Abstract
Popcorn is a food highly appreciated throughout the world, generating billions of dollars annually just in the North American market alone. Even in the face of the historical superiority of American hybrids, which occupy almost 100% of the grain production fields in Brazil, our researchers have been working to develop cultivars that combine important traits for the crop, such as tolerance to leaf diseases and high rates of grain yield and popping expansion. This work investigated the degree of genetic divergence among 40 diallel hybrids of popcorn, 16 parents and 6 elite controls using mixed models to discriminate superior materials to be adopted in the Brazilian agribusiness. Based on the individual Best Linear Unbiased Prediction (BLUP) of each of the 15 variables analyzed, the Unweighted Pair-Group Method using Arithmetic Averages (UPGMA) and Tocher clusters were performed, and the study of Pearson correlation was carried out. The results pointed out that there is genetic variability among the genotypes evaluated and that the best candidates for composing superior genotypes are in the combination between the P10×L77 parents and/or between the P7 and L88 lines. Linear correlations showed that earlier flowering and taller genotypes exhibited an association with materials more tolerant to Exserohilum turcicum intensity.
1. Introduction
Popcorn is a food globally appreciated, with the U.S. being the main producer country of both seeds and grains, as well as having the largest consumer market, handling approximately USD 2 billion dollars per year [1].
Regarding the Brazilian domestic market, the monetary value of popcorn grain is approximately three times higher than that of common corn [2]. Such difference is related to the high cost of grain production, leading to the importation of seeds primarily from the U.S. and Argentina [3]. To give greater autonomy to the Brazilian demand for popcorn grains, breeding procedures by hybrid selection become of utmost relevance, particularly linked to studies with materials of different climatic adaptations for discrimination of genotypes more tolerant to diseases, one of the main obstacles for the adaptation of crops in the tropical environment [4,5].
Regardless of this, the cultivation of popcorn has been advancing in Brazilian regions with little climate variation, as in Campo Novo do Parecis, state of Mato Grosso. This municipality has shown itself as the most productive in the country by combining the practical knowledge of the producers, the adequate pre- and post-harvest handling, and the agronomic potential of imported seeds. According to information from the Ministry of Agriculture, Livestock, and Food Supply (MAPA), there are at present 154 records of popcorn cultivars, while common corn records total 6314 [6]. Considering this numerical discrepancy, it is evident that there is the need to enhance the research on popcorn breeding in Brazil to make the domestic market less dependent on imported seeds.
In the current economic scenario, production costs make it very difficult for the private sector to invest, and the large seed companies prefer to import hybrids rather than partner with the public sector seed companies. Such partnerships would be of great importance to foster further development and production of domestic hybrids with superior popcorn quality. At the end of the 1990s, the main reasons why the country offered the domestic market and its producers low-quality popcorn were the low rate of investment in research and the lack of regulatory legislation for the commercialization of the grains [7]. Today, 24 years have passed and the research on popcorn breeding is still scarce, with few breeders to leverage the production of superior genetic materials to be used in the Brazilian seed industry.
In any event, until public–private partnerships are in place, some public research institutions are working to help reduce the dependence of the country on North American and Argentinean popcorn seeds, which are found in almost 100% of the main grain production fields of Brazilian growers. To compete with hybrid seeds that produce up to six tonnes per hectare, researchers of this special kind of corn need to be more objective in defining lines of studies consistent with the possible factors that directly or indirectly increase the productive cost of the species.
When the final goal of an investigation is the development of elite hybrids, the initial steps of genetic divergence studies within and between base populations are of utmost importance. Having established the most genetically divergent clusters, it is possible to reliably determine which parents will be involved in the final crosses and then enjoy the heterotic effect after the demanding but rewarding hybridization process.
Various studies of popcorn diversity have been successfully conducted [8,9,10,11,12,13,14,15,16,17,18,19], raising the expectation of obtaining superior genotypes. When considering analysis methods, and somewhat putting aside the usual strict independence of errors of the analysis of variance (ANOVA), a modeling that is appropriate to be used because it is a bit more flexible, but no less applicable, is the Restricted Maximum Likelihood (REML) method. This methodology is usually best applied in more complex experimental procedures, such as in predicting the genetic values of several possible selection candidates by providing the variance components with good accuracy. Resende et al. [20] state that, as the name implies, the best linear and unbiased predictor of genetic values is the BLUP (Best Linear Unbiased Predictor). The flexibility of the BLUP method is in the association of a linear model that is mixed, in other words, composed of fixed and random effect factors, disregarding the mean and the error that have always fixed and random effects, respectively. Lima et al. [1] analyzed the selective accuracy of controls regarding partially inbred S3 progenies of popcorn following the BLUP methodology and efficiently estimated the combinatorial abilities of the genotypes involved for all the variables evaluated. An interesting and useful piece of information to obtain is the Pearson [21] correlation coefficient, this being a linear measurement estimate between two variables. The correlation establishes how one variable behaves in a scenario where another is varying and, thus, infers whether there is any relationship between the variability of the two. Therefore, it becomes possible to evaluate what happens to one variable (x) while another variable (y) increases or decreases its magnitude, and this relationship may be quantified by means of a coefficient. This information is useful precisely because it makes it possible to quantify the relationships between pairs of variables, making evaluation processes easier. Another interesting analysis is the correlation network, in which it becomes possible to pre-define clusters of related variables and verify how they are interrelated.
This work aimed to infer the degree of genetic divergence among forty diallel hybrids, sixteen parents and six controls with different soil and climate adaptations as to morpho-agronomic and disease-related characteristics, positioning them against six controls as a premise for registering and releasing superior genotypes for Brazilian agribusiness.
2. Materials and Methods
2.1. Obtaining Diallel Hybrids
Sixteen lines of popcorn in the seventh generation of self-fertilization (S7) were crossed in a circular diallel scheme based on the methodology proposed by Kempthorne and Curnow [22], in the Colégio Agrícola Antônio Sarlo, in the municipality of Campos dos Goytacazes, located in the Northern Region of Rio de Janeiro State, Brazil (Supplementary Material—Table S1).
The whole procedure to obtain the hybrids was performed in a manually directed way, following the methodology applied in the Laboratory of Plant Genetic Breeding (in Portuguese, LMGV) of the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), specifically: after establishing all 40 hybrid combinations to be carried out, ears of each female parent were covered with plastic bags before a single stigma-style structure emerged from the leaf to avoid any contamination. When these style-stigma structures, properly protected, presented an adequate growth size, the tassels of the respective male parents, at an ideal flowering growth stage (up to 50% anthesis), were covered with Kraft paper bags suitable for pollination.
The directed crosses were made the day after the covering of the tassels, using the paper bags with the pollen grains of each male parent to quickly and carefully pollinate and subsequently protect the ears of each female parent. The pollinations were performed on approximately 40 ears per cross to achieve an adequate number of seeds for the evaluation of the hybrids in the following step.
2.2. Hybrid Evaluation
The evaluation of 62 treatments (40 single hybrids, 16 parental lines and 6 controls—3 hybrids and 3 varieties) (Supplementary Material—Table S2) was conducted in the same environment in which the hybrids were obtained.
This experiment was designed according to the statistical model proposed by Hallauer et al. [23], in randomized blocks with four repetitions. The seeds were sown at a depth of 0.05 m in 5.00 m rows with 0.90 m spacing between the rows and 0.20 m between the plants, totaling a stand of 26 plants. Fifteen traits were evaluated, which included the ones related to leaf diseases and those related to the morpho-agronomic ones.
The disease screening method was performed in a natural way, for both diseases. The leaf diseases (Exserohilum turcicum and Bipolaris maydis) were evaluated as to the incidence of leaf lesions on the whole plant for E. turcicum (IET) and for B. maydis (IBM), and the degree of severity was sampled on the median portion of the adaxial surface of the first ear leaf (SET—for E. turcicum and SBM—for B. maydis), both performed visually and by the same evaluator.
The diagrammatic scale proposed by Agroceres [24] was used to evaluate incidence, in which: grade 1 = 0% of incidence, grade 2 = 0.5% of incidence, grade 3 = 10% of incidence, grade 4 = 30% of incidence, grade 5 = 50% of incidence, grade 6 = 70% of incidence, grade 7 = 80% of incidence, grade 8 = 90% of incidence, and grade 9 = 100% of incidence on the plant. As for the evaluation of severity, specific classification scales were used for each of the diseases. For E. turcicum, the scale suggested by Vieira et al. [25] was adopted to estimate the percentage of lesion in the leaf area, with values of 0.5, 1.6, 5, 15, 37, 66, 87, and 96%. Regarding B. maydis, the severity was estimated following the diagrammatic scale proposed by James [26], with percentages of 1, 5, 25, and 50% of lesion in the leaf area. Both disease evaluations were carried out on five plants per plot, with the five initial and final plants of the plot being eliminated, considering the fifteen central plants as the useful plot. Two evaluations were made, with fifteen-day intervals between them, and conducted during the male flowering season.
The morpho-agronomic traits evaluated were: plant height (PH) and ear height (EH), both in centimeters; number of plants (NP); number of ears (NE); grain yield (GY) quantified by weighing the threshed grains, expressed in t ha−1; ear weight (EW), obtained by weighing the ears of each plot still with the cob and represented in g; number of ears diseased (NED); a 100-grain weight (100GW), expressed in g; popping expansion (PE), determined in two repetitions per treatment, from the expansion of 30 g of grains per repetition, in a microwave oven at maximum power of 1000 watts for two minutes and ten seconds, and subsequently measured the volume of expanded popcorn in a test tube, and expressed in mL·g−1; volume of expanded popcorn per hectare (VP), resulting from the product between average grain yield and popping expansion and expressed in m3·ha−1 [27]; days to flowering (DF), calculated by counting the number of days from sowing until 50% of the plot has reached male ripening. It should be emphasized that traits NE, GY, and PE had their values adjusted according to the ideal stand of each plot, in other words, considering 26 plants [28].
2.3. Statistical Analysis
To conduct the study of genetic diversity among the 62 treatments, firstly, genetic values were estimated by linear mixed model estimates [20]. The genetic evaluation of the accession clusters was derived from the statistical model y = Xu + Zg + e, in which y, u, g, and e are data vectors, of the overall mean effect (fixed), genotypic effect (random), and errors (random), respectively; X and Z are the incidence matrices for u and g, respectively. The mixed model equations applied for the estimate of the overall mean and the prediction of genetic values were: , in which I is an identity matrix, is the genotypic variance, and s the residual variance.
The statistical model used in the Rbio statistical program [29] to obtain the BLUPs was of order 4: Y = m + B + A + e, in which the experimental design considered is the randomized complete block (RCBD) scheme with fix effects for blocks and random effects for genotypes and error/residue. To obtain the genetic parameters, the statistical likelihood ratio test (LRT) was performed by means of deviation analysis, and the treatment effect evaluated by the chi-square test with one degree of freedom. From the individual BLUPs, the distance matrix between the accessions was generated using the average Euclidean distance for standardized data between pairs of matrices ( and ), with this distance being a measure of dissimilarity defined by the following expression: , in which is the value of character k related to matrix I, and represents the same character k for matrix j. After obtaining the dissimilarity matrix, two clustering methods were established, the hierarchical (Unweighted Pair-Group Method using Arithmetic Averages) and the Tocher optimization ones [30]. The UPGMA method is commonly used to delimit clusters by means of a dendrogram, the matrix distance between pairs of genotypes being represented by , according to the following example:
in which d11 = d22 = … = dnn = 0.
For determining the optimal number of clusters (cut-off point) in the UPGMA dendrogram, the Mojena method [31] was selected, as it is based on the relative size of the fusion levels (distances). Based on the UPGMA clustering method as well, the relative contribution of each trait evaluated to the genetic divergence by the Singh method [32] was determined.
In the non-hierarchical method of Tocher, the clusters are established by the distance between individual k and the cluster formed by individuals ij, determined by d(ij)k = dik + djk, in which the entry, or not, of individual k in the cluster is made, considering that:
- if: , individual k is included in the cluster;
- if: , individual k is not included in the cluster.
The number of individuals that constitute the original cluster is considered as n, and θ is the greatest value of the set of the smallest distances between individuals. In the Tocher methodology, clustering is based on the formation of clusters in which the distances within the clusters are smaller than the distances between clusters.
The analysis of simple linear correlation (Pearson) was conducted with significance evaluated by the t test among the fifteen traits analyzed, and the magnitude of the coefficient values (r) interpreted according to Devore [33]. Correlation graphs between the fifteen variables and a correlation network were finally plotted. To establish the network of correlations, the traits were arranged into two clusters, referred to as yield and disease. The yield cluster was composed of traits PH, EH, NE, GY, EW, NED, 100GW, and VP. The disease cluster was formed by traits IET, IBM, SET, SBM, NP, PE, and DF. All statistical analyses were conducted by means of RStudio [34], Genes [35], and Rbio [29] software.
3. Results
Table 1 shows the values of the statistical likelihood ratio test (LRT) of deviation analysis, chi-square and genetic parameters, evaluating sixty-two popcorn genotypes. According to the LRT test, the treatment effect (genotypes) was significant for thirteen traits evaluated, NE and NED did not differentiate this effect, according to the chi-square test.
Table 1.
Values of the statistical likelihood ratio test (LRT) of deviation analysis, chi-square and genetic parameters, evaluating 62 popcorn genotypes.
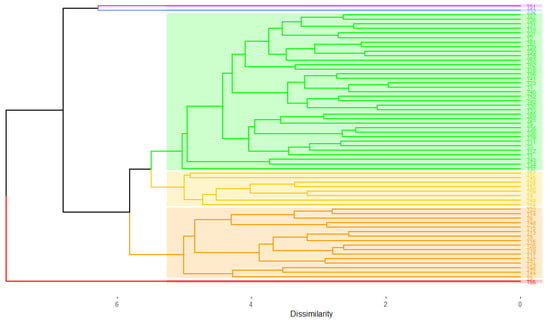
After estimating the individual BLUPs for each trait, the standardized Mean Euclidean Distance matrix was determined among the sixty-two genotypes evaluated. The dendrogram obtained by the UPGMA cluster analysis provided the formation of six clusters (Figure 1), three of which were made up of three large clusters, and another three, each consisting of only one genotype. The cophenetic correlation coefficient of the clustering formed was 0.70.
Figure 1.
Dendrogram obtained by the UPGMA method for sixty-two popcorn genotypes evaluated considering morpho-agronomic and disease resistance traits.
Among the three largest clusters, one was composed of 8 genotypes (G3); one of 16 genotypes (G2); the last one, of 36 (G4) (Table 2). The three treatments and/or genotypes that proved to be most divergent were T55 (line L88), T32 (hybrid P10 × L77), and T51 (line P7), these being allocated to clusters G1, G5, and G6, respectively (Table 1).
Following the Singh [32] method, the UPGMA clustering performed based on the Euclidean distance by unstandardized means indicated that the trait that contributed the most to establish divergence was ear weight (EW), with a magnitude of 99.43%. The remaining 0.57% was distributed among the other traits.
According to the non-hierarchical Tocher optimization method, seven clusters were formed, with two of them containing the largest number of genotypes (G1 and G2), two clusters with two genotypes (G3 and G4), and three clusters each consisting of only one genotype (G5, G6, and G7) (Table 3). There was a relative coincidence between the two clustering methods, as using the Tocher method, genotypes T32 (P10 × L77) and T55 (L88) again did not cluster with the other genotypes analyzed, and T51(P7) clustered with only one other genotype T34 (P10 × P1).
Table 3.
Description of the clusters of popcorn genotypes formed according to Tocher optimization method.
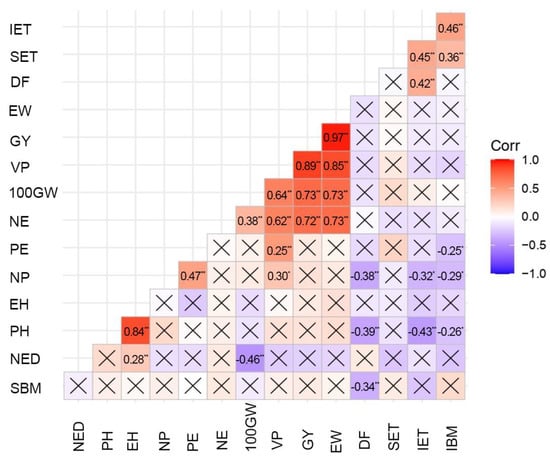
Among the 15 traits analyzed, the Pearson linear correlations showed that, of the 105 correlations obtained, 28 were significant at the 5 or 1% significance level, which is approximately 26% of the estimates. Of the 28 significant correlations, 11 were of low magnitude (39.3%), 9 were moderate (32.1%), 7 expressed a strong relation (25%), and only 1 proved to be very strong (3.6%) (Figure 2).
Figure 2.
Estimates of simple linear correlations among 15 traits evaluated in genotypes of popcorn. * and ** indicate significance at 5% and 1% probability level, respectively. Boxes with an “X” indicate no significant correlation (p < 0.05). On the right side of the correlogram, the color legend shows the correlation coefficients and the corresponding colors and “-“ indicates negative values. NED – number of ears diseased; PH—plant height; EH—ear height; NP—number of plants; PE—popping expansion; NE—number of ears; 100GW—a 100-grain weight; VP—volume of expanded popcorn per hectare; GY—grain yield; EW—ear weight; DF—days to flowering; SET—severity of E. turcicum; IET—incidence of E. turcicum; IBM—incidence of B. maydis; SBM—severity of B. maydis.
Strong correlations of positive magnitude are seen among traits related to yield in popcorn. As an example, the correlations between GY and traits EW (0.97) and VP (0.89) are highlighted; EW, in turn, correlated in a positive way with VP. (0.85). The latter, to a lesser magnitude, correlated with PE (0.51). As well as these, a correlation of 0.84 was observed between PH and EH. As for the negative correlations, all were considered weak. To exemplify this, the correlations between NP and DF (−0.38), IET (−0.32), and IBM (−0.29) are highlighted. PH also exhibited negative associations with DF (−0.39), IET (−0.43), and IBM (−0.26). Even though considered weak, the largest negative correlation, of magnitude −0.46, could be seen between NED and 100GW.
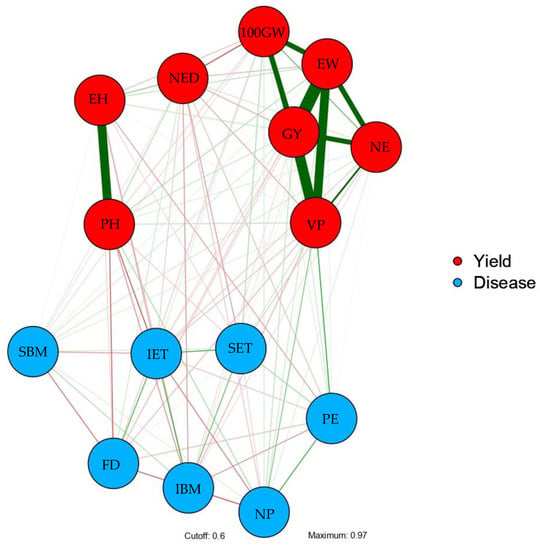
When it comes to the correlation network, of all the traits that were allocated within the red-colored network, PH and EH had a strong relationship and were also closer to the trait network designated by yield than to the disease network. 100GW, EW, GY, NE, and VP interrelated most closely, and with greater magnitude, within the whole yield network. When analyzing the part of the network designated as disease, it is evidently noted that diseases were most strongly related to DF, NP and, interestingly, also to PE (Figure 3).
Figure 3.
Correlation network obtained among 15 related traits from the Pearson linear correlation. Yield Group: PH—plant height; EH—ear height; NED—number of ears diseased; 100GW—a 100-grain weight; EW—ear weight; GY—grain yield; NE—number of ears; VP—volume of expanded popcorn per hectare. Disease group: SET—severity of E. turcicum; IET—incidence of E. turcicum; IBM—incidence of B. maydis; SBM—severity of B. maydis; NP—number of plants; PE—popping expansion; DF—days to flowering.
4. Discussion
In more recent years, mixed models have been increasingly employed in breeding programs involving annual crops since the robustness of this methodology had already been demonstrated in many perennial species regarding the estimates of genetic parameters [36,37,38]. Some examples of studies involving annual crops include soybean [39], common maize [40,41], and popcorn [1,42,43]. Given the potentiality of the statistical technique and the demand for reliable superior popcorn genotypes to be used in the seed industry, the REML/BLUP methodology was used to examine the genetic divergence among six controls and forty diallel hybrids and to indicate traits associated with greater resistance of these genotypes to diseases caused by E. turcicum and B. maydis, which have a great impact on the reduction in yield in popcorn culture.
When it comes to genetic diversity among the material in question, the cut-off point, at 5.13, used according to the Mojena [31] methodology allowed the constitution of six clusters in the cluster analysis based on the UPGMA method (Figure 1). This cut-off point proved to be efficient to evaluate the phenotypic association among landraces and to determine clusters of popcorn genotypes in a work developed by Cheim et al. [44]. Studies on diversity in the genus Zea based on morpho-agronomic variables have been successfully performed to form clusters based on the UPGMA methodology [41,45,46,47,48]. The cophenetic correlation coefficient of 0.70 demonstrated a good consistency of the clustering pattern, and it is such to estimate a linear correlation between the distance matrix elements and the elements of the cophenetic matrix. High cophenetic correlation coefficients have also been seen in other genetic divergence work in maize [48,49], with this being considered a good predictor to corroborate the consistency of the clustering pattern performed [35].
Among the genotypes investigated, those that proved to be most divergent were lines L88 (T55) and P7 (T51), as well as the hybrid P10 × L77 (T32), each forming a single cluster. Line L88 was developed by the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF) in 2009 and is derived from the variety Viçosa-Viçosa. This open-pollinated variety was developed by the Universidade Federal de Viçosa (UFV) and refers to a synthetic population from the cross between local varieties and North American hybrids. In a previous study conducted by Santos et al. [50], who analyzed the gene effects of the incidence and severity of B. maydis and E. turcicum in fifty-six diallel hybrids of popcorn evaluated in two growing seasons in the Northern Region of the state of Rio de Janeiro, Brazil, it was verified that of the nine hybrids with the best phenotypic response for the diseases and also for traits GY and PE, six had as one of the parents the lines L88 or L77, demonstrating that, besides being divergent, such lines expressed favorable dominance effects when in crosses for the traits under study.
As for the diallel hybrid P10 × L77 (T32), it comes from the combination between the lines P10 and L77, respectively, originating from the modified simple hybrid IAC 112 and the variety (OPV) ‘Viçosa-Viçosa’. Unfortunately, the production of IAC 112 was discontinued by the Instituto Agronômico de Campinas (IAC, São Paulo State, Brazil), but it demonstrated itself to be a great living fighter, not only worthy of carrying the IAC name, but also for being a genotype that raised the level of yield and grain expansion among hybrid popcorn cultivars of national origin. Bakes et al. [51] conducted two experiments in the Northern Plateau of Santa Catarina State, Brazil, to evaluate the performance of 25 popcorn genotypes, and IAC 112 held the first place for both grain yield and popping expansion. In addition, this valuable single modified hybrid expressed low grades for severity of three diseases—Phaeosphaeria maydis, Exserohilum turcicum, and Puccinia sorghi.
In a study very similar to this presented here, but evaluating the genetic merit of diallel lines and hybrids for E. turcicum, B. maydis, and P. polisora, along with grain yield and popping expansion of twenty-eight simple hybrids from a complete diallel, Santos et al. [52] found that, of the three most promising hybrids both for the three diseases and for GY and PE, one of them came from the cross between L77 and the line L61, which reinforces the good agronomic performance and interesting genetic divergence between the L77 genotype and the others.
Last but not least, the line P7 (T51) has its origin in the triple hybrid ‘Zaeli’. Originally from North America, this elite genotype has a temperate climate genealogy, and the lines from this hybrid have proven to be good gene carriers for disease tolerance in different environments. Amaral Júnior et al. [53] evaluated the reaction of 25 popcorn lines in environments with different phosphorus levels as to the incidence and severity of B. maydis and observed that among the most tolerant lines to the severity of the disease analyzed, either for environments with high or low phosphorus level, line P7 distinguished itself among the four genotypes with the best response.
One finding that drew attention was that, of the eight genotypes that composed one of the clusters, seven are lines (87.5%) originating both from hybrids and OPVs (P1—triple hybrid Zélia; L54—Beija-Flor variety; L61 and L70—BRS-Angela; L76, L77, and L80—‘Viçosa-Viçosa’) (Supplementary Material—Table S2 and Table 2). Additionally concerning this cluster, it should be noted that, according to the hierarchical clustering method used, the lines L54, L76, L77, and L80, developed from the ‘Beija-Flor’ and ‘Viçosa-Viçosa’ varieties, possibly remained closer because they were obtained from varieties developed by the same institution, the UFV.
Still considering the UPGMA method, another significant finding was that the largest cluster formed (G4) included four of the six controls used, of which three (T60—UENF14; T62—BRS-Angela; T61—UFVM 2 Barão de Viçosa) are open-pollinated varieties, and the fourth control (T41–IAC 125) is a topcross hybrid also originating from a cross between a simple hybrid and an OPV. This largest number of genotypes in G4 is consistent with the genetic structure present in a variety, in other words, germplasm with high variability.
Regarding the clustering based on the Tocher optimization method, seven clusters were formed. Two of these clusters gathered two genotypes (G3: P10 × P1 and L51; G4: P1 and L54), and three gathered each with only one genotype, being: L61 allocated in G5; P10 × L77 allocated in G6; L88, in G7. This result emphasizes the possibility of indicating the most divergent genotypes (L88—T55; L61—T57; L49—T54; P7—T51) as parents to be used in base-crosses to compose superior hybrids for the main morpho-agronomic traits of popcorn and for higher tolerance to the leaf diseases evaluated.
The existence of correlation among the quantitative traits was verified by means of the Pearson correlation coefficient (Figure 2). Considering that some traits that present the possibility of indirect selection for the economic traits of greater importance in popcorn, GY and PE, need to express positive and high magnitude correlation estimates, as it happened with GY × EW (r = 0.97), VP × GY (r = 0. 89), VP × EW (r = 0.85), and PH × EH (r = 0.84). An interesting correlation to be analyzed, and that was moderate, occurred between PE × VP (r = 0.51), whose relevance is in the fact that VP involves a direct relation with PE and GY, which was verified in this study as very weak and not significant at 5% probability (r = 0.11).
In analyzing the correlations between the traits related to the diseases studied and the other traits evaluated, it is found that the significant relations showed magnitudes of moderate to weak, which makes any inference reasonably reliable. When only the magnitudes of moderate relation were considered, it was possible to verify that IET was positively associated with IBM, SET, and DF and negatively with PH (Figure 2). In this way, the intensity estimates of E. turcicum tended to coincide with those of B. maydis intensity, as well as with those of E. turcicum severity and the number of days to flowering, while taller plants tended to express lower magnitudes of IET. It may be inferred, therefore, that earlier flowering and taller genotypes were, on average, more tolerant to E. turcicum intensity.
In a relevant study on the fungus E. turcicum, which causes North Corn Leaf Blight (NCLB) in U.S. corn crops, Ding et al. [54] inferred, by evaluating a panel of 999 lines for 56,110 SNPs, that the gene control of this causal agent is a complex trait controlled by several genes with relatively low effect. The authors concluded, then, that the pyramiding of these genes is an opportunity to achieve the desired stable resistance to this disease.
When analyzing the correlation network (Figure 3), first, it is important to understand that the green and red lines connecting each trait represent positive and negative correlations, respectively. The thickness of the line represents the value of the coefficient, in other words, the closer to the unit, the thicker the line is. Among the traits allocated within the network depicted by the red color, the PH and EH exhibited a strong association and were closer to the network of traits composed of the yield cluster than to the network of the cluster called disease. In turn, traits 100GW, EW, GY, NE, and VP showed an interrelation in a closer manner and with higher magnitude within all the yield networks. By considering the part of the disease network, it can be clearly seen that the diseases were significantly related to DF, NP and, curiously, to PE. The network revealed itself to be very informative indeed, becoming a very interesting analytical procedure to conceive the levels of association between all variables in a single plan.
5. Conclusions
On the basis of the UPGMA clustering methodology, the best candidates for use in breeding programs to obtain greater tolerance to leaf diseases (E. turcicum and B. maydis), in association with the main agronomic traits of popcorn, were hybrid P10 × L77 or line P7, the latter as one of the contrasting parents, and line L88 complementing the cross. An option worth testing would be to obtain a new topcross hybrid using P10 × L77 as the donor parent and the Viçosa-Viçosa variety, from which the L88 line was obtained, as the receptor population.
This scientific evidence suggests that crosses involving parents P7, L88, and L77 may be good stepping-stones in the gene pyramid against E. turcicum. Therefore, they deserve attention when the goal of the study refers to the composition of contrasting genotypes and, simultaneously, with satisfactory phenotypic responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12070910/s1, Table S1: Scheme of crosses in circulating diallel between 16 lines of popcorn with S = 5.; Table S2: Description of 16 lines used as parents to obtain diallel hybrids and six popcorn controls.
Author Contributions
Conceptualization, G.F.P., G.S.M., A.T.d.A.J., R.F.A., L.L.B., J.S.S., M.G.P., G.d.A.G. and R.F.D.; methodology, G.F.P., G.S.M., A.T.d.A.J., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B., F.N.V., M.G.P., G.d.A.G. and R.F.D.; software, G.F.P., G.S.M., R.F.A., L.L.B., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B. and F.N.V.; validation, G.F.P., G.S.M., R.F.A., L.L.B., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B. and F.N.V.; formal analysis, G.F.P., G.S.M., M.G.P., G.d.A.G. and R.F.D.; investigation, G.F.P., G.S.M., A.T.d.A.J., R.F.A., L.L.B., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B., F.N.V., M.G.P., G.d.A.G. and R.F.D.; resources, G.F.P. and A.T.d.A.J.; data curation, G.F.P., G.S.M., R.F.A., L.L.B., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B. and F.N.V.; writing—original draft preparation, G.F.P., G.S.M., A.T.d.A.J., R.F.A., L.L.B., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B., F.N.V., M.G.P., G.d.A.G. and R.F.D.; writing—review and editing, G.F.P., G.S.M., A.T.d.A.J., R.F.A., L.L.B., J.S.S., S.H.K., V.J.d.L., T.d.O.S., R.B.B., F.N.V., M.G.P., G.d.A.G. and R.F.D.; visualization, G.F.P., G.S.M. and A.T.d.A.J.; supervision, A.T.d.A.J., R.F.A., L.L.B., M.G.P., G.d.A.G. and R.F.D.; project administration, A.T.d.A.J.; funding acquisition, A.T.d.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—grant number 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lima, V.J.; Viana, A.P.; Amaral Junior, A.T.; Kamphorst, S.H.; Leite, J.T.; Santos, P.H.A.D.; Bispo, R.B.; Santos, T.O. Exploring the use of testers to maximize selection accuracy of partially inbred S3 popcorn progenies. Rev. Bras. Cienc. Agrar. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- CEASA/RS—Centro de Abastecimento do Rio Grande do Sul. Boletim de Preços de Atacado. Available online: https://ceasa.rs.gov.br/ (accessed on 22 March 2022).
- SFAGRO. Milho Pipoca Atrai Produtores Que Buscam Maior Renda e Diversificação de Culturas. Available online: https://www.agrolink.com.br/noticias/milho-pipoca-atrai-produtores-que-buscam-maior-renda-e-diversificacao-de-culturas_404907.html#:~:text=Custo%20e%20produtividade&text=O%20custo%20de%20produ%C3%A7%C3%A3o%20ficou,custo%20de%20semente%20mais%20alto (accessed on 2 June 2022).
- Oliveira, G.H.F.; Amaral, C.B.; Revolti, L.T.M.; Buzinaro, R.; Moro, G.V. Genetic variability in popcorn synthetic population. Acta Sci. Agron. 2019, 41, 2–9. [Google Scholar] [CrossRef]
- Kurosawa, R.N.F.; Amaral Junior, A.T.; Vivas, M.; Almeida, R.N.; Vivas, J.M.S.; Lima, V.J.; Silveira, S.F. Diallel analysis for resistance to northern leaf blight in popcorn under contrasting nitrogen availability. Agron. J. 2021, 113, 1029–1038. [Google Scholar] [CrossRef]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Registro Nacional de Cultivares RNC. Available online: https://www.agricultura.gov.br/guia-de-servicos/registro-nacional-de-cultivares-rnc (accessed on 22 March 2022).
- Pacheco, C.A.P.; Gama, E.E.G.E.; Guimarães, P.E.O.; Santos, M.X.; Ferreira, A.S. Estimativas de parâmetros genéticos nas populações CMS 42 e CMS 43 de milho pipoca. Pesqui. Agropecuária Bras. 1998, 33, 1995–2001. [Google Scholar]
- Miranda, G.V.; Coimbra, R.R.; Godoy, C.L.; Souza, L.V.; Guimarães, L.J.M.; Melo, A.V. Potencial de melhoramento e divergência genética de cultivares de milho-pipoca. Pesqui. Agropecuária Bras. 2003, 38, 681–688. [Google Scholar] [CrossRef]
- Rinaldi, D.A.; Pipolo, V.C.; Gerage, A.C.; Ruas, C.F.; Fonseca Junior, N.S.; Souza, A.; Souza, S.G.H.; Garbuglio, D.D. Correlação entre heterose e divergência genética estimadas por cruzamentos dialélicos e marcadores moleculares RAPD em populações de milho-pipoca. Bragantia 2007, 66, 183–192. [Google Scholar] [CrossRef]
- Silva, T.A.; Pinto, R.J.B.; Scapim, C.A.; Mangolin, C.A.; Machado, M.F.P.S.; Carvalho, M.S.N. Genetic divergence in popcorn genotypes using microsatellites in bulk genomic DNA. Crop Breed. Appl. Biotechnol. 2009, 9, 31–36. [Google Scholar] [CrossRef][Green Version]
- Arnhold, E.; Silva, R.G.; Viana, J.M.S. Seleção de linhagens S5 de milho-pipoca com base em desempenho e divergência genética. Acta Sci. Agron. 2010, 32, 279–283. [Google Scholar] [CrossRef]
- Saavedra, J.; Aparecida Silva, T.; Mora, F.; Scapim, C.A. Bayesian analysis of the genetic structure of a Brazilian popcorn germplasm using data from simple sequence repeats (SSR). Chil. J. Agric. Res. 2013, 73, 04–05. [Google Scholar] [CrossRef]
- Pena, G.F.; Amaral Júnior, A.T.; Ribeiro, R.M.; Ramos, H.C.C.; Boechat, M.S.B.; Santos, J.S.; Mafra, G.S.; Kamphorst, S.H.; Lima, V.J.; Vivas, M.; et al. Inference of genetic diversity in popcorn S3 progenies. Genet. Mol. Res. 2016, 15, gmr.15028456. [Google Scholar] [CrossRef]
- Vaz-De-Melo, A.; Colombo, G.A.; Vale, J.C.; Santana, W.D.; Fernandes, M.S. Estratégias de seleção entre progênies meios-irmãos de milho pipoca no cerrado Tocantinense. Rev. Bras. Tecnol. Apl. Ciências Agrárias 2017, 10, 41–50. [Google Scholar] [CrossRef][Green Version]
- Guimaraes, A.G.; Amaral Junior, A.T.; Almeida Filho, J.E.; Pena, G.F.; Vittorazzi, C.; Pereira, M.G. Population structure and impact of recurrent selection on popcorn using EST-SSR markers. Acta Sci. Agron. 2018, 40, e35218. [Google Scholar] [CrossRef]
- Oliveira, N.C.; Suzukawa, A.K.; Pereira, C.B.; Santos, H.V.; Hanel, A.; Albuquerque, F.A.; Scapim, C.A. Popcorn Genotypes Resistance To Fall Armyworm. Ciência Rural 2018, 48, e20170378. [Google Scholar] [CrossRef]
- Vittorazzi, C.; Amaral Júnior, A.T.; Guimarães, A.G.; Silva, F.H.L.; Pena, G.F.; Daher, R.F.; Gerhardt, I.F.S.; Oliveira, G.H.F.; Santos, P.H.A.D.; Souza, Y.P.; et al. Evaluation of genetic variability to form heterotic groups in popcorn. Genet. Mol. Res. 2018, 17, gmr18083. [Google Scholar] [CrossRef]
- Mafra, G.S.; Amaral Júnior, A.T.; Almeida Filho, J.E.; Vivas, M.; Santos, P.H.A.D.; Santos, J.S.; Pena, G.F.; Lima, V.J.; Kamphorst, S.H.; Oliveira, F.T.; et al. SNP-based mixed model association of growth- and yield-related traits in popcorn. PLoS ONE 2019, 14, e0218552. [Google Scholar] [CrossRef]
- Castro, C.R.; Gonçalves, L.S.A.; Pinto, R.J.B.; Scapim, C.A.; Baba, V.Y.; Zeffa, D.M.; Kuki, M. Genetic diversity and diallel analysis of elite popcorn lines. Rev. Cienc. Agron. 2022, 53, 2022. [Google Scholar] [CrossRef]
- Resende, M.D.V. Métodos Estatísticos Ótimos na Análise de Experimentos de Campo; Embrapa Florestas: Colombo, RS, Brazil, 2004; 57p. [Google Scholar]
- Pearson, K. Notes on the history of correlation. Biometrika 1920, 13, 25–45. [Google Scholar] [CrossRef]
- Kempthorne, O.; Curnow, R.N. The partial diallel cross. Biometrics 1961, 17, 229–250. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, J.M.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding, 1st ed.; Springer: New York, NY, USA, 2010; 580p. [Google Scholar]
- Agroceres. Guia Agroceres de Sanidade, 1st ed.; Sementes Agroceres: São Paulo, Brazil, 1996; 72p. [Google Scholar]
- Vieira, R.A.; Mesquini, R.M.; Silva, C.N.; Hata, F.T.; Tessmann, D.J.; Scapim, C.A. A new diagrammatic scale for the assessment of northern corn leaf blight. Crop Prot. 2014, 56, 55–57. [Google Scholar] [CrossRef]
- James, W.C. A Manual of Assessment Keys of Plant Diseases, 1st ed.; Canada Department of Agriculture Publication: Saint Paul, MN, USA, 1971; 80p. [Google Scholar]
- Amaral Júnior, A.T.; dos Santos, A.; Gerhardt, I.F.S.; Kurosawa, R.N.F.; Moreira, N.F.; Pereira, M.G.; Gravina, G.A.; Silva, F.H.L. Proposal of a super trait for the optimum selection of popcorn progenies based on path analysis. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Schmildt, E.R.; Cruz, C.D.; Zanuncio, J.C.; Pereira, P.R.G.; Ferrão, R.G. Avaliação de métodos de correção do estande para estimar a produtividade em milho. Pesqui. Agropecuária Bras. 2001, 36, 1011–1018. [Google Scholar] [CrossRef]
- Bhering, L.L. Rbio: A tool for biometric and statistical analysis using the R platform. Crop Breed. Appl. Biotechnol. 2017, 17, 187–190. [Google Scholar] [CrossRef]
- Rao, C.R. Advanced Statistical Methods in Biometric Research; John Willey: New York, NY, USA, 1952; 390p. [Google Scholar]
- Mojena, R. Hierarquical grouping method and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant Breed. 1981, 41, 237–245. [Google Scholar]
- Devore, J.L. Probabilidade e Estatística: Para Engenharia e Ciências, 1st ed.; Thomson Pioneira: São Paulo, Brazil, 2006; 706p. [Google Scholar]
- R Core Team. R: A Language and ENVIRONMENT for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2021. Available online: http://www.R-project.org/ (accessed on 30 March 2022).
- Cruz, C.D. GENES—A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Viana, A.P.; Resende, M.D.V. Genética Quantitativa No Melhoramento de Fruteiras, 1st ed.; Editora Interciência: Rio de Janeiro, Brazil, 2014; 122p. [Google Scholar]
- Rodrigues, H.S.; Cruz, C.D.; Macêdo, J.L.; Resende, M.D.V.; Lopes, R.; Borém, A. Genetic variability and progeny selection of peach palm via mixed models (REML/BLUP). Acta Sci. Agron. 2017, 39, 2. [Google Scholar] [CrossRef][Green Version]
- Resende, M.D.V.; Alves, R.S. Linear, generalized, hierarchical, bayesian and random regression mixed models in genetics/genomics in plant breeding. Funct. Plant Breed. J. 2020, 2, 2. [Google Scholar] [CrossRef]
- Volpato, L.; Alves, R.S.; Teodoro, P.E.; Resende, M.D.V.; Nascimento, M.; Nascimento, A.C.C.; Ludke, W.H.; Silva, F.L.; Borém, A. Multi-trait multi-environment models in the genetic selection of segregating soybean progeny. PLoS ONE 2019, 14, e0215315. [Google Scholar] [CrossRef]
- Crevelari, J.A.; Pereira, M.G.; Azevedo, F.H.V.; Vieira, R.A.M. Genetic improvement of silage maize: Predicting genetic gain using selection indexes and best linear unbiased prediction. Rev. Cienc. Agron. 2019, 50, 197–204. [Google Scholar] [CrossRef]
- Kaefer, K.A.C.; Schuelter, A.R.; Schuster, I.; Marcolin, J.; Vendruscolo, E.C.G. Identification and characterization of maize lines resistant to leaf diseases. Semin. Ciências Agrárias 2019, 40, 517–526. [Google Scholar] [CrossRef]
- Viana, J.M.S.; Valente, M.S.F.; Scapim, C.A.; Resende, M.D.V.; Silva, F.F. Genetic evaluation of tropical popcorn inbred lines using BLUP. Maydica 2011, 56, 273–281. [Google Scholar]
- Freitas, I.L.J.; Amaral Júnior, A.T.; Viana, A.P.; Pena, G.F.; Cabral, P.D.S.; Vittorazzi, C.; Silva, T.R.C. Ganho Genético Avaliado Com Índices De Seleção E Com REML/Blup Em Milho-Pipoca. Pesqui. Agropecuária Bras. 2013, 48, 1464–1471. [Google Scholar] [CrossRef][Green Version]
- Cheim, L.M.G.; Costa, F.M.; Silva, N.C.A.; Caneppele, C.; Cesar, A.L.T.M.S.; Rossignoli, P.A.; Faria, A.M.M. Characterization of the seeds of a landrace popcorn (Zea mays L. subsp. mays) cropped in an organic system via Family Farming. Res. Soc. Dev. 2021, 10, e7110817141. [Google Scholar] [CrossRef]
- Kurosawa, R.N.F.; Vivas, M.; Amaral Junior, A.T.; Ribeiro, R.M.; Miranda, S.B.; Pena, G.F.; Leite, J.T.; Mora, F. Popcorn germplasm resistance to fungal diseases caused by Exserohilum turcicum and Bipolaris maydis. Bragantia 2018, 77, 35–47. [Google Scholar] [CrossRef]
- Sousa, A.M.C.B.; Chavaglia, A.C.; Civardi, E.A.; Pinto, J.F.N.; Reis, E.F. Genetic dissimilarity for resistance to foliar diseases associated with the agronomic potential in maize. Rev. Caatinga 2020, 33, 936–944. [Google Scholar] [CrossRef]
- Souza, R.; Ogliari, J.B.; Pinto, T.T. Analysis of on farm conservation of sweet corn in a diversity microcenter of Zea mays L. in Southern Brazil. Maydica 2020, 65, 1–11. [Google Scholar]
- Cordeiro, A.G.M.; Lima, J.S.; Pena, G.F.; Rossi, A.A.B.; Godinho, V.P.C.; Guimaraes, P.E.O. Diversidade genética entre genótipos de milho (Zea mays L.) a partir de caracteres morfoagronômicos. Rev. Ciências Agro-Ambient. 2021, 19, 126–131. [Google Scholar] [CrossRef]
- Pacheco, R.F.C.; Guimarães, A.G.; Oliveira, J.R.; Saraiva, E.A.; Santos, G.M.F.; Costa, M.R.; Guimarães, C.G. Caracterização morfológica e divergência genética de populações de milho crioulo do Alto Vale do Jequitinhonha. Rev. Agrar. Acad. 2019, 2, 15–26. [Google Scholar] [CrossRef]
- Santos, J.S.; Vivas, M.; Amaral Júnior, A.T.; Ribeiro, R.M.; Mafra, G.S.; Pena, G.F. Gene effects from Bipolaris maydis incidence and severity on popcorn. Rev. Bras. Ciências Agrárias 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Backes, R.L.; Balbinot Junior, A.A.; Sawazaki, E.; Gallotti, G.J.M.; Miranda, G.V. Desempenho de genótipos de milho pipoca no Planalto Norte Catarinense. Agropecuária Catarin. 2007, 20, 1, 78–81. [Google Scholar]
- Santos, J.S.; Souza, Y.P.; Vivas, M.; Amaral Junior, A.T.; Almeida, J.E.; Mafra, G.S.; Viana, A.P.; Gravina, G.A.; Ferreira, F.R.A. Genetic merit of popcorn lines and hybrids for multiple foliar diseases and agronomic properties. Funct. Plant Breed. J. 2020, 2, 33–47. [Google Scholar] [CrossRef]
- Amaral Junior, A.T.; Poltronieri, T.P.S.; Santos, P.H.D.; Vivas, M.; Gerhardt, I.F.S.; Carvalho, B.M.; Freitas, C.S.; Silveira, S.F. Reaction of popcorn lines (S7) cultivated in distinct phosphorus levels to Bipolaris maydis infection. Summa Phytopathol. 2019, 45, 18–22. [Google Scholar] [CrossRef]
- Ding, J.; Ali, F.; Chen, G.; Li, H.; Mahuku, G.; Yang, N.; Narro, L.; Magorokosho, C.; Makumbi, D.; Yan, J. Genome-wide association mapping reveals novel sources of resistance to northern corn leaf blight in maize. BMC Plant Biol. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).