Contributions of Plant Litter Decomposition to Soil Nutrients in Ecological Tea Gardens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Arrangement
2.3. Sample Collection and Testing
2.4. Data Analysis
2.5. Data Processing
3. Results
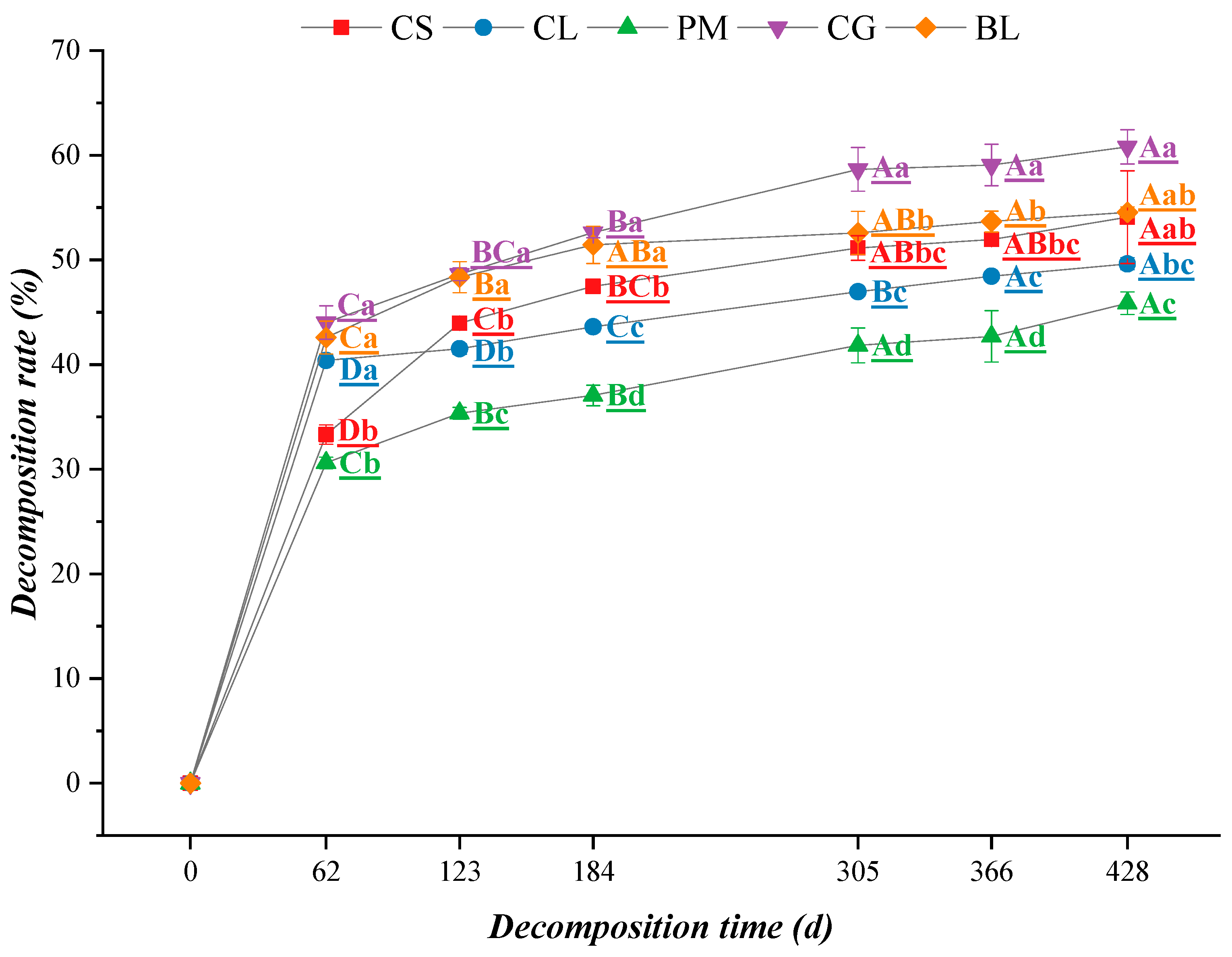
3.1. Decomposition Characteristics of Different Litters
3.1.1. Decomposition Rate
3.1.2. Decomposition Rate (k)
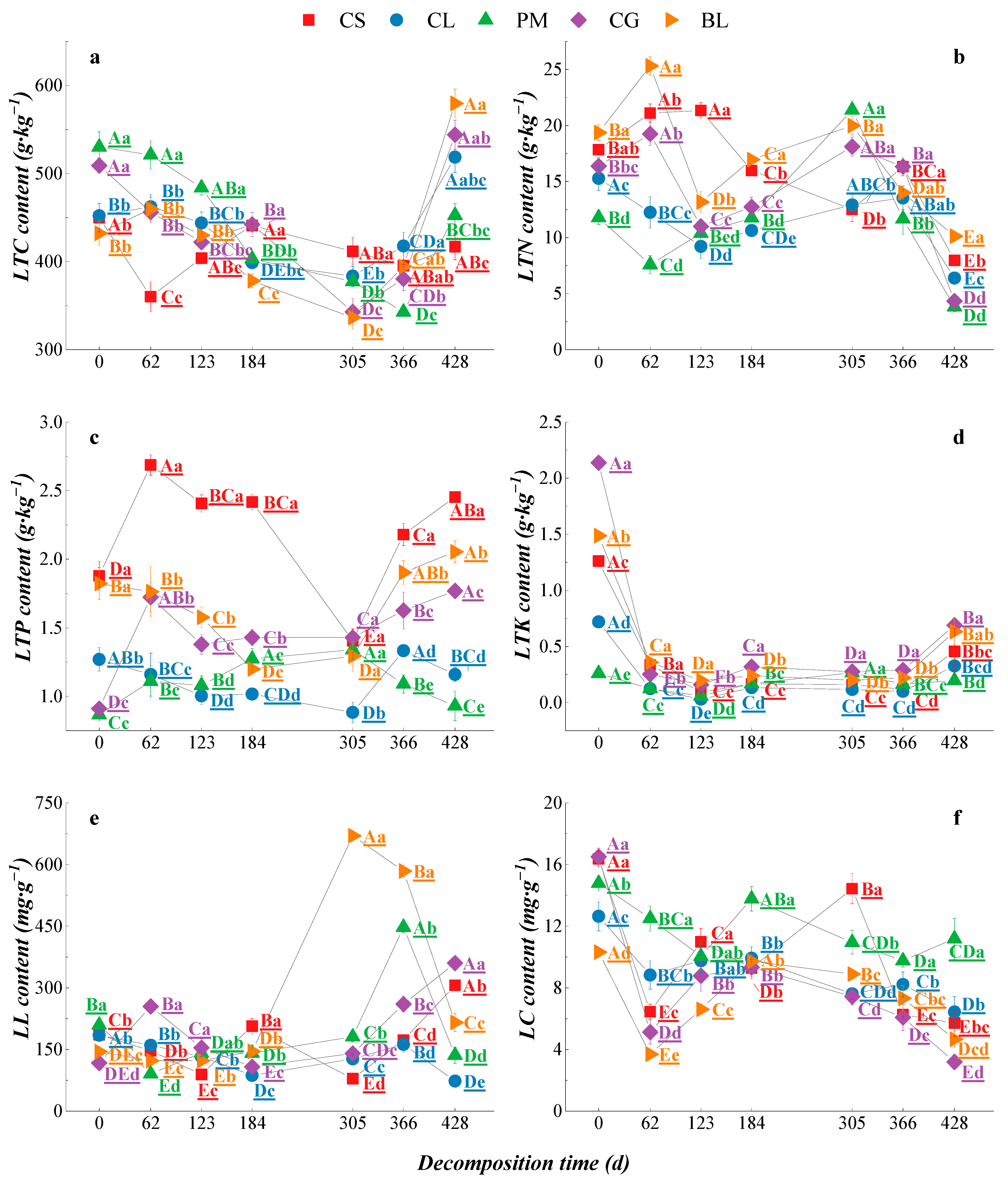
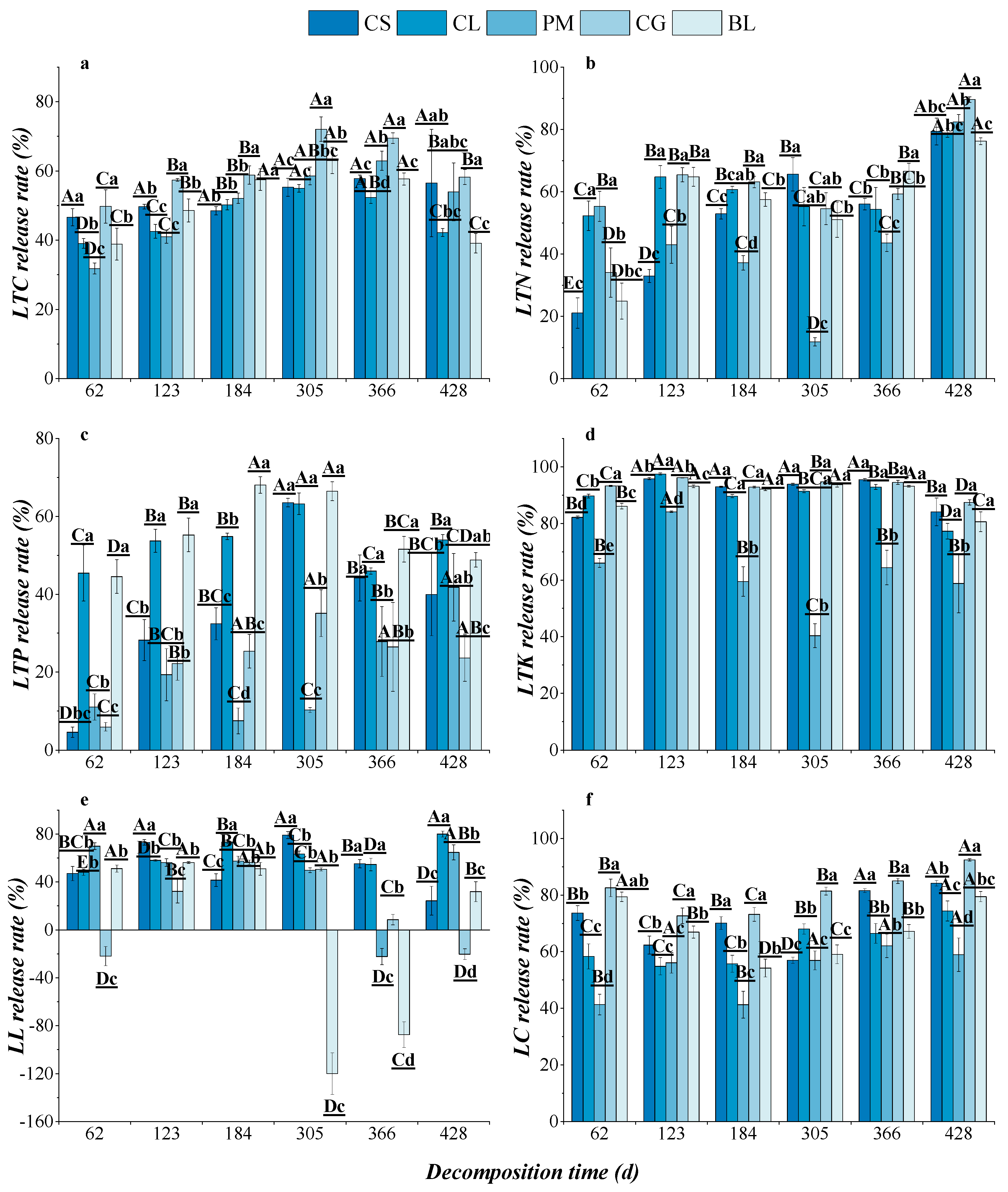
3.2. Nutrient Dynamics of Different Litter Decompositions
3.2.1. Dynamic Changes in the Nutrient Content of Litter
3.2.2. Dynamic Changes in Nutrient Release from Litter
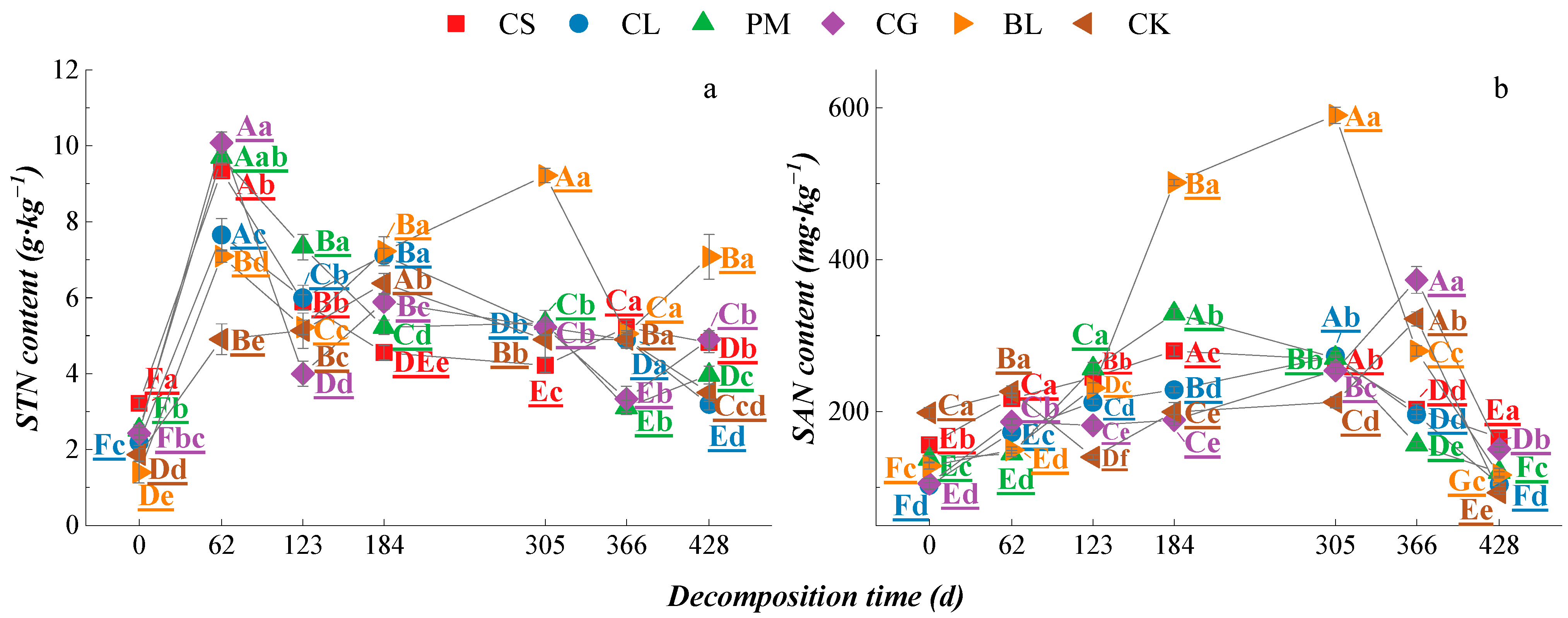
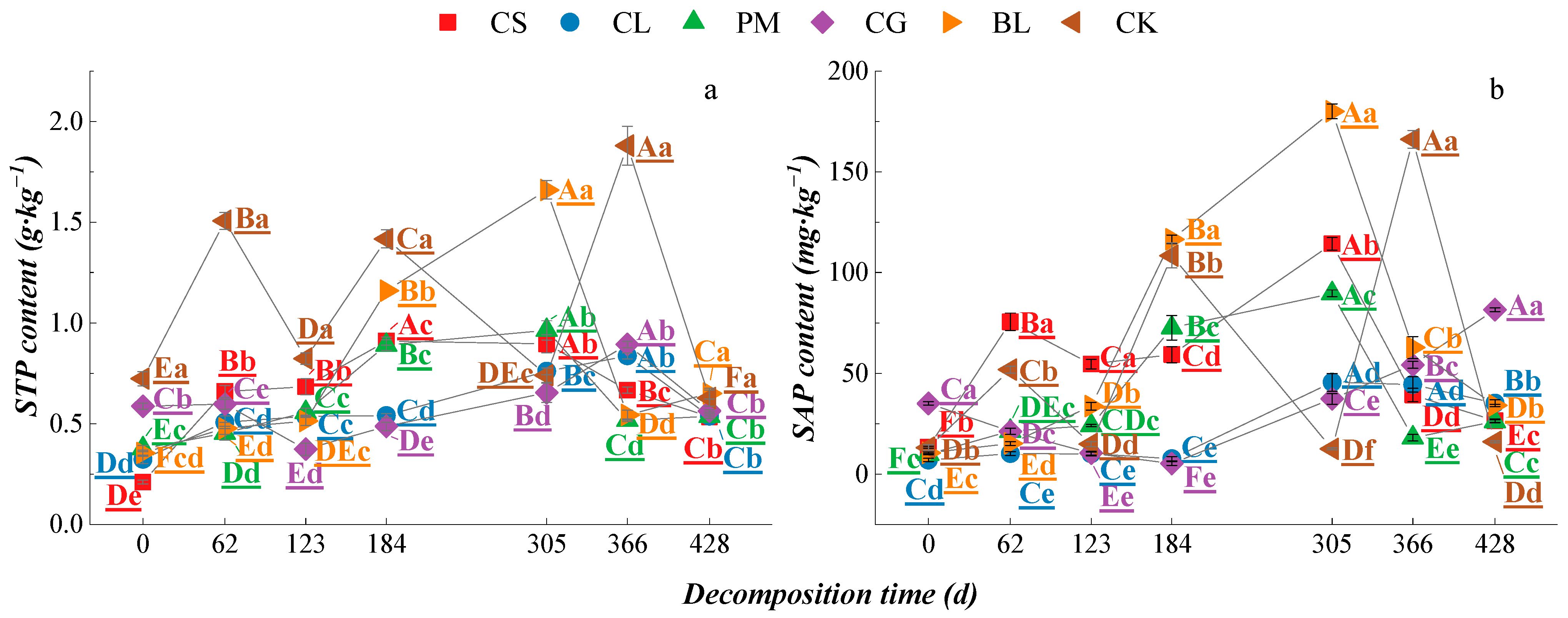
3.3. Soil Nutrient Characteristics of Different Litters during the Process of Decomposition
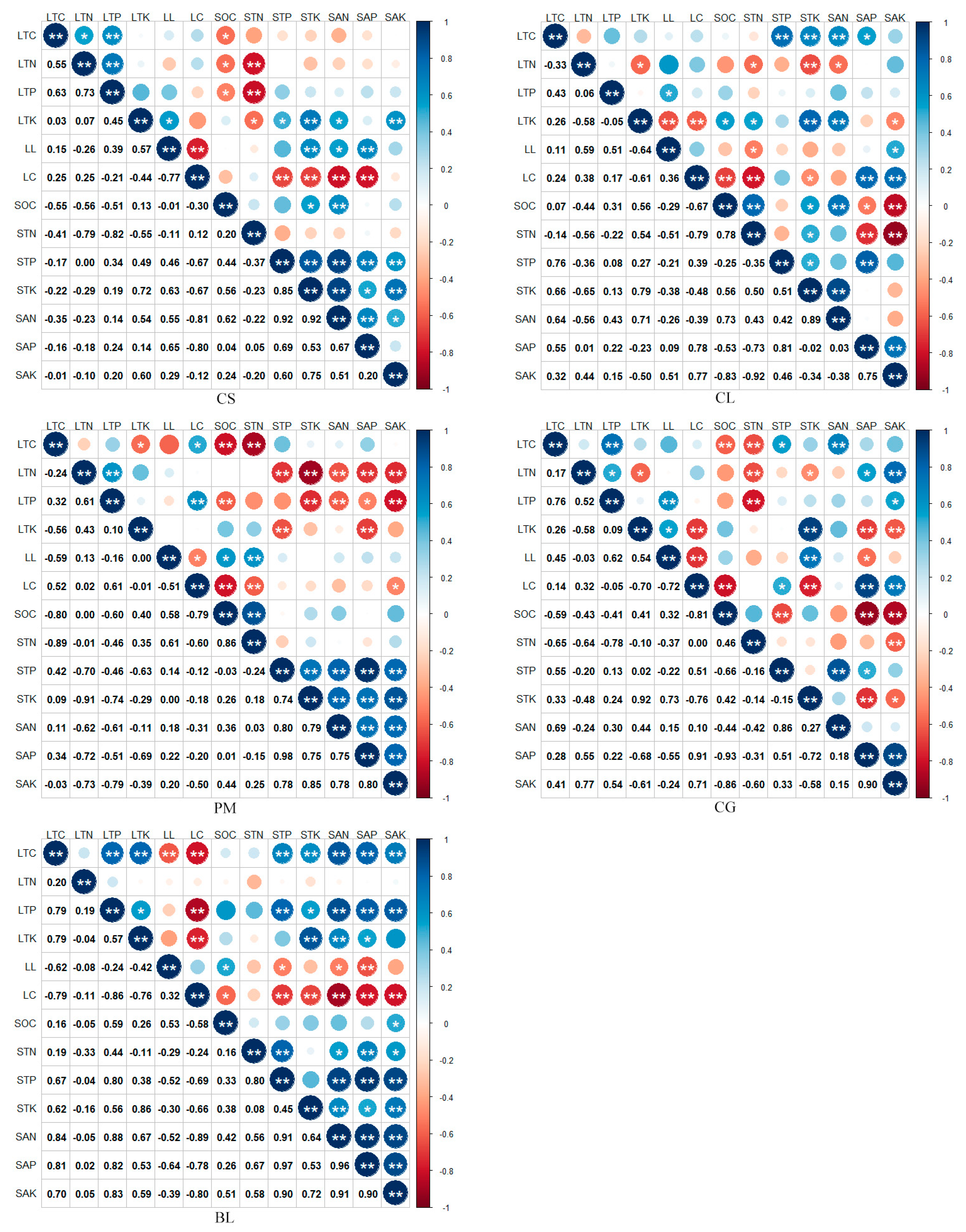
3.4. Relationship between Nutrient Release from Litter and Soil Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hornbach, D.J.; Shea, K.L.; Dosch, J.J.; Thomas, C.L.; Gartner, T.B.; Aguilera, A.G.; Anderson, L.J.; Geedey, K.; Mankiewicz, C.; Pohlad, B.R.; et al. Decomposition of Leaf Litter from Native and Nonnative Woody Plants in Terrestrial and Aquatic Systems in the Eastern and Upper Midwestern U.S.A. Am. Midl. Nat. 2021, 186, 51–75. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982, 63, 621–626. [Google Scholar]
- Lanuza, O.; Casanoves, F.; Delgado, D.; van den Meersche, K. Leaf litter stoichiometry affects decomposition rates and nutrient dynamics in tropical forests under restoration in Costa Rica. Restor. Ecol. 2019, 27, 549–558. [Google Scholar] [CrossRef]
- Chen, F.-S.; Wang, G.G.; Fang, X.-M.; Wan, S.-Z.; Zhang, Y.; Liang, C. Nitrogen deposition effect on forest litter decomposition is interactively regulated by endogenous litter quality and exogenous resource supply. Plant Soil 2019, 437, 413–426. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ilvesniemi, H.; Berg, B.; Kutsch, W.; Yang, Y.-S.; Ma, X.-Q.; Westman, C.J. Aboveground litterfall in Eurasian forests. J. For. Res. 2003, 14, 27–34. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosyst; Springer: New York, NY, USA, 2011. [Google Scholar]
- Berg, B.; Mcclaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Mao, J.; Tang, Y.; Yu, X.; Wang, Y.; He, Q.; Ran, L.; Liu, D. Research Progress on the Construction Model of Ecological Tea Garden in China. Tillage Cultiv. 2010, 30, 9–10. [Google Scholar] [CrossRef]
- Castle, S.; Rejmánková, E.; Foley, J.; Parmenter, S. Hydrologic alterations impact plant litter decay rate and ecosystem resilience in Mojave wetlands. Restor. Ecol. 2019, 27, 1094–1104. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Yu, L.; Zhou, C.; Yan, L.; Cai, G. Research on Leaf Llitter Decomposition and Hydrological Characteristics of Dominant Tree Species in the Caohai Wetland Watershed. J. Soil Water Conserv. 2014, 28, 98–103. [Google Scholar] [CrossRef]
- Bai, G.; Wang, Z.; Li, Y.; Cao, G.; Yang, Y.; Xue, L.; Ji, M.; Xing, Y. Effects of Short-term Litter Management on Soil Nutrient Changes in Larix principis-rupprechtii Plantation. J. Inn. Mong. For. Sci. Technol. 2021, 47, 30–32. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.; Wu, J.; Fu, X.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Baietto, A.; Hernández, J.; Del Pino, A. Comparative Dynamics of Above-Ground Litter Production and Decomposition from Eucalyptus grandis Hill ex Maiden and Pinus taeda L., and Their Contribution to Soil Organic Carbon. Forests 2021, 12, 349. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Antignani, V.; Capodilupo, M.; Mazzoleni, S. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 2010, 331, 481–496. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, J.; Zhang, Z.; Ren, F.; Chen, L.; He, J. Changes in litter quality induced by nutrient addition alter litter decomposition in an alpine meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2016, 6, 34290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, J.; Wang, K.; Song, Y.; Zhang, Y.; Zhang, Z.; Pan, T. Characteristics of litter return and nutrient dynamic change in four typical forests in the subalpine of central Yunnan province. J. Cent. South Univ. For. Technol. 2021, 41, 134–144. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecol. Soc. Am. 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Liu, Y.; Guo, K.; Zhao, H.; Qiao, X.; Wang, S.; Zhang, L.; Cai, X. Mixing litter from deciduous and evergreen trees enhances decomposition in a subtropical karst forest in southwestern China. Soil Biol. Biochem. 2016, 101, 44–54. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and Litter Decomposition in Terrestrial Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W.; Nicholsona, K.S.; Bonner, K.I.; Watsona, R.N. Response of soil microbial biomass dynamics, activity and plant litter decomposition to agricultural intensification over a seven-year period. Soil Biol. Biochem. 1999, 31, 1707–1720. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Zhang, Y.; Fu, X.; Tsang, Y.; Wu, J.; Le, Y. Variable decomposition of two plant litters and their effects on the carbon sequestration ability of wetland soil in the Yangtze River estuary. Geoderma 2018, 319, 230–238. [Google Scholar] [CrossRef]
- Sun, Z.; Mou, X.; Zhang, D.; Sun, W.; Hu, X.; Tian, L. Impacts of burial by sediment on decomposition and heavy metal concentrations of Suaeda salsa in intertidal zone of the Yellow River estuary, China. Mar. Pollut. Bull. 2017, 116, 103–112. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Dieye, T.; Assigbetse, K.; Diedhiou, I.; Sembene, M.; Dieng, A.; Gueye, M.; Masse, D. The effect of Jatropha curcas L. leaf litter decomposition on soil carbon and nitrogen status and bacterial community structure (Senegal). J. Soil Sci. Environ. Manag. 2016, 7, 32–44. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, F.; Yang, W.; Zhao, Y.; He, W.; Tan, B. Foliar litter nitrogen dynamics as affected by forest gap in the alpine forest of eastern Tibet Plateau. PLoS ONE 2014, 9, e97112. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liu, M.; Yuan, Z.; Zhang, S.; Li, C. Effects of different simulative sediment depths on litter decomposition and nutrient dynamic change of several annual herbaceous plants. Acta Ecol. Sin. 2020, 40, 7755–7766. [Google Scholar] [CrossRef]
- Moretto, A.S.; Distel, R.A. Decomposition of and nutrient dynamics in leaf litter and roots of Poa ligularis and Stipa gyneriodes. J. Arid. Environ. 2003, 55, 503–514. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.L.; Lin, G.G.; Gao, Y.C.; Fang, Y.T.; Zeng, D.H. Mixing effects of understory plant litter on decomposition and nutrient release of tree litter in two plantations in Northeast China. PLoS ONE 2013, 8, e76334. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, T.K.B.; Bustamante, M.M.D.C.; Kozovits, A.R. Diversity of shrub tree layer, leaf litter decomposition and N release in a Brazilian Cerrado under N, P and N plus P additions. Environ. Pollut. 2011, 159, 2236–2242. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Deng, C.; Chen, Y.; He, R.; Zhang, J.; Liu, Y. Relationships between decomposition rate of leaf litter and initial quality across the alpine timberline ecotone in Western Sichuan, China. Chin. J. Appl. Ecol. 2015, 26, 3602–3610. [Google Scholar] [CrossRef]
- Steffensen, J.P.; Andersen, K.K.; Bigler, M.; Clausen, H.B.; Dahl-Jensen, D.; Fischer, H.; Goto-Azuma, K.; Hansson, M.; Johnsen, S.J.; Jouzel, J.; et al. High-Resolution Greenland Ice Core Data Show Abrupt Climate Change Happens in Few Years. Science 2008, 321, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Hong, M.; Huo, L.; Ye, H.; Zhao, B.; De, H. Effects of exogenous nitrogen input and water change on litter decomposition in a desert grassland. Chin. J. Appl. Ecol. 2018, 9, 3167–3174. [Google Scholar] [CrossRef]
- Maisto, G.; De Marco, A.; Meola, A.; Sessa, L.; Virzo De Santo, A. Nutrient dynamics in litter mixtures of four Mediterranean maquis species decomposing in situ. Soil Biol. Biochem. 2011, 43, 520–530. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J. For. Res. 2004, 9, 23–31. [Google Scholar] [CrossRef]
- Xu, X.-N.; Shibata, H.; Enoki, T. Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: Dynamics of mineral nutrients. J. For. Res. 2006, 17, 1–6. [Google Scholar] [CrossRef]
- Moro, M.J.; Domingo, F. Litter Decomposition in Four Woody Species in a Mediterranean Climate: Weight Loss, N and P Dynamics. Ann. Bot. 2000, 86, 1065–1071. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Gao, X.; Duan, X.; Cao, Y.; Zhao, H.; Wang, J. Analysis on pH and Major Soil Nutrients of Tea Gardens in Key Tea Producing Areas of Guizhou. Southwest China J. Agric. Sci. 2015, 28, 286–291. [Google Scholar] [CrossRef]
- Dong, J.; Bian, J.; Zhu, Q.; Luo, Y. Relationship between tea aroma and soil conditions. J. Zhejiang Univ. (Agric. Life Sci.) 2013, 39, 309–317. [Google Scholar] [CrossRef]
- Liu, S.; Hu, L.; Chu, S.; Cao, Z.; Zeng, S. Decomposition characteristics of three forest litters and their effects on acidity and nutrient content in lateritic red soil. J. Plant Resour. Environ. 2013, 22, 11–17. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, D.; Tian, K.; Yu, H. Biomass production and litter decomposition of lakeshore plants in Napahai wetland, Northwestern Yunnan Plateau, China. Acta Ecol. Sin. 2013, 33, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yu, Y.; Li, Z.; Chen, J. Discussion on the Construction of Ecological Tea Garden and the reduction of Pesticide residues in Tea. Tea Sci. Technol. 2010, 51, 32–34. [Google Scholar] [CrossRef]



| Litter Type | LTC /g·kg−1 | LTN /g·kg−1 | LTP /g·kg−1 | LTK /g·kg−1 | LL /mg·g−1 | LC /mg·g−1 | C/N Ratio | LL/N Ratio |
|---|---|---|---|---|---|---|---|---|
| Camellia sinensis | 450.01 ± 19.24 b | 17.85 ± 1.40 ab | 1.88 ± 0.10 a | 1.26 ± 0.04 c | 171.40 ± 34.70 a | 16.37 ± 0.54 a | 25.35 ± 2.86 bc | 10.49 ± 1.41 b |
| Cunninghamia lanceolata | 452.12 ± 14.05 b | 15.28 ± 1.07 c | 1.27 ± 0.09 b | 0.72 ± 0.01 d | 184.74 ± 11.31 a | 12.64 ± 0.95 c | 29.7 ± 2.74 b | 12.16 ± 1.50 b |
| Pinus massoniana | 530.34 ± 16.94 a | 11.78 ± 1.62 d | 0.87 ± 0.05 c | 0.26 ± 0.01 e | 195.06 ± 25.34 a | 14.79 ± 0.49 b | 45.49 ± 5.48 a | 18.01 ± 2.40 a |
| Cinnamomum glanduliferum | 509.17 ± 8.5 a | 16.38 ± 0.74 bc | 0.91 ± 0.03 c | 2.14 ± 0.01 a | 117.20 ± 15.57 b | 16.51 ± 0.55 a | 31.11 ± 0.96 b | 7.19 ± 1.30 c |
| Betula luminifera | 432.22 ± 13.73 b | 19.37 ± 0.70 a | 1.82 ± 0.12 a | 1.49 ± 0.01 b | 159.05 ± 25.38 ab | 10.31 ± 0.14 d | 22.33 ± 0.62 d | 7.46 ± 0.47 c |
| Litter Type | Regression Equation | k | R2 | Half-Life/a | Turnover Period/a |
|---|---|---|---|---|---|
| Camellia sinensis | y = 78.305 × 10−0.045t | 0.045 b | 0.8697 ** | 1.31 ± 0.20 b | 5.68 ± 0.88 b |
| Cunninghamia lanceolata | y = 75.665 × 10−0.035t | 0.035 c | 0.9963 ** | 1.67 ± 0.03 a | 7.20 ± 0.12 a |
| Pinus massoniana | y = 82.056 × 10−0.033t | 0.033 c | 0.9791 ** | 1.75 ± 0.05 a | 7.57 ± 0.23 a |
| Cinnamomum glanduliferum | y = 73.000 × 10−0.052t | 0.052 a | 0.9658 ** | 1.11 ± 0.09 c | 4.79 ± 0.38 c |
| Betula luminifera | y = 71.900 × 10−0.040t | 0.040 bc | 0.8425 * | 1.44 ± 0.04 b | 6.24 ± 0.16 b |
| Litter Type | Factor | Explanation Rate/% | P |
|---|---|---|---|
| Camellia sinensis | LTC release rate | 43.75 | 0.005 |
| LTN release rate | 31.88 | 0.027 | |
| LTP release rate | 44.61 | 0.005 | |
| LTK release rate | 53.36 | 0.002 | |
| LL release rate | 58.58 | 0.002 | |
| LC release rate | 39.34 | 0.023 | |
| Cunninghamia lanceolata | LTC release rate | 62.02 | 0.002 |
| LTN release rate | 40.74 | 0.010 | |
| LTP release rate | 55.56 | 0.003 | |
| LTK release rate | 51.39 | 0.003 | |
| LL release rate | 48.00 | 0.005 | |
| LC release rate | 51.78 | 0.003 | |
| Pinus massoniana | LTC release rate | 76.96 | 0.003 |
| LTN release rate | 45.63 | 0.012 | |
| LTP release rate | 57.96 | 0.003 | |
| LTK release rate | 64.55 | 0.003 | |
| LL release rate | 52.24 | 0.003 | |
| LC release rate | 75.22 | 0.003 | |
| Cinnamomum glanduliferum | LTC release rate | 59.94 | 0.004 |
| LTN release rate | 58.64 | 0.004 | |
| LTP release rate | 60.82 | 0.003 | |
| LTK release rate | 46.70 | 0.005 | |
| LL release rate | 23.13 | 0.107 | |
| LC release rate | 55.23 | 0.004 | |
| Betula luminifera | LTC release rate | 38.34 | 0.013 |
| LTN release rate | 19.69 | 0.120 | |
| LTP release rate | 42.59 | 0.006 | |
| LTK release rate | 28.76 | 0.036 | |
| LL release rate | 89.33 | 0.001 | |
| LC release rate | 11.59 | 0.355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yang, R.; Peng, X.; Hou, C.; Ma, J.; Guo, J. Contributions of Plant Litter Decomposition to Soil Nutrients in Ecological Tea Gardens. Agriculture 2022, 12, 957. https://doi.org/10.3390/agriculture12070957
Liu S, Yang R, Peng X, Hou C, Ma J, Guo J. Contributions of Plant Litter Decomposition to Soil Nutrients in Ecological Tea Gardens. Agriculture. 2022; 12(7):957. https://doi.org/10.3390/agriculture12070957
Chicago/Turabian StyleLiu, Shaqian, Rui Yang, Xudong Peng, Chunlan Hou, Juebing Ma, and Jiarui Guo. 2022. "Contributions of Plant Litter Decomposition to Soil Nutrients in Ecological Tea Gardens" Agriculture 12, no. 7: 957. https://doi.org/10.3390/agriculture12070957






