67, 79, 93, 105,121) | 0.40 ± 0.06 (100%) | 2.41 ± 0.88 (100%) |
| C39 | 26.10 | 1051 | 3338-55-4 | 1,3,6-Octatriene, 3,7-dimethyl-((Z)-β-ocimene) (53, 67, 79, 93, 105,121) | 96.03 ± 2.71 (100%) | 37.63 ± 12.32 (100%) |
| C40 | 26.59 | 1058 | 934-80-5 | Benzene, 4-ethyl-1,2-dimethyl-(91, 105, 119) | n.d. | 0.06 ± 0.06 (60%) |
| C41 | 28.38 | 1081 | 2870-04-4 | Benzene, 2-ethyl-1,3-dimethyl-(91, 105, 119) | n.d. | 0.04 ± 0.04 (60%) |
| C42 | 28.60 | 1084 | 1124-11-4 | Pyrazine, tetramethyl-(54, 136) | n.d. | 0.16 ± 0.15 (60%) |
| C43 | 30.07 | 1104 | 124-19-6 | Nonanal (57, 70, 82, 98) | 0.04 ± 0.04 (60%) | n.d. |
| C44 | 30.08 | 1105 | 821-55-6 | 2-Nonanone (58, 71, 124, 142) | n.d. | 1.92 ± 1.57(100%) |
| C45 | 34.16 | 1173 | 91-20-3 | Naphthalene (102, 128) | n.d. | 0.33 ± 0.33 (60%) |
| C46 | 37.86 | 1242 | 17057-82-8 | 1H-Indene, 2,3-dihydro-1,2-dimethyl-(91, 115, 131, 146) | n.d. | 0.03 ± 0.03 (60%) |
| C47 | 39.42 | 1274 | 700-12-9 | Benzene, pentamethyl (115, 133, 148) | n.d. | 0.05 ± 0.05 (60%) |
| C48 | 39.97 | 1285 | 264-09-5 | Benzocycloheptatriene (115, 139, 142) | n.d. | 0.22 ± 0.22 (60%) |
| C49 | 40.55 | 1297 | 112-12-9 | 2-Undecanone (58, 71, 85, 95) | n.d. | 0.07 ± 0.06 (60%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liolios, V.; Kanelis, D.; Tananaki, C.; Rodopoulou, M.-A. A Comparative Study of Healthy and American Foulbrood-Infected Bee Brood (Apis mellifera L.) through the Investigation of Volatile Compounds. Agriculture 2022, 12, 812. https://doi.org/10.3390/agriculture12060812
Liolios V, Kanelis D, Tananaki C, Rodopoulou M-A. A Comparative Study of Healthy and American Foulbrood-Infected Bee Brood (Apis mellifera L.) through the Investigation of Volatile Compounds. Agriculture. 2022; 12(6):812. https://doi.org/10.3390/agriculture12060812
Chicago/Turabian StyleLiolios, Vasilios, Dimitrios Kanelis, Chrysoula Tananaki, and Maria-Anna Rodopoulou. 2022. "A Comparative Study of Healthy and American Foulbrood-Infected Bee Brood (Apis mellifera L.) through the Investigation of Volatile Compounds" Agriculture 12, no. 6: 812. https://doi.org/10.3390/agriculture12060812
APA StyleLiolios, V., Kanelis, D., Tananaki, C., & Rodopoulou, M.-A. (2022). A Comparative Study of Healthy and American Foulbrood-Infected Bee Brood (Apis mellifera L.) through the Investigation of Volatile Compounds. Agriculture, 12(6), 812. https://doi.org/10.3390/agriculture12060812






