Using Smoke Condensed Liquids from Pruned Fruit-Tree Branches for Aedes Mosquito Larva Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparations of Mosquito Larvae and Fruit-Tree Branches
2.2. Smoldering Reactor Design and Operation
2.3. Analysis of SCL Ingredients
2.4. Bactericidal Tests
2.5. Change of Abundance over Time (Preservation of the SCL)
2.6. Larvicidal Test
2.7. Estimated Larva’s LC50 at a Certain Treatment Time
2.8. Effects of pH and Dissolved Oxygen (DO)
2.9. Data Quality
3. Results and Discussion
3.1. SCL Properties
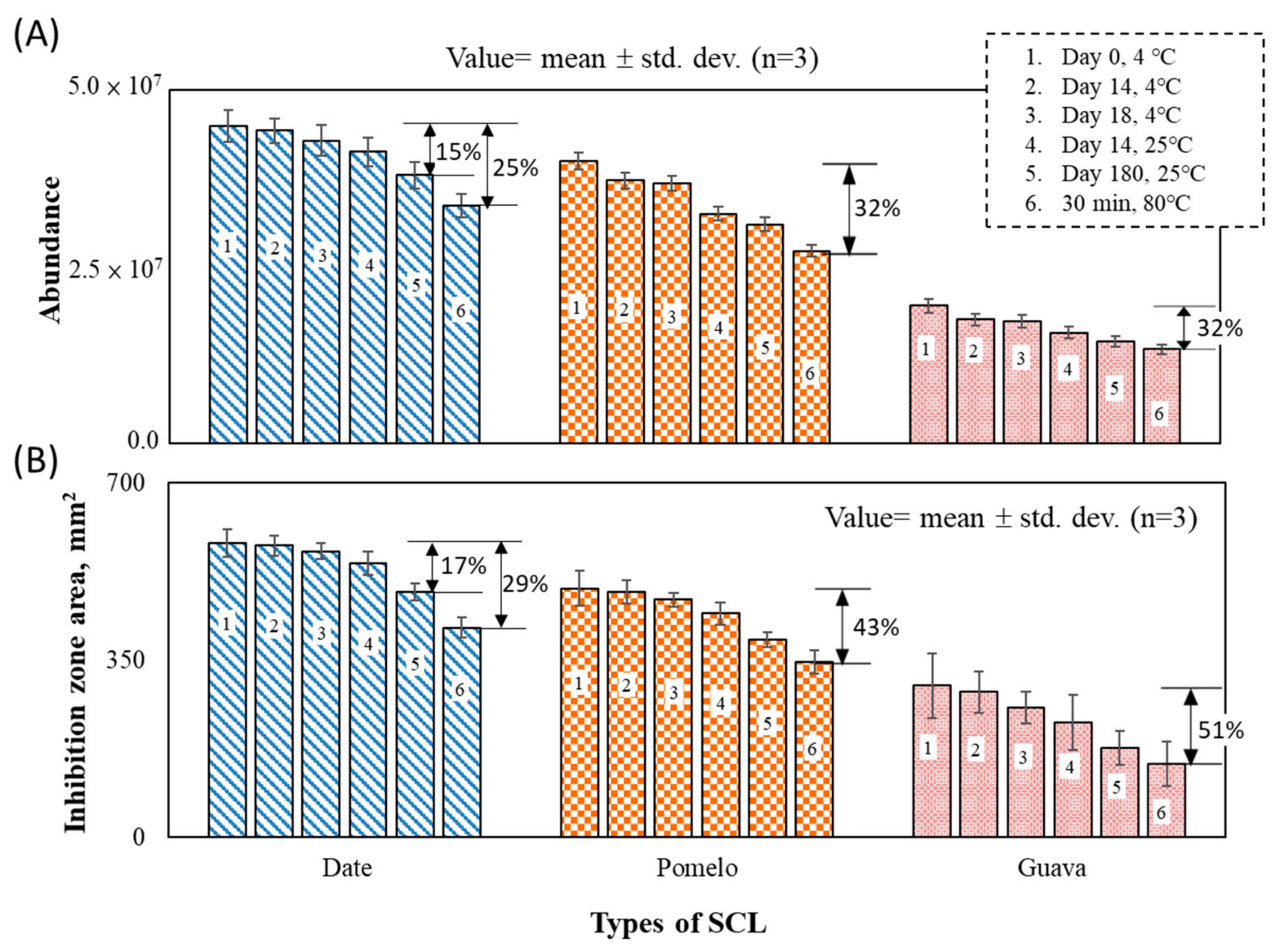
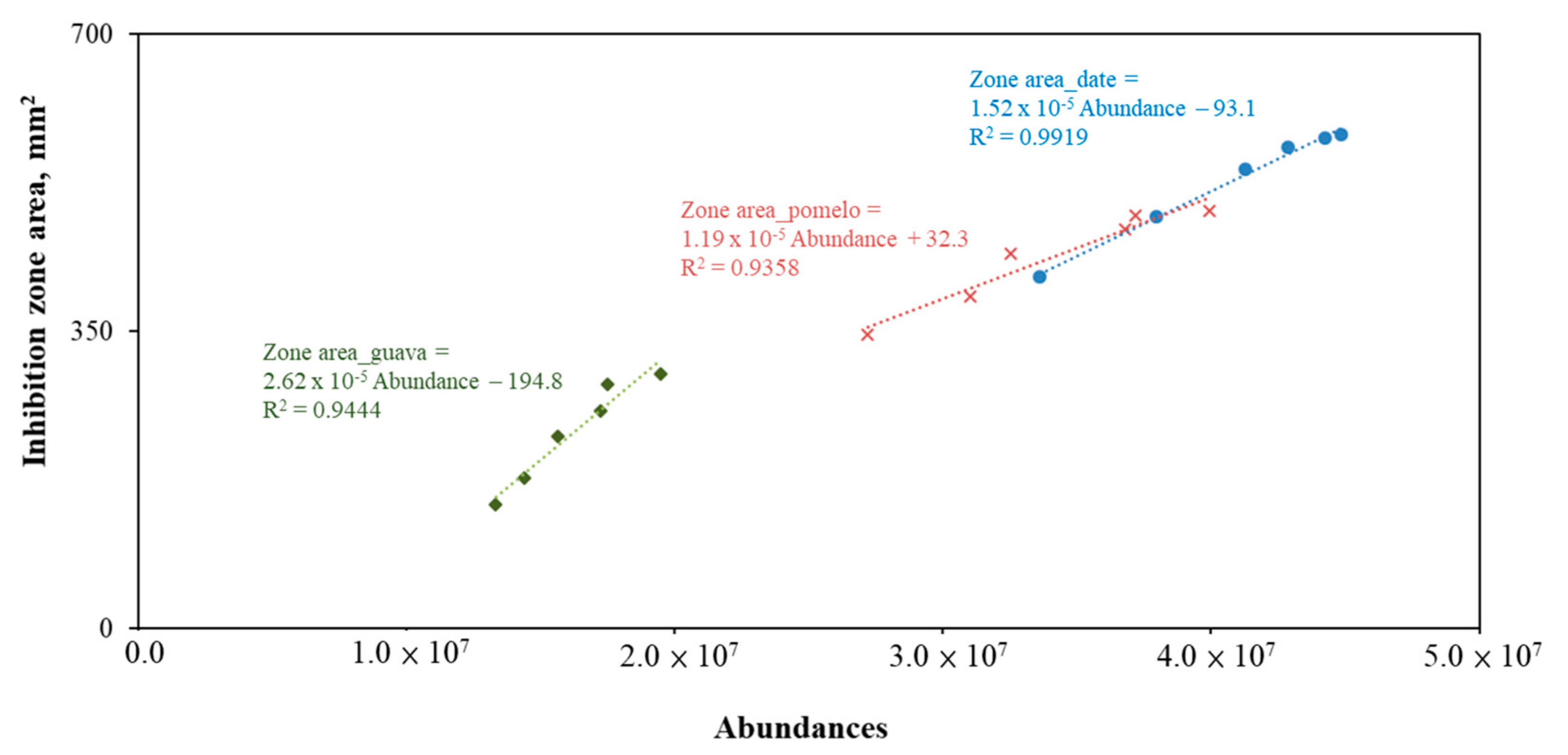
3.2. SCL Bactericidal Effect
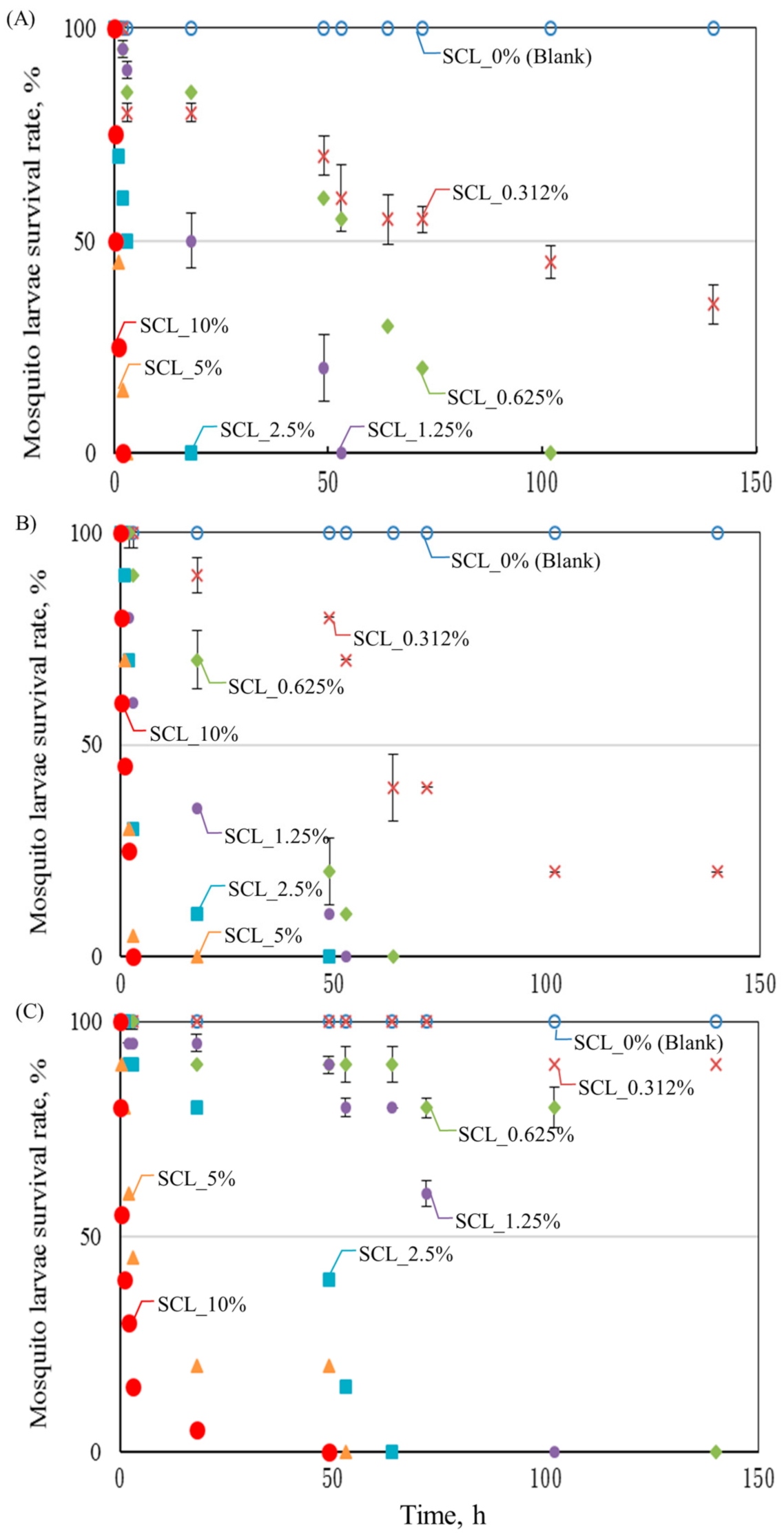
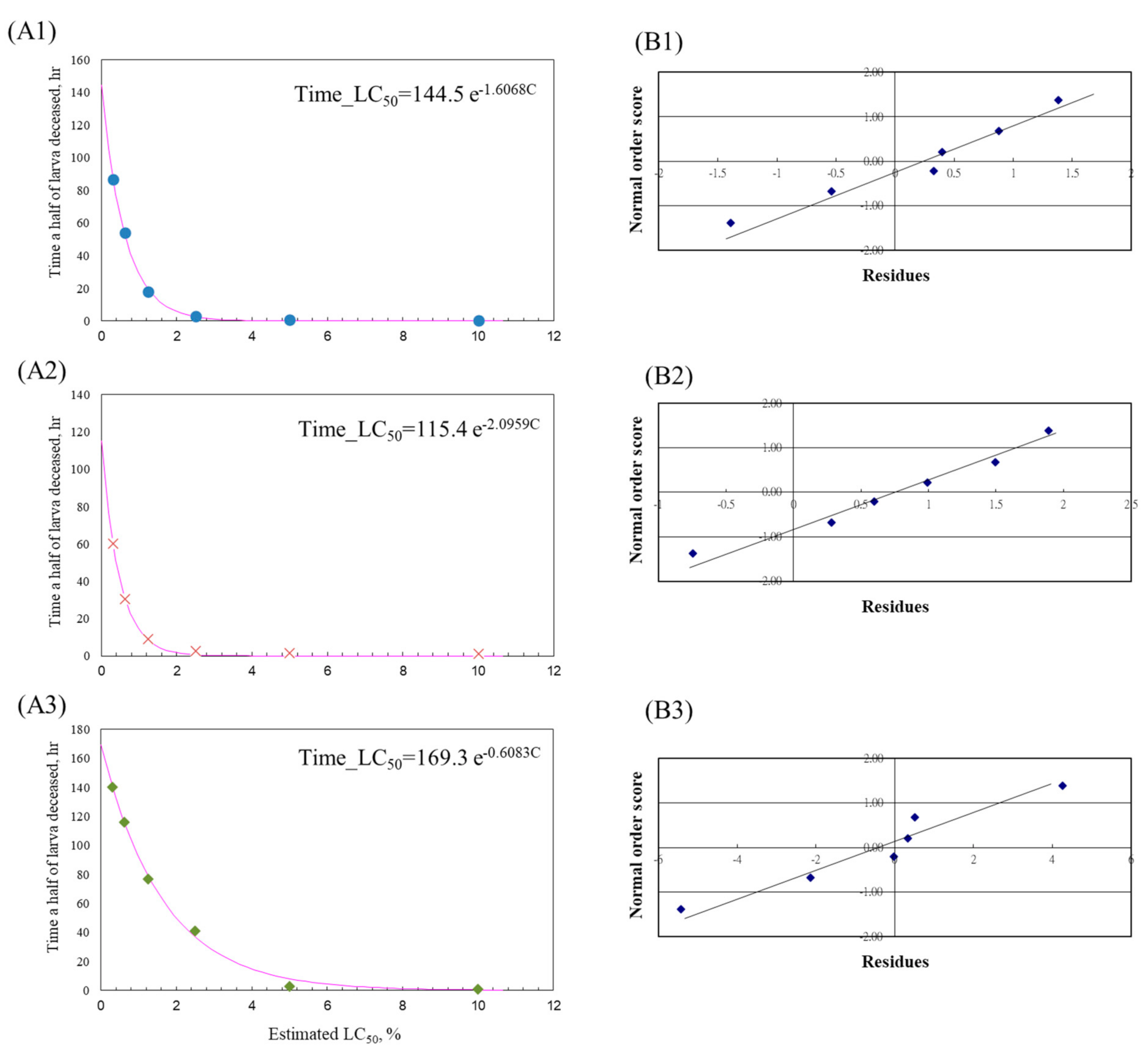
3.3. SCL Larvicide Effect
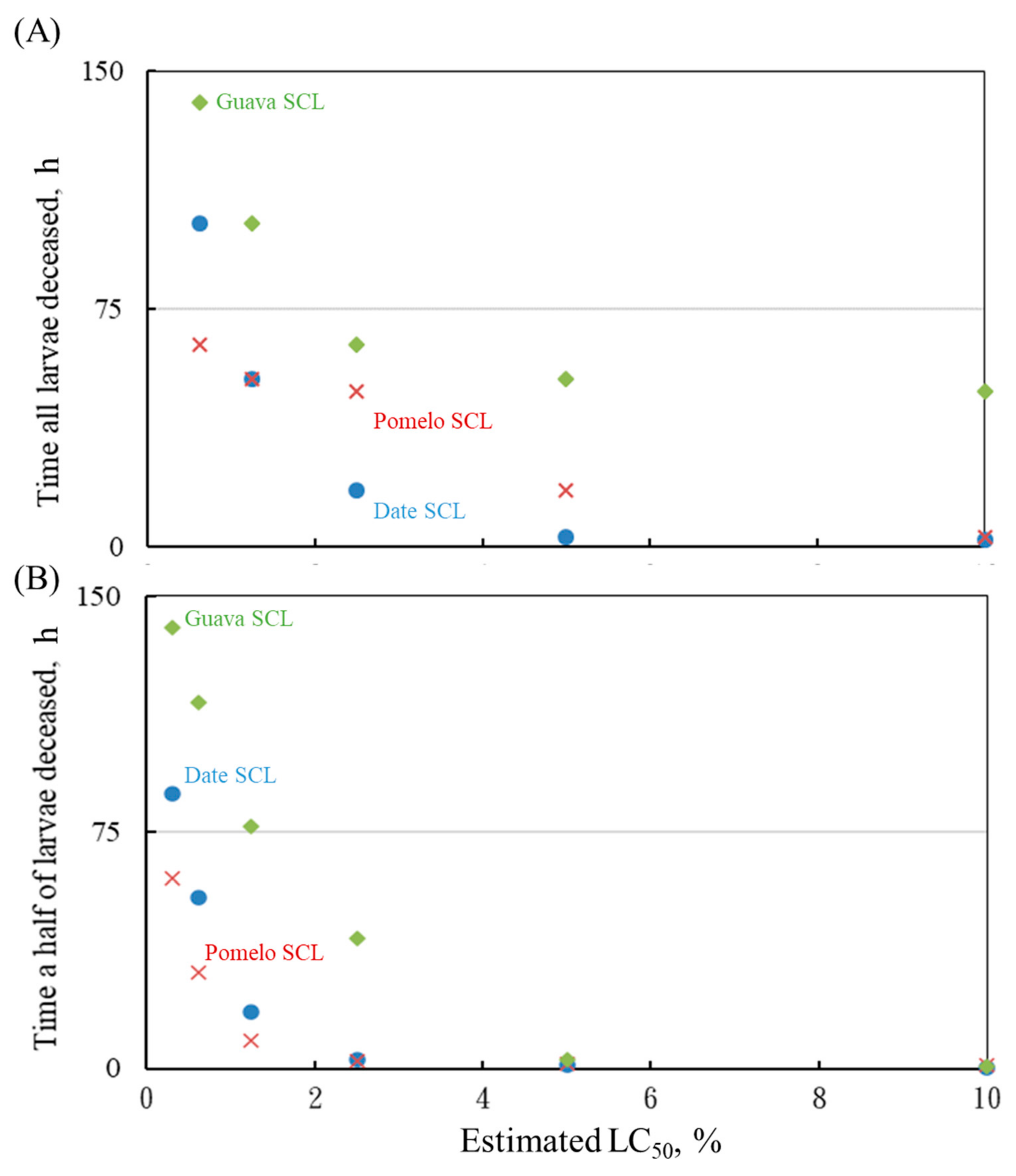
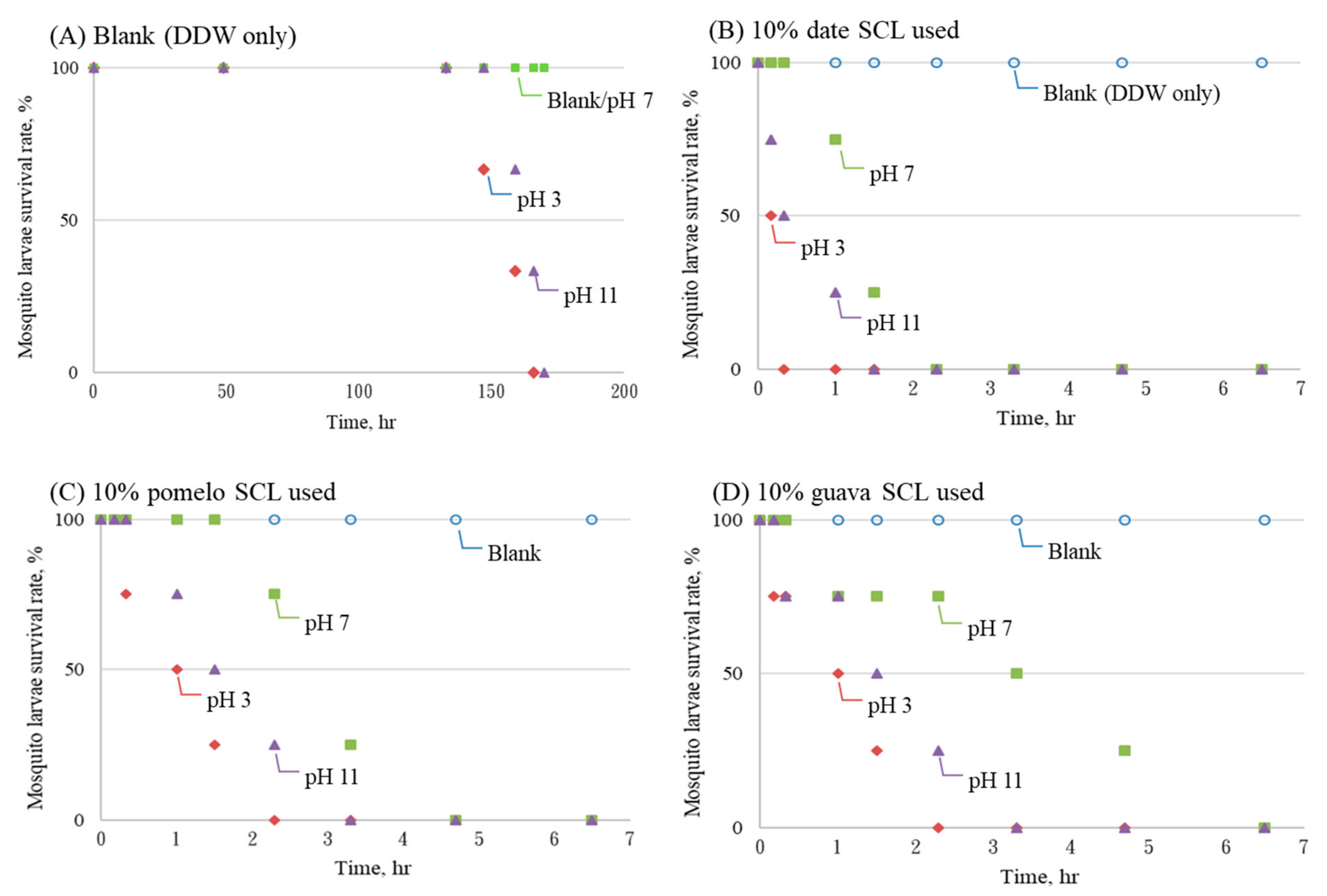
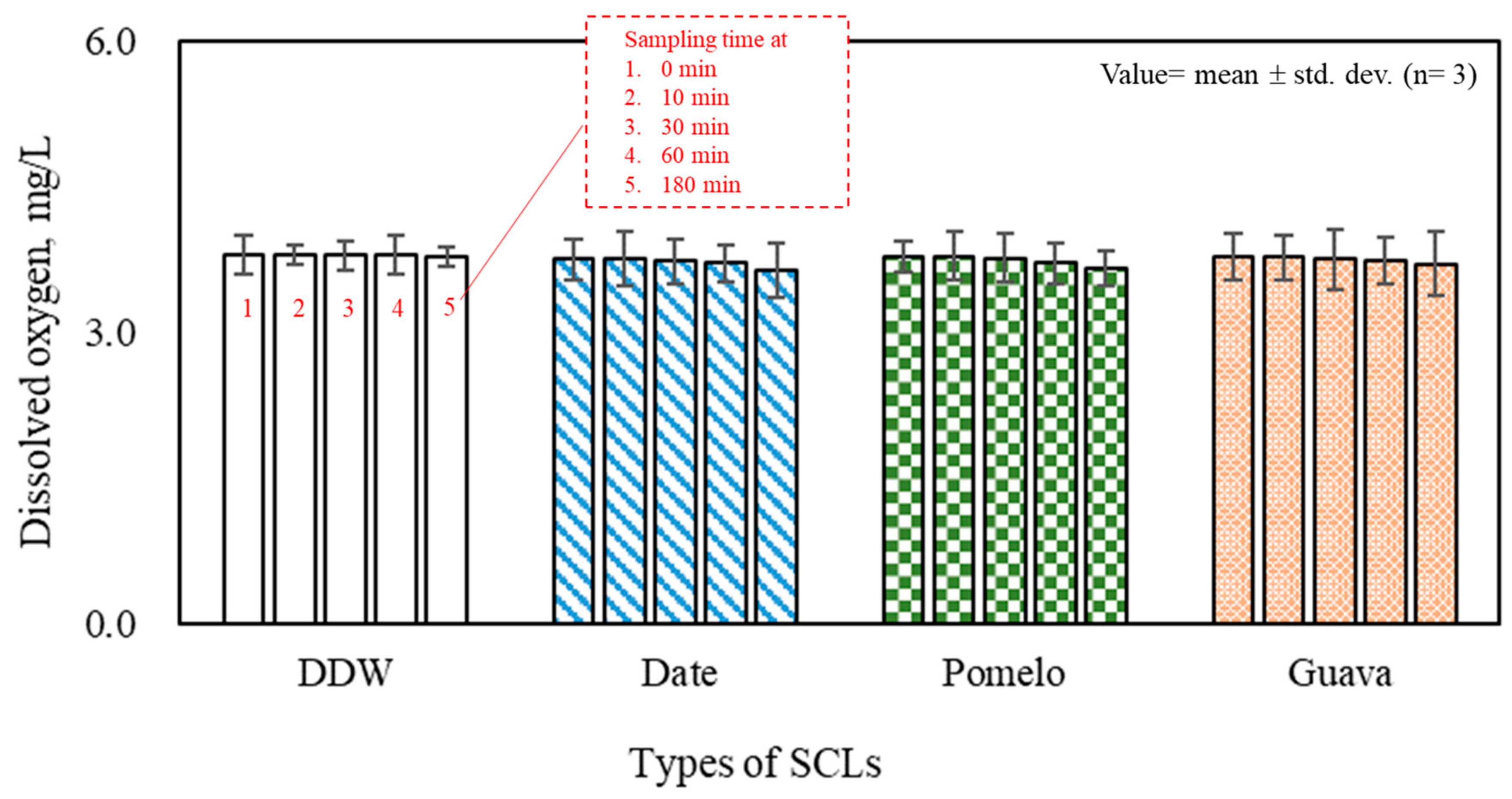
3.4. Other Factors of Larvicidal Effects
4. Conclusions
- (1)
- The SCL generated from the three fruit-tree branches appeared to be acidic, free of heavy metals, and consisted of complex yet mostly similar organo-compounds.
- (2)
- The abundance of the SCL compounds was in the order (large to small) of date, pomelo, and guava SCLs. The greater abundance of the SCL liquid, the greater the bactericidal effect. At ten percent of the date and pomelo SCLs, 1.44 and 1.13 times (compared with the 75% ethanol) bactericidal efficiencies resulted, respectively.
- (3)
- A highly positive correlation existed between the SCL abundance and its bactericidal effect. At extreme volatilization conditions (i.e., heated at 80 °C for 30 min), around a 30% loss of bactericidal effectiveness was observed in the 10% date SLC case. At ambient temperature, the date and pomelo abundances lost less than 20% in 180 days, which indicated the possibility of long-term preservation.
- (4)
- The larvicidal effect was positively proportional to the amount of the SCL used as well. At 10% of the SCL, all of the tested larvae were killed in 2–3 h while using the date and pomelo SCLs. A first-order mathematic model showed satisfaction in describing the relationship of time required to reach 50% of larval mortality and the content of SCL.
- (5)
- A first-order mathematic model with known parameters was able to predict the time and SCL concentration required to reach a 50% larval mortality. The dose–effect relationship between SCL concentration and larval mortality was obvious.
- (6)
- High or low liquid pHs enhanced the larvicidal effect of the SCL; however, the abundance of the SCL was a more dominant factor in mosquito larval mortality. In addition, liquid DO barely changed when 10% of the SCL was spiked.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- TWCDC. Dengue Fever; Taiwan Centers for Disease Control: Taipei, Taiwan, 2021. Available online: https://www.cdc.gov.tw/En/Category/ListContent/bg0g_VU_Ysrgkes_KRUDgQ?uaid=9_Oq7OYHa-l8B05iUwyVvQ (accessed on 8 September 2021).
- Pratheeba, T.; Vivekanandhan, P.; Faeza, A.K.N.; Natarajan, D. Chemical constituents and larvicidal efficacy of Naringi crenulata (Rutaceae) plant extracts and bioassay guided fractions against Culex quinquefasciatus mosquito (Diptera: Culicidae). Biocatal. Agric. Biotechnol. 2019, 19, 101137. [Google Scholar] [CrossRef]
- Baskar, K.; Sudha, V.; Nattudurai, G.; Ignacimuthu, S.; Duraipandiyan, V.; Jayakumar, M.; Al-Dhabi, N.A.; Benelli, G. Larvicidal and repellent activity of the essential oil from Atalantia monophylla on three mosquito vectors of public health importance, with limited impact on non-target zebra fish. Physiol. Mol. Plant Pathol. 2018, 101, 197–201. [Google Scholar] [CrossRef]
- Khader, S.Z.A.; Ahmed, S.S.Z.; Venkatesh, K.P.; Chinnaperumal, K.; Nayaka, S. Larvicidal potential of selected indigenous lichens against three mosquito species?ulex quinquefasciatus, Aedes aegypti and Anopheles stephensi. Chin. Herb. Med. 2018, 10, 152. [Google Scholar] [CrossRef]
- Louis, M.L.M.; Pushpa, V.; Balakrishna, K.; Ganesan, P. Mosquito larvicidal activity of Avocado (Persea americana Mill.) unripe fruit peel methanolic extract against Aedes aegypti, Culex quinquefasciatus and Anopheles stephensi. S. Afr. J. Bot. 2020, 133, 1–4. [Google Scholar] [CrossRef]
- Monisha, D.; Sivasankar, V.; Mylsamy, P.; Paulraj, M.G. Mosquito Larvicidal activity of Enhalus acoroides (L.f) Royle and Halophila ovalis (R. Br) Hook. f. against the deadly vectors Aedes aegypti and Culex quinquefasciatus. S. Afr. J. Bot. 2020, 133, 63–72. [Google Scholar] [CrossRef]
- Kala, S.; Sogan, N.; Verma, P.; Naik, S.N.; Agarwal, A.; Patanjali, P.K.; Kumar, J. Nanoemulsion of cashew nut shell liquid bio-waste: Mosquito larvicidal activity and insights on possible mode of action. S. Afr. J. Bot. 2019, 127, 293–300. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Chanthini, K.M.-P.; Karthi, S.; Vasantha-Srinivasan, P.; Ponsankar, A.; Sivanesh, H.; Stanley-Raja, V.; Shyam-Sundar, N.; Narayanan, K.R.; Senthil-Nathan, S. Toxic effect of essential oil and its compounds isolated from Sphaeranthus amaranthoides Burm. f. against dengue mosquito vector Aedes aegypti Linn. Pestic. Biochem. Physiol. 2019, 160, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S.; et al. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crop. Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Chicory (Cichorium intybus) and wormwood (Artemisia absinthium) extracts exhibit strong larvicidal activity against mosquito vectors of malaria, dengue fever, and filariasis. Parasitol. Int. 2018, 67, 781–786. [Google Scholar] [CrossRef]
- Johnson, M.; Maharaja, P.; Murugesan, S.; Janakiraman, N.; Menezes, I.R.A.; da Costa, J.G.M.; Barbosa, C.R.S.; Coutinho, H.D.M. Larvicidal activity of some medicinal plant extracts against filariasis fever mosquito, Culex quinquefasciatus (Say.) (Diptera: Culicidae). Comp. Immunol. Microbiol. Infect. Dis. 2018, 61, 1–4. [Google Scholar] [CrossRef]
- El-Sheikh, T.M.Y.; Al-Fifi, Z.I.; Alabboud, M.A. Larvicidal and repellent effect of some Tribulus terrestris L., (Zygophyllaceae) extracts against the dengue fever mosquito, Aedes aegypti (Diptera: Culicidae). J. Saudi Chem. Soc. 2012, 20, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Vivekanandhan, P.; Senthil-Nathan, S.; Shivakumar, M.S. Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiol. Mol. Plant Pathol. 2018, 101, 156–162. [Google Scholar] [CrossRef]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V. Mosquito larvicidal activity of methyl-p-hydroxybenzoate isolated from the leaves of Vitex trifolia Linn. Acta Trop. 2011, 120, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Saokaew, S.; Kiatsomboon, I.; Piromyaporn, M.; Sivasupaporn, N.; Pusparajah, P.; Chan, K.G.; Goh, B.H.; Lee, L.H.; Phayak, T.; Kengkla, K.; et al. Repellent And Larvicidal Activity Of Thai Medicinal Plants Against Mosquitoes: A Systematic Review And Meta-Analysis. Value Health 2017, 20, A681. [Google Scholar] [CrossRef]
- Cheng, S.S.; Lin, C.Y.; Chung, M.J.; Liu, Y.H.; Huang, C.G.; Chang, S.T. Larvicidal activities of wood and leaf essential oils and ethanolic extracts from Cunninghamia konishii Hayata against the dengue mosquitoes. Ind. Crop. Prod. 2013, 47, 310–315. [Google Scholar] [CrossRef]
- Thongwat, D.; Lamlertthon, S.; Pimolsri, U.; Bunchu, N. Larvicidal activity of endocarp and seed crude extracts of Dracaena loureiri Gagnep against Aedes aegypti (L.) mosquito. Asian Pac. J. Trop. Biomed. 2017, 7, 222–226. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Huang, C.-G.; Chen, W.-J.; Kuo, Y.-H.; Chang, S.-T. Larvicidal activity of tectoquinone isolated from red heartwood-type Cryptomeria japonica against two mosquito species. Bioresour. Technol. 2008, 99, 3617–3622. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Larvicidal activity of the essential oil from Amomum subulatum Roxb. (Zingiberaceae) against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae), and non-target impact on four mosquito natural enemies. Physiol. Mol. Plant Pathol. 2018, 101, 219–224. [Google Scholar] [CrossRef]
- Chong, S.-L.; Hematpoor, A.; Hazni, H.; Sofian-Azirun, M.; Litaudon, M.; Supratman, U.; Murata, M.; Awang, K. Mosquito larvicidal limonoids from the fruits of Chisocheton erythrocarpus Hiern. Phytochem. Lett. 2019, 30, 69–73. [Google Scholar] [CrossRef]
- Park, D.H.; Choi, J.Y.; Lee, S.-H.; Kim, J.H.; Park, M.G.; Kim, J.Y.; Wang, M.; Kim, H.J.; Je, Y.H. Mosquito larvicidal activities of farnesol and farnesyl acetate via regulation of juvenile hormone receptor complex formation in Aedes mosquito. J. Asia-Pac. Entomol. 2020, 23, 689–693. [Google Scholar] [CrossRef]
- Hapairai, L.K.; Mysore, K.; Sun, L.; Li, P.; Wang, C.-W.; Scheel, N.D.; Lesnik, A.; Scheel, M.P.; Igiede, J.; Wei, N.; et al. Characterization of an adulticidal and larvicidal interfering RNA pesticide that targets a conserved sequence in mosquito G protein-coupled dopamine 1 receptor genes. Insect Biochem. Mol. Biol. 2020, 120, 103359. [Google Scholar] [CrossRef]
- Castelino, P.A.; Naik, P.; Dasappa, J.P.; Sujayraj, R.S.; Chandra, K.S.; Chaluvaiah, K.; Nair, R.; Kumari, M.S.; Kalthur, G.; Adiga, S.K. Synthesis of novel thiadiazolotriazin-4-ones and study of their mosquito-larvicidal and antibacterial properties. Eur. J. Med. Chem. 2014, 84, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, R.; Zheng, L.; Zhou, Y.; Wang, L.; Zhao, F.; Hassan, B.; Xu, Y. Toxicity and repellency of two anthranilates against Aedes albopictus Skuse (Diptera: Culicidae). Acta Trop. 2019, 200, 105171. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Bhavya, K.S.; Angalene, J.L.A.; Roshini, S.M.; Preethi, R.; Steffi, S.M.; Raji, P.; Kumar, S.S. Utilization of gum polysaccharide of Araucaria heterophylla and Azadirachta indica for encapsulation of cyfluthrin loaded super paramagnetic iron oxide nanoparticles for mosquito larvicidal activity. Int. J. Biol. Macromol. 2019, 153, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Pathalam, G.; Rajendran, H.A.D.; Appadurai, D.R.; Gandhi, M.R.; Michael, G.P.; Savarimuthu, I.; Naif, A.A.-D. Isolation and molecular characterization of actinomycetes with antimicrobial and mosquito larvicidal properties. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Kim, I.-H.; Ensign, J.; Kim, D.-Y.; Jung, H.-Y.; Kim, N.-R.; Choi, B.-H.; Park, S.-M.; Lan, Q.; Goodman, W.G. Specificity and putative mode of action of a mosquito larvicidal toxin from the bacterium Xenorhabdus innexi. J. Invertebr. Pathol. 2017, 149, 21–28. [Google Scholar] [CrossRef]
- Dhanasekaran, D.; Latha, S.; Suganya, P.; Panneerselvam, A.; Kumar, T.S.; Alharbi, N.S.; Arunachalam, C.; Alharbi, S.A.; Thajuddin, N. Taxonomic identification and bioactive compounds characterization of Psilocybe cubensis DPT1 to probe its antibacterial and mosquito larvicidal competency. Microb. Pathog. 2020, 143, 104138. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, G.D.; Kumar, V. Mosquito-larvicidal BinA toxin displays affinity for glycoconjugates: Proposal for BinA mediated cytotoxicity. J. Invertebr. Pathol. 2018, 156, 29–40. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, G.D.; Kumar, V. Receptor protein of Lysinibacillus sphaericus mosquito-larvicidal toxin displays amylomaltase activity. Insect Biochem. Mol. Biol. 2018, 93, 37–46. [Google Scholar] [CrossRef]
- Sharma, M.; Hire, R.S.; Hadapad, A.B.; Gupta, G.D.; Kumar, V. Polyethylene Glycol Conjugation Enhances Mosquito-Larvicidal Activity of Lysinibacillus Sphaericus BinA Protein. Biophys. J. 2017, 112 (Suppl. 1), 49a. [Google Scholar] [CrossRef] [Green Version]
- Bhuvaneswari, R.; Xavier, R.J.; Arumugam, M. Larvicidal property of green synthesized silver nanoparticles against vector mosquitoes (Anopheles stephensi and Aedes aegypti). J. King Saud Univ. Sci. 2016, 28, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Denver, CO, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Denver, CO, USA, 2017. [Google Scholar]
- TWEPA. Heterotrophic Plate Count (NIEA E203.56B Method); Taiwan Environmental Protection Agency: Taipei, Taiwan, 2021. Available online: https://www.epa.gov.tw/niea/F664644BCCE9FB43 (accessed on 20 September 2021).
- ASM. Kirby-Bauer Disk Diffusion Susceptibility Test; American Society of Microbiology: Washington, DC, USA, 2009; Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf (accessed on 20 September 2020).
- Akolgo, G.A.; Essandoh, E.O.; Gyamfi, S.; Atta-Darkwa, T.; Kumi, E.N.; de Freitas Maia, C.M.B. The potential of a dual purpose improved cookstove for low income earners in Ghana–Improved cooking methods and biochar production. Renew. Sustain. Energy Rev. 2018, 82, 369–379. [Google Scholar] [CrossRef]
- Gitau, K.J.; Mutune, J.; Sundberg, C.; Mendum, R.; Njenga, M. Factors influencing the adoption of biochar-producing gasifier cookstoves by households in rural Kenya. Energy Sustain. Dev. 2019, 52, 63–71. [Google Scholar] [CrossRef]
- Zhou, Q.; Houge, B.A.; Tong, Z.; Gao, B.; Liu, G. An in-situ Technique for Producing Low-Cost Agricultural Biochar. Pedosphere 2018, 28, 690–695. [Google Scholar] [CrossRef]



| Sample Types | pH | TN (%) | TP (mg/L) | K (mg/L) | Moisture (%) | Organic Portion (%) | Fix Portion (%) |
|---|---|---|---|---|---|---|---|
| SCL_date | 2.9 ± 0.15 | <0.01 | <0.2 | 4.3 ± 0.5 | 98.9 ± 0.2 | 1.1 ± 0.1 | 0.01 ± 0.006 |
| SCL_pomelo | 2.6 ± 0.18 | <0.01 | <0.2 | 2.3 ± 0.6 | 99.4 ± 0.2 | 0.6 ± 0.1 | 0.01 ± 0.006 |
| SCL_guava | 2.6 ± 0.13 | <0.01 | <0.2 | 1.8 ± 0.2 | 99.8 ± 0.2 | 0.2 ± 0.1 | 0.00 ± 0.000 |
| Eluted Time (min) | Standardized SCL Abundance @ and Percent of Total in Parenthesis | Compounds/Remarks | ||
|---|---|---|---|---|
| Date | Pomelo | Guava | ||
| 4.367 | 1,803,840 (4.0) | 1,695,168 (4.2) | - | Pyrazole, 1,4-dimethyl- |
| 7.448 | - | 1,077,328 (2.7) | 823,903 (4.2) | 2(5H)-Furanone |
| 9.233 | 390,152 (0.9) | - | - | Phenol |
| 10.274 | 2,270,628 (5.1) | 2,027,954 (5.1) | 1,359,437 (7.0) | 1,2-Cyclopentanedione, 3-methyl- |
| 11.227 | 1,346,975 (3.0) | - | - | Phenol, 3-methyl- |
| 11.468 | 5,047,432 (11.2) | 4,907,177 (12.3) | 2,671,629 (13.7) | Phenol, 2-methoxy- |
| 12.044 | 1,368,125 (3.0) | 571,430 (1.4) | 921,379 (4.7) | Maltol |
| 13.394 | 1,855,644 (4.1) | 1,600,951 (4.0) | 1,203,184 (6.2) | Creosol |
| 13.572 | 4,318,762 (9.6) | 3,347,830 (8.4) | 1,310,554 (6.7) | Catechol |
| 14.571 | 3,140,422 (7.0) | 2,525,284 (6.3) | 1,340,465 (6.9) | 1,2-Benzenediol, 3-methoxy- |
| 14.806 | 1,597,987 (3.6) | 1,244,550 (3.1) | 544,757 (2.8) | Phenol, 4-ethyl-2-methoxy- |
| 15.947 | 7,201,005 (16.0) | 5,289,310 (13.2) | 5,735,301 (29.4) | Phenol, 2,6-dimethoxy- |
| 19.483 | 600,658 (1.3) | 327,768 (0.8) | - | Phenol, 2,6-dimethoxy-4-(2-propenyl)- |
| Abundance of major compounds (% of total) | 30,941,630(68.9%) | 24,614,750 (61.6%) | 15,910,610 (81.6%) | The abundance of major ingredients (Percent of the major ingredients with respect to the total) |
| Abundance of phenolics (% of total) | 16,184,210 (36.1%) | 12,838,647 (32.1%) | 8,951,687 (45.9%) | The abundance of phenolic ingredients (Percent of the major ingredients with respect to the total) |
| Total abundance | 44,881,227 (100) | 39,970,517 (100) | 19,503,967 (100) | Total abundance = total ions of the GC-MS chromatogram |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.-S.; Shen, M.-W.; Chen, S.-T. Using Smoke Condensed Liquids from Pruned Fruit-Tree Branches for Aedes Mosquito Larva Control. Agriculture 2022, 12, 825. https://doi.org/10.3390/agriculture12060825
Yang D-S, Shen M-W, Chen S-T. Using Smoke Condensed Liquids from Pruned Fruit-Tree Branches for Aedes Mosquito Larva Control. Agriculture. 2022; 12(6):825. https://doi.org/10.3390/agriculture12060825
Chicago/Turabian StyleYang, Dun-Sheng, Meng-Wei Shen, and Shyi-Tien Chen. 2022. "Using Smoke Condensed Liquids from Pruned Fruit-Tree Branches for Aedes Mosquito Larva Control" Agriculture 12, no. 6: 825. https://doi.org/10.3390/agriculture12060825
APA StyleYang, D.-S., Shen, M.-W., & Chen, S.-T. (2022). Using Smoke Condensed Liquids from Pruned Fruit-Tree Branches for Aedes Mosquito Larva Control. Agriculture, 12(6), 825. https://doi.org/10.3390/agriculture12060825








