Simulating Spring Barley Yield under Moderate Input Management System in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design, Management System, and Growing Conditions
2.3. Collected Data Set
- List of quantitative and qualitative data:
- ―
- Constant—the constant obtained during the analysis (called regression constant),
- ―
- Yield as the amount of seeds as tons of seed dry matter per hectare (dt ha−1) across 3 years (2016–2018) and across all locations (as a genetic potential of the genotype),
- ―
- NPK sum—NPK fertilization, sum,
- ―
- N sum—nitrogen mineral fertilization N -sum,
- ―
- Compl—soil complex valuation classes according to the soil quality evaluation system in Poland compatible with regulations of the Council of Ministers; class reflects the agricultural value of soils and a lower class means more fertile soils; (converted into a synthetic indicator according IUNG-PIB Pulawy),
- ―
- LT-lodgbfhar—lodging tendency before harvesting,
- ―
- Disease resistance: PM (powdery mildew), NB—net blotch, BBR (barley brown rust), SB (rhynchosporium); disease resistance was scored on 1–9 scale (9—no symptoms of the disease).
- Weather environmental variables
- ―
- The sum of rainfall: o1—in January, o2—in February, o3—in March, o4—in April, o5—in May, o6—in June, and o7—in July,
- ―
- Average monthly ground temperature: tg1—in January, tg2—in February, tg3—in March, tg4—in April, tg5—in May, tg6—in June, and tg7—in July,
- ―
- Average daily air temperature: tp1—in January, tp2—in February, tp3—in March, tp4—in April, tp5—in May, tp6—in June, tp7—in July.
2.4. Calculation Methodology
3. Results
3.1. Identification of Weather Environmental Variables Used in Multivariate Regression Analysis
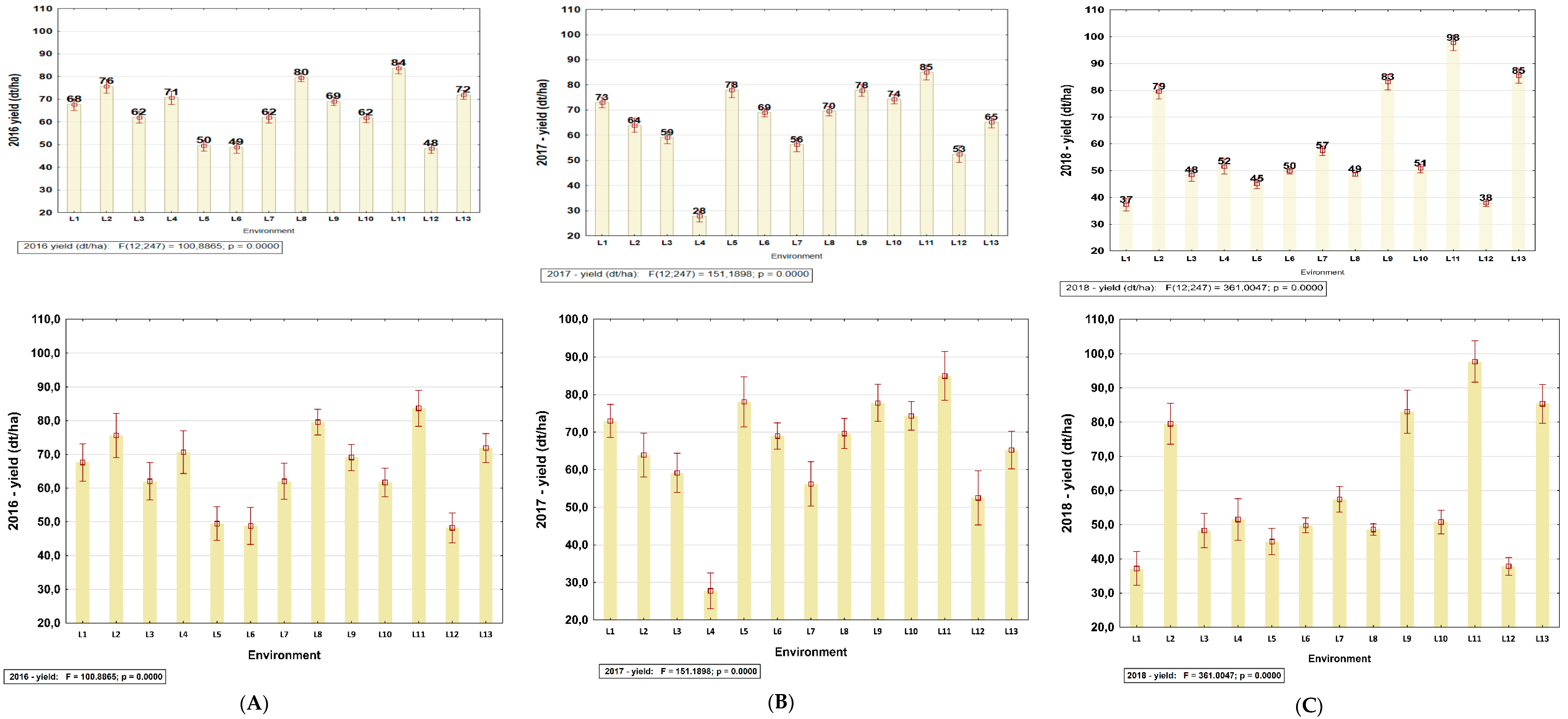
3.2. Identification of Yield Information for Site–Years Used in this Study
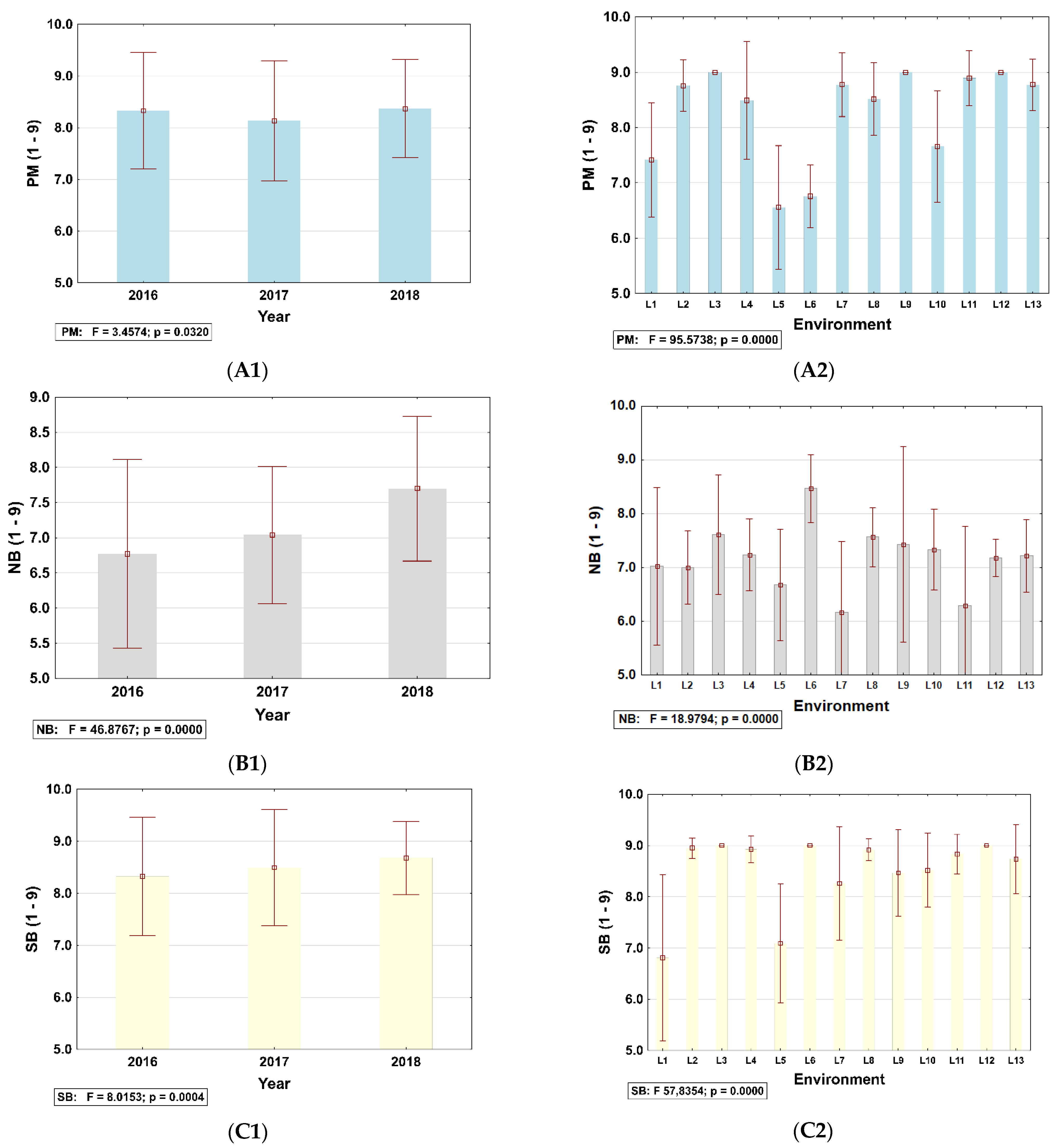
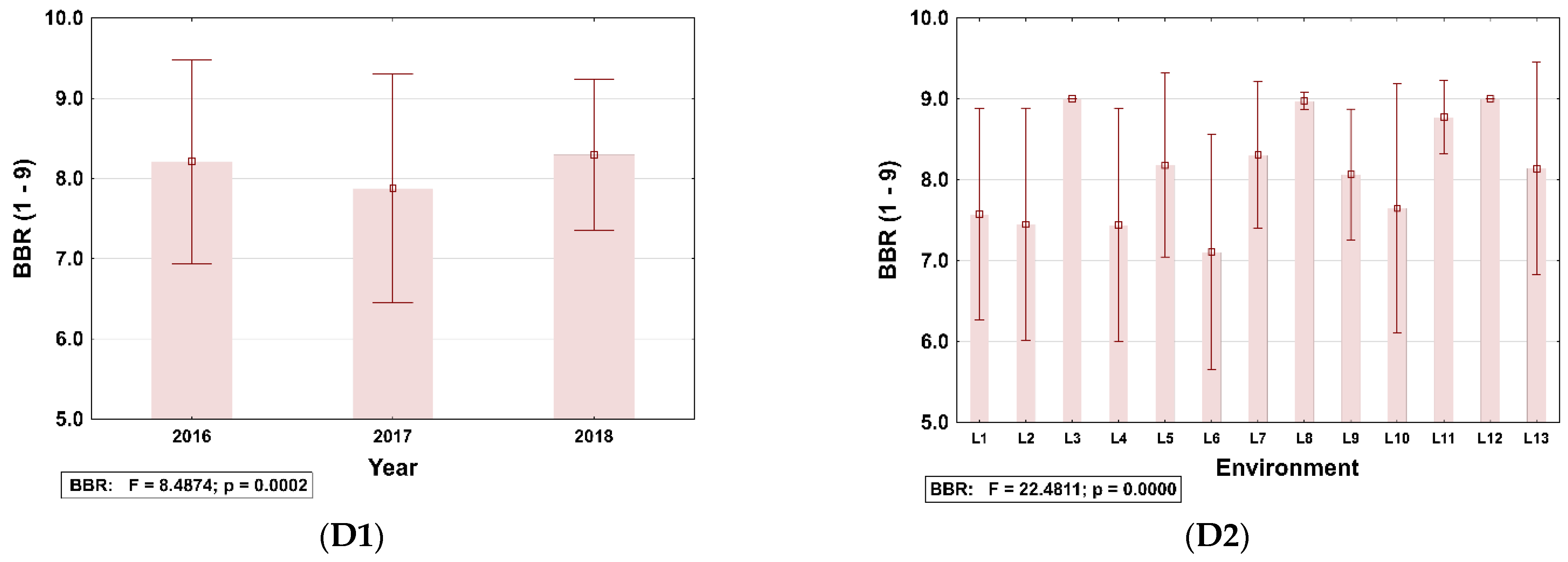
3.3. Identification of Disease Information for Site–Years Used in This Study
3.4. Impact of Diseases on Yield Potential
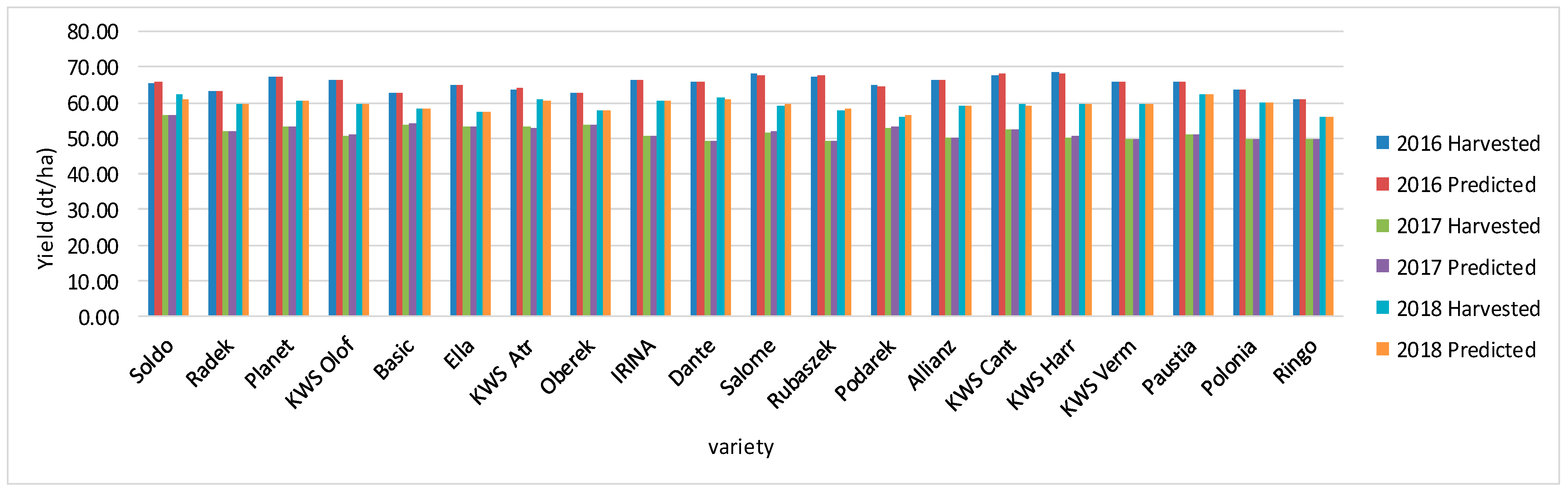
3.5. Regression Models
- (1)
- Ten traits have a similar effect (in the prediction model they have the same sign) on the yield of almost all varieties (i.e., u from 17 to 20). This is, e.g., a lodging tendency (LT) that occurred in the prediction model for 18 cultivars with a plus sign, and we write: (18-). Other traits are: o1 (19-), o2 (20+), tg3 (17+), tg4 (17-), tp3 (18-) and tp6 (17).
- (2)
- Twenty traits have a similar effect on the yield of more than half of the studied cultivars: o4 (16-), o7 (16-), tp2 (15+), tp4 (13+), tp7 (14+).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakellariou, M.; Mylona, P.V. New Uses for Traditional Crops: The Case of Barley Biofortification. Agronomy 2020, 10, 1964. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Pessarakli, M.; Mozafari, A.A.; Sohrabi, F.; Moradi, M.; Marvasti, F.B. Screening barley varieties tolerant to drought stress based on tolerant indices. J. Plant Nutr. 2021, 45, 739–750. [Google Scholar] [CrossRef]
- Wade, R.N.; Donaldson, S.M.; Karley, A.J.; Johnson, S.N.; Hartley, S.E. Uptake of silicon in barley under contrasting drought regimes. Plant Soil 2022. [Google Scholar] [CrossRef]
- López-Castañeda, C.; Richards, R. Variation in temperate cereals in rainfed environments I. Grain yield, biomass and agronomic characteristics. Field Crop. Res. 1994, 37, 51–62. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Christopher, J.; Devoil, P.; Hammer, G. The role of root architectural traits in adaptation of wheat to water-limited environments. Funct. Plant Biol. 2006, 33, 823–837. [Google Scholar] [CrossRef] [Green Version]
- Ingvordsen, C.H. Climate Change Effects on Plant Ecosystems—Genetic Resources for Future Barley Breeding. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2014. [Google Scholar]
- Rodrigues, P.C. An overview of statistical methods to detect and understand genotype-by-environment interaction and QTL-by-environment interaction. Biom. Lett. 2018, 55, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Vanga, S.K.; Saxena, R.; Orsat, V.; Raghavan, V. Effect of climate change on the yield of cereal crops: A review. Climate 2018, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Salem, M. Genotype by Environment Interactions for Yield-Related Traits in Tunisian Barley (Hordeum vulgare L.) Accessions under a Semiarid Climate. Acta Agrobot. 2021, 73, 1–10. [Google Scholar] [CrossRef]
- Nkurunziza, L.; Watson, C.A.; Öborn, I.; Smith, H.G.; Bergkvist, G.; Bengtsson, J. Socio-ecological factors determine crop performance in agricultural systems. Sci. Rep. 2020, 10, 4232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, L.; Yin, L.; Wang, Z.; Zheng, D. Evaluation of Sustainable and Analysis of Influencing Factors for Agriculture Sector: Evidence From Jiangsu Province, China. Front. Environ. Sci. 2022, 10, 836002. [Google Scholar] [CrossRef]
- De Temmerman, L.; Fangmeier, A.; Craigon, J. European Journal of Agronomy: Preface. Eur. J. Agron. 2002, 17, 231–232. [Google Scholar] [CrossRef]
- Nurminiemi, M.; Madsen, S.; Rognli, O.A.; Bjørnstad, Ã.; Ortiz, R. Analysis of the genotype-by-environment interaction of spring barley tested in the Nordic Region of Europe: Relationships among stability statistics for grain yield. Euphytica 2002, 127, 123–132. [Google Scholar] [CrossRef]
- Irmak, J.W.; Jones, W.D.; Batchelor, S.; Irmak, K.J.; Boote, J.O. Paz Artificial Neural Network Model as a Data Analysis Tool in Precision Farming. Trans. ASABE 2006, 49, 2027–2037. [Google Scholar] [CrossRef]
- Sroka, W.; Sulewski, P. Ocena przydatności wybranych metod prognozowania plonów rośln. Rocz. Nauk Roln. Ser. G Ekon. Roln 2008, 9, 68–82. [Google Scholar]
- Hochman, Z.; Van Rees, H.; Carberry, P.S.; Hunt, J.R.; McCown, R.L.; Gartmann, A.; Holzworth, D.; Van Rees, S.; Dalgliesh, N.P.; Long, W.; et al. Re-inventing model-based decision support with Australian dryland farmers. 4. Yield Prophet® helps farmers monitor and manage crops in a variable climate. Crop Pasture Sci. 2009, 60, 1057–1070. [Google Scholar] [CrossRef] [Green Version]
- Derejko, A.; Madry, W.; Gozdowski, D.; Rozbicki, J.; Golba, J.; Piechocinski, M.; Studnicki, M. Wpływ odmian, miejscowości i intensywności uprawy oraz ich interakcji na plony pszenicy ozimej w doświadczeniach PDO. Biul. Inst. Hod. Aklim. Roślin 2011, 259, 131–146. [Google Scholar]
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crop. Res. 2017, 202, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Mądry, W.; Derejko, A.; Studnicki, M.; Paderewski, J.; Gacek, E. Response of winter wheat cultivars to crop management and environment in post-registration trials. Czech J. Genet. Plant Breed. 2017, 53, 76–82. [Google Scholar] [CrossRef]
- Wysokiej, W.W.; Oraz, T.; Suszy, S. Grażyna Podolska. ZESZYT 2018, 57, 9–21. [Google Scholar] [CrossRef]
- Cammarano, D.; Holland, J.; Ronga, D. Spatial and temporal variability of spring barley yield and quality quantified by crop simulation model. Agronomy 2020, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Iwanska, M.; Paderewski, J.; Stepien, M.; Rodrigues, P.C. Adaptation of winter wheat cultivars to different environments: A case study in Poland. Agronomy 2020, 10, 632. [Google Scholar] [CrossRef]
- Araya, A.; Prasad, P.V.V.; Gowda, P.H.; Djanaguiramana, M. Climate Risk Management Modeling the effects of crop management on food barley production under a midcentury changing climate in northern Ethiopia. Clim. Risk Manag. 2021, 32, 100308. [Google Scholar] [CrossRef]
- Wajid, A.; Hussain, K.; Ilyas, A.; Habib-Ur-Rahman, M.; Shakil, Q.; Hoogenboom, G. Crop Models: Important Tools in Decision Support System to Manage Wheat Production under Vulnerable Environments. Agriculture 2021, 11, 1166. [Google Scholar] [CrossRef]
- Wójcik-Gront, E.; Studnicki, M. Long-term yield variability of triticale (×triticosecale wittmack) tested using a cart model. Agric. 2021, 11, 92. [Google Scholar] [CrossRef]
- Gardi, M.W.; Memic, E.; Zewdu, E.; Graeff-Hönninger, S. Simulating the effect of climate change on barley yield in Ethiopia with the DSSAT-CERES-Barley model. Agron. J. 2022, 114, 1128–1145. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Craufurd, P.Q.; Ellis, R.H.; Porter, J.R.; Vara Prasad, P.V. Temperature variability and the yield of annual crops. Agric. Ecosyst. Environ. 2000, 82, 159–167. [Google Scholar] [CrossRef]
- Palosuo, T.; Kersebaum, K.C.; Angulo, C.; Hlavinka, P.; Moriondo, M.; Olesen, J.E.; Patil, R.H.; Ruget, F.; Rumbaur, C.; Takáč, J.; et al. Simulation of winter wheat yield and its variability in different climates of Europe: A comparison of eight crop growth models. Eur. J. Agron. 2011, 35, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Babushkina, E.A.; Belokopytova, L.V.; Zhirnova, D.F.; Shah, S.K.; Kostyakova, T.V. Climatically driven yield variability of major crops in Khakassia (South Siberia). Int. J. Biometeorol. 2018, 62, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Troy, T.J.; Devineni, N. Stochastically modeling the projected impacts of climate change on rainfed and irrigated US crop yields. Environ. Res. Lett. 2019, 14, 74021. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, N.R.; Drummond, S.T.; Lund, E.D.; Sudduth, K.A.; Buchleiter, G.W. Soil Electrical Conductivity and Topography Related to Yield for Three Contrasting Soil–Crop Systems. Agron. J. 2003, 95, 483. [Google Scholar] [CrossRef]
- Mueller, L.; Schindler, U.; Mirschel, W.; Graham Shepherd, T.; Ball, B.C.; Helming, K.; Rogasik, J.; Eulenstein, F.; Wiggering, H. Assessing the productivity function of soils. A review. Agron. Sustain. Dev. 2010, 30, 601–614. [Google Scholar] [CrossRef]
- Hussain, K.; Wongleecharoen, C.; Hilger, T.; Ahmad, A.; Kongkaew, T.; Cadisch, G. Modelling resource competition and its mitigation at the crop-soil-hedge interface using WaNuLCAS. Agrofor. Syst. 2016, 90, 1025–1044. [Google Scholar] [CrossRef]
- Szewrański, S.; Kazak, J.; Żmuda, R.; Wawer, R. Indicator-based assessment for soil resource management in the Wrocław larger urban zone of Poland. Polish J. Environ. Stud. 2017, 26, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, E.I. Learning algorithms for fuzzy cognitive maps—A review study. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2012, 42, 150–163. [Google Scholar] [CrossRef]
- Akhtar, H.; Lupascu, M.; Sukri, R.S.; Smith, T.E.L.; Cobb, A.R.; Swarup, S. Significant sedge-mediated methane emissions from degraded tropical peatlands. Environ. Res. Lett. 2021, 16, 014002. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef] [Green Version]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Juroszek, P.; Von Tiedemann, A. Plant pathogens, insect pests and weeds in a changing global climate: A review of approaches, challenges, research gaps, key studies and concepts. J. Agric. Sci. 2013, 151, 163–188. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Akbas, B.; Andreasen, C.B.; Arendse, W.; Bluemel, S.; Dachbrodt-Saaydeh, S.; Fuchs, A.; Jansen, J.P.; Kiss, J.; Kudsk, P.; et al. A call for stakeholders to boost integrated pest management in Europe: A vision based on the three-year European research area network project. Int. J. Pest Manag. 2018, 64, 352–358. [Google Scholar] [CrossRef]
- Morris, C.E.; Moury, B. Revisiting the Concept of Host Range of Plant Pathogens. Annu. Rev. Phytopathol. 2019, 57, 63–90. [Google Scholar] [CrossRef]
- Çelik Oğuz, A.; Karakaya, A. Genetic Diversity of Barley Foliar Fungal Pathogens. Agronomy 2021, 11, 434. [Google Scholar] [CrossRef]
- Brzozowski, L.; Mazourek, M. A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 2018, 10, 2023. [Google Scholar] [CrossRef] [Green Version]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian barley industry. Australas. Plant Pathol. 2010, 39, 85–96. [Google Scholar] [CrossRef]
- Agostinetto, L.; Casa, R.T.; Bogo, A.; Sachs, C.; Reis, E.M.; Kuhnem, P.R. Critical yield-point model to estimate damage caused by brown spot and powdery mildew in barley. Ciênc. Rural 2014, 44, 957–963. [Google Scholar] [CrossRef]
- Pinnschmidt, H.O.; Hovmøller, M.S.; Oøstergård, H. Approaches for field assessment of resistance to leaf pathogens in spring barley varieties. Plant Breed. 2006, 125, 105–113. [Google Scholar] [CrossRef]
- Østergård, H.; Kristensen, K.; Pinnschmidt, H.O.; Hansen, P.K.; Hovmøller, M.S. Predicting spring barley yield from variety-specific yield potential, disease resistance and straw length, and from environment-specific disease loads and weed pressure. Euphytica 2008, 163, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Kiær, L.P.; Skovgaard, I.M.; Østergård, H. Effects of inter-varietal diversity, biotic stresses and environmental productivity on grain yield of spring barley variety mixtures. Euphytica 2012, 185, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Mehta, S.; Aggarwal, S.K.; Tiwari, M. Barley, Disease Resistance, and Molecular Breeding Approaches; Springer: Cham, Switzerland, 2019; Chapter 11; ISBN 9783030207281. [Google Scholar]
- Czembor, J.H.; Czembor, E.; Suchecki, R.; Watson-Haigh, N.S. Genome-Wide Association Study for Powdery Mildew and Rusts Adult Plant Resistance in European Spring Barley from Polish Gene Bank. Agronomy 2022, 12, 7. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Brändle, U.; Koller, B.; Limpert, E.; McDermott, J.M.; Müller, K.; Schaffner, D. Barley mildew in Europe: Population biology and host resistance. Euphytica 1992, 63, 125–139. [Google Scholar] [CrossRef]
- Dreiseitl, A. Differences in powdery mildew epidemics in spring and winter barley based on 30-year variety trials. Ann. Appl. Biol. 2011, 159, 49–57. [Google Scholar] [CrossRef]
- Tucker, M.A.; Jayasena, K.; Ellwood, S.R.; Oliver, R.P. Pathotype variation of barley powdery mildew in Western Australia. Australas. Plant Pathol. 2013, 42, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Dreiseitl, A. Specific resistance of barley to powdery mildew, its use and beyond. A concise critical review. Genes 2020, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Cynthia Ge, C.; Wentzel, E.; D’Souza, N.; Chen, K.; Oliver, R.P.; Ellwoo, S.R. Adult resistance genes to barley powdery mildew confer basal penetration resistance associated with broad-spectrum resistance. Plant Genome 2021, 14, e20129. [Google Scholar] [CrossRef]
- Tucker, M.A. Adaptation of Barley Powdery Mildew (Blumeria graminis f. sp. hordei) in Western Australia to Contemporary Agricultural Practices. Ph.D. Thesis, Curtin University, Bentley, Australia, 2015. [Google Scholar]
- Arabi, M.I.E.; Jawhar, M.; Al-Safadi, B.; Mirali, N. Yield responses of barley to leaf stripe (Pyrenophora graminea) under experimental conditions in southern Syria. J. Phytopathol. 2004, 152, 519–523. [Google Scholar] [CrossRef]
- Avrova, A.; Knogge, W. Rhynchosporium commune: A persistent threat to barley cultivation. Mol. Plant Pathol. 2012, 13, 986–997. [Google Scholar] [CrossRef]
- McDonald, B.A.; Zhan, J.; Burdon, J.J. Genetic structure of Rhynchosporium secalis in Australia. Phytopathology 1999, 89, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Stefansson, T.S.; Willi, Y.; Croll, D.; McDonald, B.A. An assay for quantitative virulence in Rhynchosporium commune reveals an association between effector genotype and virulence. Plant Pathol. 2014, 63, 405–414. [Google Scholar] [CrossRef]
- Brown, J.S. Pathogenic variation among isolates of Rhynchosporium secalis from cultivated barley growing in Victoria, Australia. Euphytica 1985, 34, 129–133. [Google Scholar] [CrossRef]
- Arabi, M.I.E.; MirAli, N.; Jawhar, M.; Al-Safadi, B. The effects of barley seed infected with Pyrenophora graminea on storage protein (Hordeins) patterns. Plant Var. Seeds 2001, 14, 113–117. [Google Scholar]
- Corteill, P.J.; Rees, R.G.; Platz, G.J.; Doill-Macky, R. Effect of leaf-rust on selected Australian barleys. Australian. J. Exp. Agric. 1992, 32, 747–751. Available online: https://www.publish.csiro.au/an/EA9920747 (accessed on 2 April 2022).
- Arnast, B.; Martens, J.; Wright, G.; Burnett, P.; Sanderson, F. Incidence, importance and virulence of Puccinia hordei on barley in New Zealand. Ann. Appl. Biol. 2008, 92, 185–190. [Google Scholar] [CrossRef]
- Griffey, C.A.; Das, M.K.; Baldwin, R.E.; Waldenmaier, C.M. Yield losses in winter barley resulting from a new race of Puccinia hordei in North America. Plant Dis. 1994, 78, 256–260. [Google Scholar] [CrossRef]
- Whelan, H.G.; Gaunt, R.E.; Scott, W.R. The effect of leaf rust (Puccinia hordei) on yield response in barley (Hordeum vulgare L.) crops with different yield potentials. Plant Pathol. 1997, 46, 397–406. [Google Scholar] [CrossRef]
- Niks, R.E.; Walther, U.; Jaiser, H.; Martinez, F.; Rubiales, D.; Andersen, O.; Flath, K.; Gymer, P.; Heinrichs, F.; Jonsson, R.; et al. Resistance against barley leaf rust (Puccinia hordei) in West-European spring barley germplasm. Agronomie 2000, 20, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Czembor, J.H.; Czembor, H.J.; Attene, G.; Papa, R. Leaf rust resistance in selections from barley landraces collected in Sardinia. Plant Breed. Seed Sci. 2007, 56, 13–20. [Google Scholar]
- Czembor, H.J.; Czembor, J.H. Leaf rust resistance in spring barley cultivars and breeding lines. Plant Breed. Seed Sci. 2007, 55, 5–20. [Google Scholar]
- Torkashvand, A.M.; Ahmadi, A.; Nikravesh, N.L. Prediction of kiwifruit firmness using fruit mineral nutrient concentration by artificial neural network (ANN) and multiple linear regressions (MLR). J. Integr. Agric. 2017, 16, 1634–1644. [Google Scholar] [CrossRef] [Green Version]
- Ansarifar, J.; Wang, L.; Archontoulis, S.V. An interaction regression model for crop yield prediction. Sci. Rep. 2021, 11, 17754. [Google Scholar] [CrossRef]
- Brinkmeyer, L.; Drumond, R.; Johannes, B.; Schmidt-Thieme, L. Few Shot Forecasting of Time-Series with Heterogeneous Channels. Learning Complex Time Series Forecasting Models Usually Requires a Large Amount of Data, as Each Model Is Trained from Scratch for Each Task/Data Set. 2022. Meta-Learning for Time-Series with Heterogeneous Channels. Available online: https://arxiv.org/pdf/2204.03456.pdf (accessed on 2 April 2022).
- Jutras, P.; Quillan, R.S.; LeForte, M.J. Evidence from Middle Ordovician paleosols for the predominance of alkaline groundwater at the dawn of land plant radiation. Geology 2009, 37, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Keating, B.A. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef] [Green Version]
- Basso, B.; Liu, L.; Ritchie, J.T. A Comprehensive Review of the Models’ Performances; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 136. [Google Scholar]
- Eitzinger, J.; Trnka, M.; Hösch, J.; Žalud, Z.; Dubrovský, M. Comparison of CERES, WOFOST and SWAP models in simulating soil water content during growing season under different soil conditions. Ecol. Model. 2004, 171, 223–246. [Google Scholar] [CrossRef]
- Lamsal, A.; Welch, S.; Jones, J.W.; Crain, J. Efficient crop model parameter estimation and site characterization using large breeding trial data sets. Agric. Syst. 2017, 157, 170–184. [Google Scholar] [CrossRef]
- Akhavizadegan, F.; Ansarifar, J.; Wang, L.; Huber, I.; Archontoulis, S. V OPEN A time—Dependent parameter estimation framework for crop modeling. Sci. Rep. 2021, 11, 11437. [Google Scholar] [CrossRef] [PubMed]
- Effendi, Z.; Ramli, R.; Ghani, J.A. A back propagation neural networks for grading Jatropha curcas fruits maturity. Am. J. Appl. Sci. 2010, 7, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Kelvin, L.; Benavides-mendoza, A.; Gonz, S. Artificial Neural Network Modeling of Greenhouse Tomato Yield and Aerial Dry Matter. Agriculture 2020, 10, 97. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley and Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Kosaki, T.; Wasano, K.; Juo, A.S.R. Multivariate statistical analysis of yield-determining factors. Soil Sci. Plant Nutr. 1989, 35, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Speed, T.P.; Yu, B. Model selection and prediction: Normal regression. Sel. Work. Terry Speed 2012, 45, 308–327. [Google Scholar] [CrossRef]
- Rynkiewicz, J. General bound of overfitting for MLP regression models. Neurocomputing 2012, 90, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Niedbała, G. Application of multiple linear regression for multi-criteria yield prediction of winter wheat. J. Res. Appl. Agric. Eng. 2018, 63, 125. [Google Scholar]
- Id, L.S.; Kaczmarek, Z.; Popławska, W.; Liersch, A.; Matuszczak, M.; Bili, Z.R.; Sosnowska, K. Estimation of seed yield in oilseed rape to identify the potential of semi-resynthesized parents for the development of new hybrid cultivars. PLoS ONE 2019, 14, e0215661. [Google Scholar]
- Henric, J.F.; Legros, J.P.; Slawinskai, C.; Walczak, R.T. Yield prediction for winter wheat in eastern Poland (Grabów) using the ACCESS-II model. Int. Agrophysics 1996, 10, 239–247. [Google Scholar]
- Mańkowski, D.; Pankratz, A. Forecasting with Dynamic Regression Models; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1991. [Google Scholar]
- Mańkowski, D.; Rawlings, J.O.; Pantula, S.G.; Dickey, D.A. Applied Regression Analysis—A Research Tool, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Mańkowski, D.; Harrel, F.E., Jr. Regression Modeling Strategies; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Mańkowski, S.; TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.io (accessed on 15 May 2021).
- Mańkowski, D.; VSN International. Genstat for Windows, 21st ed.; VSN International: Hemel Hempstead, UK, 2020; Available online: Genstat.co.uk (accessed on 15 May 2021).


| Part of Poland | Part of Poland Code | Location | Location Code | Latitude (φ) | Longitude (λ) | Altitude (m) |
|---|---|---|---|---|---|---|
| North-West | NW | Białogard | L1 | 54°00′ | 15°59′ | 32.0 |
| North | N | Radostowo | L2 | 53°98′ | 18°75′ | 40.0 |
| North-East | NE | Ruska Wies | L3 | 53°47′ | 22°12′ | 15.8 |
| North-East | NE | Krzyzewo | L4 | 53°01′ | 22°46′ | 135.0 |
| Central | NE | Nowa Wies Ujska | L5 | 53°03′ | 16°75′ | 105.0 |
| Central | C | Glebokie | L6 | 52°65′ | 18°43′ | 85.0 |
| Central | C | Sulejow | L7 | 51°21′ | 19°52′ | 188.0 |
| Central | C | Kaweczyn | L8 | 52°10′ | 20°21′ | 90.0 |
| South | S | Glubczyce | L9 | 50°18′ | 17°83′ | 280.0 |
| South | S | Pawlowice | L10 | 49°57′ | ||
| Central-Weast | C | Slupia | L11 | 50°63′ | 19°96′ | 290.0 |
| East | E | Cibór Duży | L12 | 52°08′ | 23°11′ | 114.0 |
| South-East | SE | Przecław | L13 | 49°53′ | 22°44′ | 230.0 |
| Management of the Trials Across the Thirteen Environments and Three Years | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Location | Soil Complexity | 2016 | 2017 | 2018 | ||||||||||||
| N | Sum of N | P2O5 | K2O | Sum of NPK | N | Sum of N | P2O5 | K2O | Sum of NPK | N | Sum of N | P2O5 | K2O | Sum of NPK | |||
| 1 | Białogard | 4 | 300 | 120 | 60 | 120 | 300 | 300 | 110 | 60 | 120 | 290 | 300 | 120 | 60 | 120 | 300 |
| 2 | Radostowo | 1 | 300 | 94 | 42 | 112 | 248 | 300 | 80 | 60 | 102 | 242 | 300 | 88 | 70 | 105 | 263 |
| 3 | Ruska Wieś | 2 | 300 | 70 | 60 | 70 | 200 | 300 | 70 | 40 | 90 | 200 | 300 | 70 | 60 | 90 | 220 |
| 4 | Krzyżewo | 4 | 300 | 50 | 60 | 90 | 200 | 300 | 60 | 60 | 90 | 210 | 300 | 88 | 36 | 102 | 226 |
| 5 | Nowa Wieś Ujska | 4 | 300 | 90 | 70 | 105 | 265 | 300 | 90 | 48 | 80 | 218 | 300 | 108 | 24 | 24 | 156 |
| 6 | Głębokie | 2 | 300 | 70 | 30 | 80 | 180 | 300 | 72 | 24 | 70 | 166 | 300 | 72 | 24 | 68 | 164 |
| 7 | Sulejów | 2 | 300 | 91 | 25 | 70 | 186 | 300 | 96 | 30 | 70 | 196 | 300 | 120 | 40 | 70 | 230 |
| 8 | Kawęczyn | 4 | 300 | 62 | 45 | 90 | 197 | 300 | 95 | 45 | 90 | 230 | 300 | 80 | 45 | 90 | 215 |
| 9 | Cicibór | 4 | 300 | 63 | 40 | 60 | 163 | 300 | 92 | 40 | 60 | 192 | 300 | 89 | 40 | 60 | 189 |
| 10 | Głubczyce | 1 | 300 | 40 | 60 | 90 | 190 | 350 | 36 | 0 | 0 | 36 | 300 | 81 | 47 | 40.7 | 169 |
| 11 | Pawłowice | 3 | 300 | 128 | 84 | 84 | 296 | 300 | 90 | 36 | 75 | 201 | 300 | 90 | 72 | 72 | 234 |
| 12 | Słupia | 2 | 300 | 113 | 50 | 70 | 233 | 300 | 117 | 59 | 70 | 246 | 300 | 107 | 50 | 70 | 227 |
| 13 | Przecław | 2 | 300 | 60 | 40 | 60 | 160 | 300 | 80 | 60 | 90 | 230 | 300 | 103 | 70 | 105 | 278 |
| Supplementary Monthly Average Daily Air Temperature, Ground Temperature and Sum of Rainfall | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Daily Air Temperature | Average Monthly Ground Temperature | the Sum of Rainfall | |||||||||||||||||||||||||||||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |||||||||||||||||||||||||||
| Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. | Month | Average | Min. | Max. |
| I | −3.2 | −5.7 | −1.9 | I | −4.2 | −6.2 | −1.0 | I | 0.7 | −1.4 | 2.7 | I | −1.5 | −3.1 | 0.6 | I | −1.2 | −2.3 | 0.8 | I | 0.4 | −0.4 | 1.4 | I | 28.3 | 11.3 | 45.1 | I | 18.3 | 10.7 | 42.1 | I | 32.6 | 18.3 | 65.6 |
| II | 3.2 | 1.5 | 4.0 | II | −0.7 | −2.3 | 0.6 | II | −3.3 | −5.1 | −2.2 | II | 1.9 | 1.0 | 2.8 | II | −0.8 | −2.1 | 0.4 | II | −1.3 | −2.1 | −0.1 | II | 60.9 | 23.5 | 91.9 | II | 33.5 | 17.7 | 55.5 | II | 11.8 | 4.9 | 24.7 |
| III | 3.8 | 2.7 | 4.7 | III | 5.7 | 4.3 | 6.3 | III | 0.1 | −1.5 | 1.3 | III | 2.6 | 1.9 | 4.4 | III | 3.3 | 2.7 | 5.7 | III | −0.5 | −1.3 | 1.1 | III | 31.3 | 13.9 | 69.7 | III | 44.4 | 19.5 | 70.8 | III | 28.0 | 18.0 | 53.1 |
| IV | 8.5 | 7.9 | 9.8 | IV | 7.1 | 6.0 | 7.7 | IV | 12.5 | 11.0 | 13.4 | IV | 7.0 | 5.6 | 10.4 | IV | 5.8 | 5.0 | 8.7 | IV | 9.0 | 7.3 | 13.1 | IV | 32.5 | 12.8 | 57.0 | IV | 74.4 | 34.8 | 145.3 | IV | 29.4 | 9.4 | 61.4 |
| V | 14.4 | 13.3 | 15.6 | V | 13.3 | 12.4 | 14.1 | V | 16.2 | 14.5 | 17.4 | V | 13.7 | 12.0 | 18.0 | V | 12.6 | 10.7 | 16.8 | V | 15.3 | 13.1 | 17.6 | V | 45.2 | 12.2 | 108.3 | V | 51.9 | 19.6 | 111.9 | V | 41.9 | 9.4 | 68.8 |
| VI | 17.9 | 17.0 | 18.6 | VI | 17.3 | 16.4 | 18.7 | VI | 18.0 | 16.8 | 18.7 | VI | 18.2 | 16.0 | 22.2 | VI | 17.4 | 15.1 | 21.1 | VI | 18.6 | 16.5 | 20.4 | VI | 71.0 | 23.8 | 168.9 | VI | 76.8 | 26.3 | 174.4 | VI | 46.2 | 22.5 | 93.3 |
| VII | 19.1 | 18.0 | 19.9 | VII | 18.1 | 16.7 | 19.2 | VII | 20.0 | 18.1 | 21.0 | VII | 18.7 | 16.6 | 21.6 | VII | 18.0 | 16.0 | 21.0 | VII | 19.2 | 17.7 | 20.5 | VII | 154.5 | 86.0 | 408.2 | VII | 91.0 | 44.4 | 166.1 | VII | 106.4 | 80.7 | 131.2 |
| No. | Variety | Model F-Statistic | R sq. adj. | No. | Variety | Model F-Statistic | R sq. adj. |
|---|---|---|---|---|---|---|---|
| 1 | Soldo | 6.88 *** | 0.746 | 11 | Salome | 6.93 *** | 0.766 |
| 2 | Radek | 10.34 *** | 0.831 | 12 | Rubaszek | 8.73 *** | 0.785 |
| 3 | RGT Planet | 12.82 *** | 0.848 | 13 | Podarek | 10.33 *** | 0.797 |
| 4 | KWS Olof | 11.52 *** | 0.816 | 14 | Allianz | 8.62 *** | 0.822 |
| 5 | Basic | 8.53 *** | 0.79 | 15 | KWS Cantton | 12.22 *** | 0.842 |
| 6 | Ella | 7.72 *** | 0.75 | 16 | KWS Harris | 9.19 *** | 0.812 |
| 7 | KWS Atrika | 8.05 *** | 0.796 | 17 | KWS Vermont | 19.26 *** | 0.891 |
| 8 | Oberek | 9.47 *** | 0.791 | 18 | Paustian | 10.28 *** | 0.843 |
| 9 | KWS Iri | 8.22 *** | 0.774 | 19 | Polonia Staropolska | 7.93 *** | 0.745 |
| 10 | KWS Dante | 6.12 *** | 0.719 | 20 | Ringo | 7.26 *** | 0.776 |
| Trait | Model (20 Cultivars) | |||
|---|---|---|---|---|
| Estimation | t-Statistic | Standarized Estimation | ||
| Constant | Constant | −113.13 | −4.27 *** | - |
| sum of NPK | NPK | −0.083 | −6.59 *** | −0.2629 |
| sum of N | N | 0.276 | 8.45 *** | 0.3946 |
| soil cmplxexity | soilcmplx | 4.641 | 8.85 *** | 0.3131 |
| powdery mildew | PM | −1.011 | −2.86 *** | −0.0708 |
| net blotch | NB | |||
| barley rust | BR | |||
| rynchosporium | RN | −2.686 | −5.67 *** | −0.1778 |
| lodging before harvest-lodging tendency | LT | 0.526 | 2.23 ** | 0.0619 |
| mean yield accross 3 years before | YC | 1.304 | 22.06 *** | 0.9323 |
| the sum of rainfall January | r1 | −0.357 | −5.87 *** | −0.2625 |
| the sum of rainfall Febuary | r2 | 0.129 | 4.05 *** | 0.1976 |
| the sum of rainfall March | r3 | 0.452 | 9.14 *** | 0.38 |
| the sum of rainfall April | r4 | 0.181 | 5.74 *** | 0.3585 |
| the sum of rainfall May | r5 | 0.038 | 1.91 * | 0.0549 |
| the sum of rainfall June | r6 | |||
| the sum of rainfall July | r7 | 0.018 | 2.00 ** | 0.0671 |
| average monthly ground temperature January | tg1 | |||
| verage monthly ground temperature Febuary | tg2 | −6.782 | −6.70 *** | −0.6498 |
| verage monthly ground temperature March | tg3 | 21.47 | 12.08 *** | 2.2865 |
| verage monthly ground temperatureApril | tg4 | −15.139 | −10.09 *** | −1.3334 |
| verage monthly ground temperature May | tg5 | 18.46 | 10.80 *** | 1.9042 |
| verage monthly ground temperature June | tg6 | −1.63 | −1.48 * | −0.1375 |
| verage monthly ground temperature July | tg7 | −5.274 | −3.60 *** | −0.3704 |
| average daily air temperature January | ts1 | 2.678 | 4.56 *** | 0.4161 |
| average daily air temperature Febuary | ta2 | 5.455 | 8.38 *** | 0.9547 |
| average daily air temperature March | ta3 | −6.838 | −5.62 *** | −1.031 |
| average daily air temperature April | ta4 | 12.15 | 9.94 *** | 1.8219 |
| average daily air temperature May | ta5 | −7.366 | −4.15 *** | −0.6056 |
| average daily air temperature June | ta6 | −7.49 | −4.83 *** | −0.3099 |
| average daily air temperature Julay | ta7 | 9.509 | 10.18 *** | 0.635 |
| Model F-statistic | 102.55 *** | |||
| R sq. adj. | 0.786 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czembor, E.; Kaczmarek, Z.; Pilarczyk, W.; Mańkowski, D.; Czembor, J.H. Simulating Spring Barley Yield under Moderate Input Management System in Poland. Agriculture 2022, 12, 1091. https://doi.org/10.3390/agriculture12081091
Czembor E, Kaczmarek Z, Pilarczyk W, Mańkowski D, Czembor JH. Simulating Spring Barley Yield under Moderate Input Management System in Poland. Agriculture. 2022; 12(8):1091. https://doi.org/10.3390/agriculture12081091
Chicago/Turabian StyleCzembor, Elzbieta, Zygmunt Kaczmarek, Wiesław Pilarczyk, Dariusz Mańkowski, and Jerzy H. Czembor. 2022. "Simulating Spring Barley Yield under Moderate Input Management System in Poland" Agriculture 12, no. 8: 1091. https://doi.org/10.3390/agriculture12081091







