Efficiency and Effectivity of a Biological–Epidemiological Fungal Disease Management System in Wheat—A Study of 26 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area Surveyed and Survey Strategy
2.2. Sampling and Disease Assessment
2.3. Data Analyses
2.4. Statistical Analyses
3. Results
3.1. Occurrence of Wheat Foliar Diseases from 1995 to 2021 in the Fungicide Untreated Control
3.2. Threshold-Based Reduction of Disease Sevities
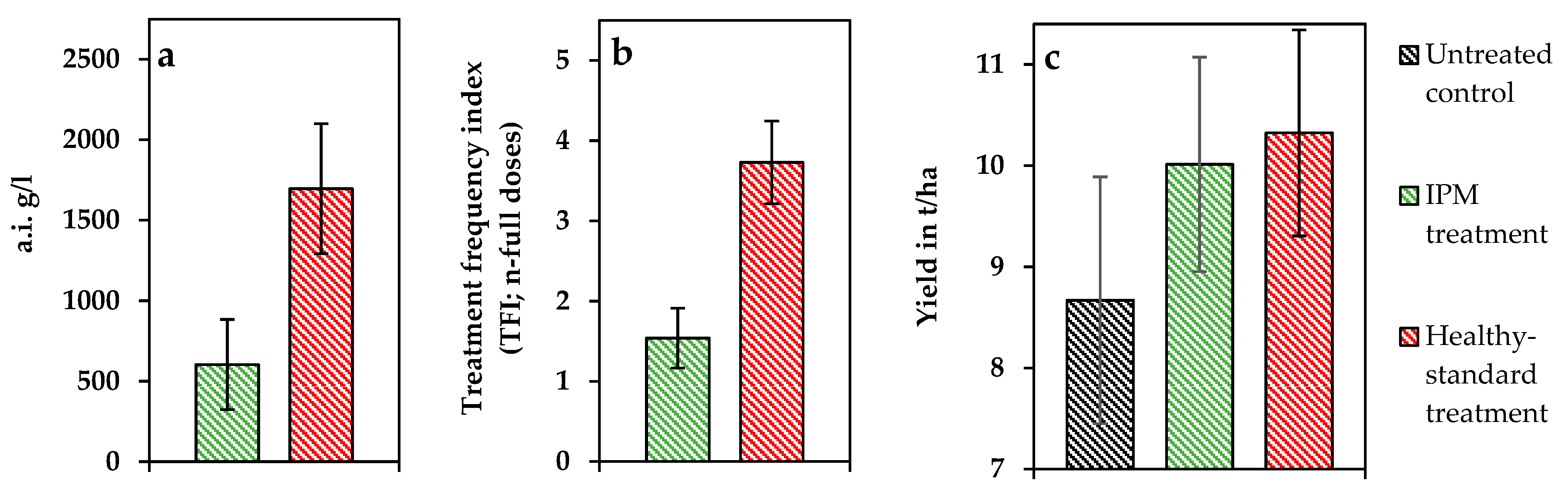
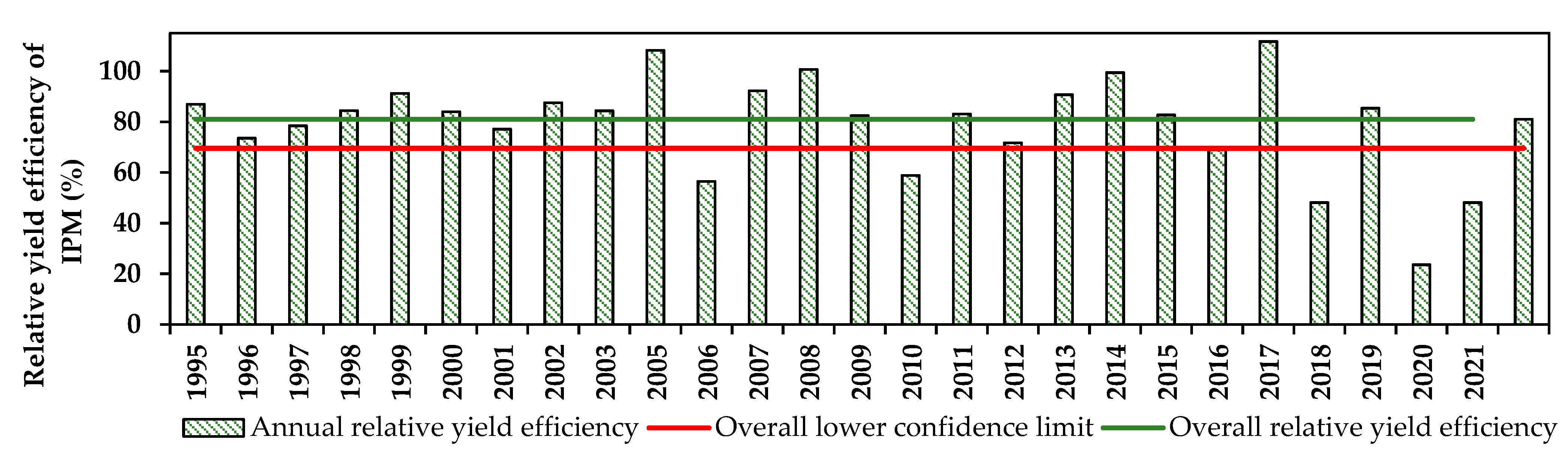
3.3. Efficiency of Fungal Disease Management
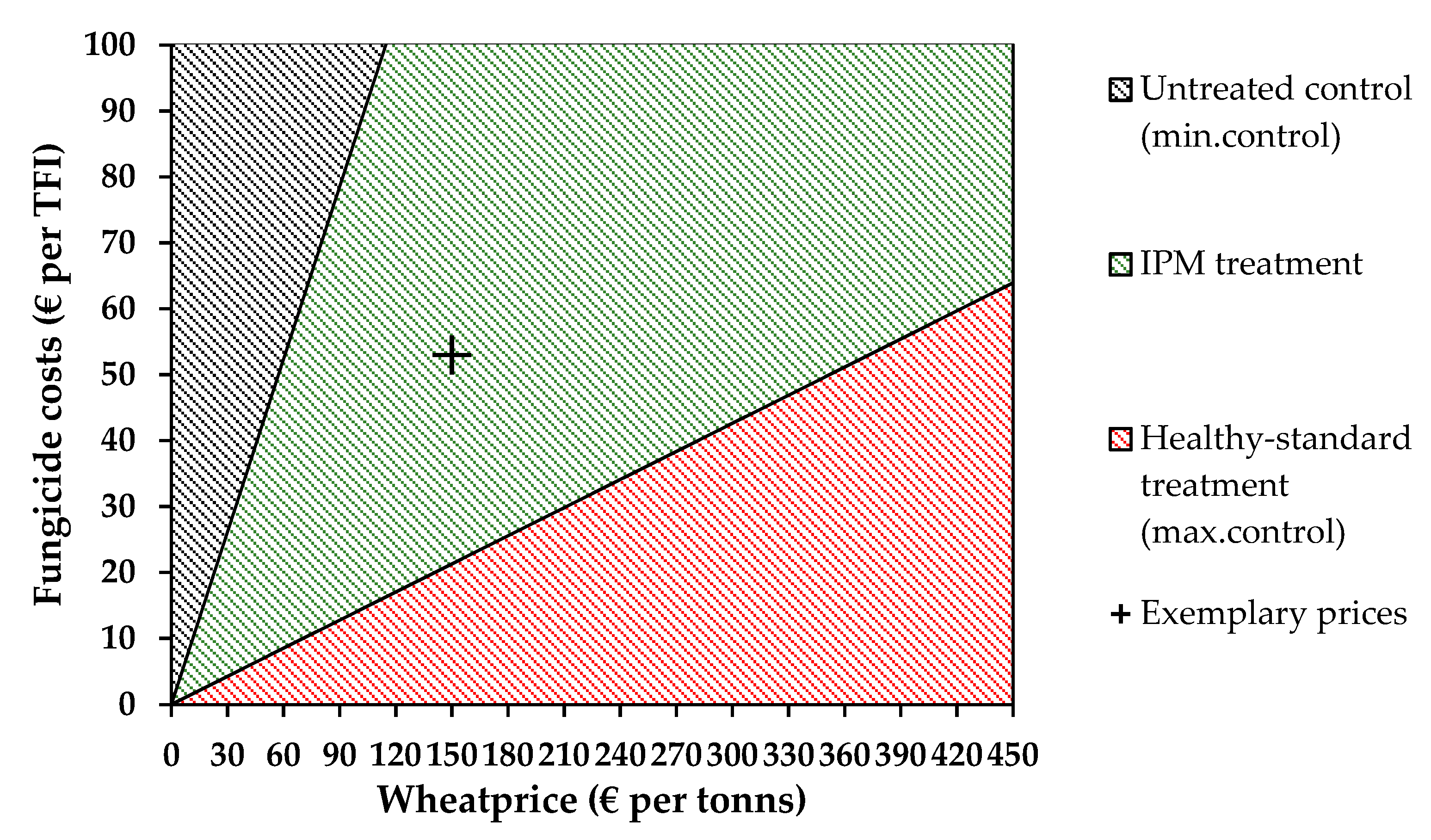
3.4. Economic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT Statistical Database; FAO: Rome, Italy, 2019. [Google Scholar]
- WHEAT. Wheat: Vital Grain of Civilization and Food Security; 2013 Annual Report; CGIAR: Washington, DC, USA, 2014. [Google Scholar]
- Bouma, E. Development of comparable agro-climatic zones for the international exchange of data on the efficacy and crop safety of plant protection products. Bull. OEPP 2005, 35, 233–238. [Google Scholar] [CrossRef]
- Poole, N.F.; Arnaudin, M.E. The role of fungicides for effective disease management in cereal crops. Can. J. Plant Pathol. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Enghiad, A.; Ufer, D.; Countryman, A.M.; Thilmany, D.D. An Overview of Global Wheat Market Fundamentals in an Era of Climate Concerns. Int. J. Agron. 2017, 2017, 3931897. [Google Scholar] [CrossRef]
- IPPC Secretariat. Protecting Plants, Protecting Life. In International Year of Plant Health—Final Report; FAO on behalf of the Secretariat of the International Plant Protection Convention: Rome, Italy, 2021; ISBN 978-92-5-135056-0. [Google Scholar]
- Birr, T.; Verreet, J.-A.; Klink, H. Prediction of deoxynivalenol and zearalenone in winter wheat grain in a maize-free crop rotation based on cultivar susceptibility and meteorological factors. J. Plant Dis. Prot. 2019, 126, 13–27. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Turner, J.A.; Chantry, T.; Taylor, M.C.; Kennedy, M.C. Changes in agronomic practices and incidence and severity of diseases in winter wheat in England and Wales between 1999 and 2019. Plant Pathol. 2021, 70, 1759–1778. [Google Scholar] [CrossRef]
- Jalli, M.; Huusela, E.; Jalli, H.; Kauppi, K.; Niemi, M.; Himanen, S.; Jauhiainen, L. Effects of Crop. Rotation on Spring Wheat Yield and Pest Occurrence in Different Tillage Systems: A Multi-Year Experiment in Finnish Growing Conditions. Front. Sustain. Food Syst. 2021, 5, 214. [Google Scholar] [CrossRef]
- Draz, I.S.; Esmail, S.M.; Abou-Zeid, M.H.; Essa, T.M. Powdery mildew susceptibility of spring wheat cultivars as a major constraint on grain yield. Ann. Agric. Sci. 2019, 64, 39–45. [Google Scholar] [CrossRef]
- Hosford, R.M., Jr.; Larez, C.R.; Hammond, J.J. Interaction of wet period and temperature on Pyrenophora tritici-repentis infection and development in wheats of differing resistance. Phytopathology 1987, 77, 1021–1027. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Henze, M.; Beyer, M.; Klink, H.; Verreet, J.-A. Characterizing Meteorological Scenarios Favorable for Septoria tritici Infections in Wheat and Estimation of Latent Periods. Plant Dis. 2007, 91, 1445–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juroszek, P.; Racca, P.; Link, S.; Farhumand, J.; Kleinhenz, B. Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risks. Plant Pathol. 2020, 69, 179–193. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Jalli, M.; Kaseva, J.; Andersson, B.; Ficke, A.; Jørgensen, L.N.; Ronis, A.; Kaukoranta, T.; Ørum, J.E.; Djurle, A. Yield increases due to fungicide control of leaf blotch diseases in wheat and barley as a basis for IPM decision-making in the Nordic-Baltic region. Eur. J. Plant Pathol. 2020, 158, 315–333. [Google Scholar] [CrossRef]
- Lázaro, E.; Makowski, D.; Vicent, A. Decision support systems halve fungicide use compared to calendar-based strategies without increasing disease risk. Commun. Earth. Environ. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Heick, T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation—Is It Indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef]
- Willocquet, L.; Meza, W.R.; Dumont, B.; Klocke, B.; Feike, T.; Kersebaum, K.C.; Meriggi, P.; Rossi, V.; Ficke, A.; Djurle, A.; et al. An outlook on wheat health in Europe from a network of field experiments. Crop. Prot. 2021, 139, 105335. [Google Scholar] [CrossRef]
- European Commission. Communication from the commission to the european parliament, the council, the european economic and social commitee of the regions A Farm to Fork Strategy for a fair, healthy and environmentally-friendly food system. Off. J. Eur. Union 2020, 381, 1–24. [Google Scholar]
- Verreet, J.-A.; Klink, H.; Hoffmann, G.M. Regional Monitoring for Disease Prediction and Optimization of Plant Protection Measuares: The IPM Wheat Model. Plant Dis. 2000, 84, 816–826. [Google Scholar] [CrossRef] [Green Version]
- DWD Climate Data Center. Vieljährige Stationsmittelwerte für die Klimareferenzperiode 1991–2020, für aktuellen Standort und Bezugsstandort. Available online: https://www.dwd.de/DE/leistungen/klimadatendeutschland/vielj_mittelwerte.html (accessed on 18 May 2021).
- Statistisches Amt für Hamburg und Schleswig-Holstein. Kreisergebnisse Schleswig-Holstein 2020—Endgültiges Ergebnis der Landwirtschaftszählung 2020–: Kennziffer: C IV-LZ 2020 SH, SK Sonderbericht Kreisdaten; Statistisches Amt für Hamburg und Schleswig-Holstein: Hamburg, Germany, 2021. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Bundessortenamt. Beschreibende Sortenliste Getreide, Mais, Ölfrüchte, Leguminosen (großkörnig), Hackfrüchte (außer Kartoffeln) 2007; Deutscher Landwirtschaftsverlag: Hannover, Germany, 2007; ISBN 0948-4167. [Google Scholar]
- Madden, L.V.; Hughes, G.; van den Bosch, F. CHAPTER 4: Temporal Analysis I: Quantifying and Comparing Epidemics. In The Study of Plant Disease Epidemics; Madden, L.V., Hughes, G., van den Bosch, F., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2017; pp. 63–116. ISBN 978-0-89054-505-8. [Google Scholar]
- Mahmood, A.; Alam, K.; Salam, A.; Iqbal, S. Effect of flag leaf removal on grain yield, its components and quality of hexaploid wheat. Cereal Res. Commun. 1991, 19, 305–310. [Google Scholar]
- Avci Birsin, M. Effects of Removal of Some Photosynthetic Structures on Some Yield Components in Wheat. Tarim Bilim. Derg. 2005, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Bürger, J.; de Mol, F.; Gerowitt, B. The “necessary extent” of pesticide use—Thoughts about a key term in German pesticide policy. Crop. Prot. 2008, 27, 343–351. [Google Scholar] [CrossRef]
- Pigeot, I.; Schäfer, J.; Röhmel, J.; Hauschke, D. Assessing non-inferiority of a new treatment in a three-arm clinical trial including a placebo. Stat. Med. 2003, 22, 883–899. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2022. [Google Scholar]
- Pinheiro, J. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; ISBN 9780387227474. [Google Scholar]
- Carroll, R.J.; Ruppert, D. Transformation and Weighting in Regression; CRC Press: London, UK, 1988; ISBN 9780203735268. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods. Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Bretz, F.; Hothorn, T.; Westfall, P.H. Multiple Comparisons Using R; Chapman & Hall/CRC Press: Boca Ranton, FL, USA, 2011; ISBN 9781584885740. [Google Scholar]
- Engel, C. Analyse der witterungsabhängigen Schadensdynamik von Weizen- pathogenen und deren schwellenorientierten Bekämpfung auf die Pflanzen—Sowie Ertragsphysiologie des Winterweizens anhand des überregionalen IPS-Monitorings Schleswig-Holstein (1995–2014). Ph.D. Thesis, Christian-Albrechts-Universiät, Kiel, Germany, 2014. [Google Scholar]
- Hamer, W.B. Spatial Prediction of the Infestation Risks of Winter Wheat by the Pathogens Blumeria graminis f. sp. tritici (Powdery mildew) and Puccinia triticina (Brown rust) in Schleswig-Holstein Using Machine Learning Techniques. In Kieler Geographische Schriften; Dünckmann, F., Duttmann, R., Hassink, R., Hoppe, W., Oppelt, N., Wehrhahn, R., Vafeidis, A., Eds.; Department of Geography at the Kiel University: Kiel, Germany, 2019; ISBN 978-3-923887-74-3. [Google Scholar]
- Kamrath, K.; Freier, B.; Beyer, N. Analyse der Kosten für die Anwendung von Pflanzenschutzmitteln in Winterweizen und Winterraps auf der Grundlage des Netzes Vergleichsbetriebe Pflanzenschutz 2007 bis 2010. J. Kult. 2012, 64, 416–420. [Google Scholar]
- Duveiller, E.; Singh, R.P.; Nicol, J.M. The challenges of maintaining wheat productivity: Pests, diseases, and potential epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Naseri, B.; Sabeti, P. Analysis of the effects of climate, host resistance, maturity and sowing date on wheat stem rust epidemics. J. Plant Pathol. 2021, 103, 197–205. [Google Scholar] [CrossRef]
- Ors, M.E.; Randoux, B.; Selim, S.; Siah, A.; Couleaud, G.; Maumené, C.; Sahmer, K.; Halama, P.; Reignault, P. Cultivar-dependent partial resistance and associated defence mechanisms in wheat against Zymoseptoria tritici. Plant Pathol. 2018, 67, 561–572. [Google Scholar] [CrossRef]
- Orellana-Torrejon, C.; Vidal, T.; Boixel, A.-L.; Gélisse, S.; Saint-Jean, S.; Suffert, F. Annual dynamics of Zymoseptoria tritici populations in wheat cultivar mixtures: A compromise between the efficacy and durability of a recently broken-down resistance gene? Plant Pathol. 2022, 71, 289–303. [Google Scholar] [CrossRef]
- Klink, H.; Verreet, J.-A.; Hasler, M.; Birr, T. Will Triazoles Still Be of Importance in Disease Control of Zymoseptoria tritici in the Future? Agronomy 2021, 11, 933. [Google Scholar] [CrossRef]
- Bankina, B.; Bimšteine, G.; Arhipova, I.; Kaņeps, J.; Stanka, T. Importance of Agronomic Practice on the Control of Wheat Leaf Diseases. Agriculture 2018, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Conner, R.L.; Kuzyk, A.D.; Su, H. Impact of powdery mildew on the yield of soft white spring wheat cultivars. Can. J. Plant Sci. 2003, 83, 725–728. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, S.; Li, S.; Wang, J.; Chen, H.; Wang, K.; Lin, Z.; Wei, Y.; Du, L.; Yan, Y. Improvement of three commercial spring wheat varieties for powdery mildew resistance by marker-assisted selection. Crop. Prot. 2019, 125, 104889. [Google Scholar] [CrossRef]
- Singh, R.P.; William, H.; Huerta-Espino, J.; Rosewarne, G. Wheat Rust in Asia: Meeting the Challenges with Old and New Technologies. In New Directions for a Diverse Planet: Proceedings of the 4th International Crop. Science Congress, Brisbane, Australia, 26 September–1 October 2004; International Crop. Science Society, Fischer, T., Turner, T., Angus, J., McIntyre, L., Robertson, M., Borrell, A., Lloyd, D., Eds.; Australian Society of Agronomy Inc.: Brisbane, Australia, 2004; ISBN 1-920842-21-7. [Google Scholar]
- van Ginkel, M.; Rajaram, S. Breeding for Durable Resistance to Diseases in Wheat: An International Perspective. In Durability of Disease Resistance; Jacobs, T., Parlevliet, J.E., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 259–272. ISBN 978-94-010-4885-9. [Google Scholar]
- Jørgensen, L.N.; Olsen, L.V. Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop. Prot. 2007, 26, 1606–1616. [Google Scholar] [CrossRef]
- Moffat, C.S.; Santana, F.M. Diseases Affecting Wheat: Tan Spot. In Integrated Disease Management of Wheat and Barley; Burleigh Dodds Series in Agricultural Science; Oliver, R., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 95–107. ISBN 978-1-78676-216-0. [Google Scholar]
- Cotuna, O.; Paraschivu, M.; Paraschivu, A.M.; Sărăţeanu, V. The influence of tillage, crop rotation and residue management on tan spot (Drechslera tritici repentis Died. Shoemaker) in winter wheat. Res. J. Agric. Sci. 2015, 47, 13–21. [Google Scholar]
- Cookson, W.R.; Murphy, D.V.; Roper, M.M. Characterizing the relationships between soil organic matter components and microbial function and composition along a tillage disturbance gradient. Soil Biol. Biochem. 2008, 40, 763–777. [Google Scholar] [CrossRef]
- Pelosi, C.; Pey, B.; Caro, G.; Cluzeau, D.; Peigné, J.; Bertrand, M.; Hedde, M. Dynamics of earthworm taxonomic and functional diversity in ploughed and no-tilled cropping systems. Soil Tillage Res. 2016, 156, 25–32. [Google Scholar] [CrossRef]
- Peigné, J.; Vian, J.F.; Payet, V.; Saby, N.P. Soil fertility after 10 years of conservation tillage in organic farming. Soil Tillage Res. 2018, 175, 194–204. [Google Scholar] [CrossRef]
- Soane, B.D.; Ball, B.C.; Arvidsson, J.; Basch, G.; Moreno, F.; Roger-Estrade, J. No-till in northern, western and south-western Europe: A review of problems and opportunities for crop production and the environment. Soil Tillage Res. 2012, 118, 66–87. [Google Scholar] [CrossRef] [Green Version]
- Fones, H.; Gurr, S.J. The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genet. Biol. 2015, 79, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torriani, S.F.F.; Melichar, J.P.E.; Mills, C.; Pain, N.; Sierotzki, H.; Courbot, M. Zymoseptoria tritici: A major threat to wheat production, integrated approaches to control. Fungal Genet. Biol. 2015, 79, 8–12. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Hovmøller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Goral, T.; et al. IPM Strategies and Their Dilemmas Including an Introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281. [Google Scholar] [CrossRef]
- Murray, G.M.; Martin, R.H.; Cullis, B.R. Relationship of the severity of Septoria tritici blotch of wheat to sowing time, rainfall at heading and average susceptibility of wheat cultivars in the area. Aust. J. Agric. Res. 1990, 41, 307. [Google Scholar] [CrossRef]
- Hardwick, N.V.; Jones, D.R.; Slough, J.E. Factors affecting diseases of winter wheat in England and Wales, 1989–1998. Plant Pathol. 2001, 50, 453–462. [Google Scholar] [CrossRef]
- Daamen, R.A.; Stol, W. Survey of cereal diseases and petts in the Netherlands. 5. Occurrence of Septoria spp. in winter wheat. Eur. J. Plant Pathol. 1992, 98, 369–376. [Google Scholar] [CrossRef]
- Boixel, A.-L.; Gélisse, S.; Marcel, T.C.; Suffert, F. Differential tolerance of Zymoseptoria tritici to altered optimal moisture conditions during the early stages of wheat infection. J. Plant. Pathol. 2022, 104, 495–507. [Google Scholar] [CrossRef]
- Beyer, M.; Marozsak, B.; Dam, D.; Parisot, O.; Pallez-Barthel, M.; Hoffmann, L. Enhancing septoria leaf blotch forecasts in winter wheat II: Model architecture and validation results. J. Plant. Dis. Prot. 2022, 129, 45–51. [Google Scholar] [CrossRef]
- Beyer, M.; Pallez-Barthel, M.; Dam, D.; Hoffmann, L.; El Jarroudi, M. Enhancing septoria leaf blotch forecasts in winter wheat I: The effect of temperature on the temporal distance between critical rainfall periods and the breaking of the control threshold. J. Plant. Dis. Prot. 2022, 129, 37–44. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Nielsen, G.C.; Ørum, J.E.; Jensen, J.E.; Pinnschmidt, H.O. Integrating Disease Control in Winter Wheat—Optimizing Fungicide Input. Outlooks Pest Man. 2008, 19, 206–213. [Google Scholar] [CrossRef]
- Kvakkestad, V.; Steiro, Å.L.; Vatn, A. Pesticide Policies and Farm Behavior: The Introduction of Regulations for Integrated Pest Management. Agriculture 2021, 11, 828. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; van Nistelrooy, J.G.M.; Kema, G.H.J.; De Waard, M.A. Multiple mechanisms account for variation in base-line sensitivity to azole fungicides in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2003, 59, 1333–1343. [Google Scholar] [CrossRef]
- Cools, H.J.; Bayon, C.; Atkins, S.; Lucas, J.A.; Fraaije, B.A. Overexpression of the sterol 14α-demethylase gene (MgCYP51) in Mycosphaerella graminicola isolates confers a novel azole fungicide sensitivity phenotype. Pest Manag. Sci. 2012, 68, 1034–1040. [Google Scholar] [CrossRef]
- Cools, H.J.; Fraaije, B.A. Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag. Sci. 2013, 69, 150–155. [Google Scholar] [CrossRef]
- Leroux, P.; Albertini, C.; Gautier, A.; Gredt, M.; Walker, A.-S. Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14 alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2007, 63, 688–698. [Google Scholar] [CrossRef]
- Leroux, P.; Walker, A.-S. Multiple mechanisms account for resistance to sterol 14α-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2011, 67, 44–59. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Temporal Changes in Sensitivity of Zymoseptoria tritici Field Populations to Different Fungicidal Modes of Action. Agriculture 2021, 11, 269. [Google Scholar] [CrossRef]
- Kildea, S.; Marten-Heick, T.; Grant, J.; Mehenni-Ciz, J.; Dooley, H. A combination of target-site alterations, overexpression and enhanced efflux activity contribute to reduced azole sensitivity present in the Irish Zymoseptoria tritici population. Eur. J. Plant Pathol. 2019, 154, 529–540. [Google Scholar] [CrossRef]
- Mäe, A.; Fillinger, S.; Sooväli, P.; Heick, T.M. Fungicide Sensitivity Shifting of Zymoseptoria tritici in the Finnish-Baltic Region and a Novel Insertion in the MFS1 Promoter. Front. Plant Sci. 2020, 11, 385. [Google Scholar] [CrossRef] [Green Version]
- Kildea, S.; Dooley, H.; Phelan, S.; Mehenni-Ciz, J.; Spink, J. Developing fungicide control programmes for blotch in Irish winter wheat crops. In Modern Fungicides and Antifungal Compounds VIII: Proceedings of the 18th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 24–28 April 2016; Deising, H.B., Fraaije, B.A., Mehl, A., Oerke, H., Sierotzki, H., Stammler, G., Eds.; DPG Publisher: Braunschweig, Germany, 2017; ISBN 9783941261150. [Google Scholar]
- Strobel, D.; Bryson, R.; Roth, J.; Stammler, G. Field performance of DMI fungicides against Zymoseptoria tritici across Europe—Compromized by further sensitivity shift? In Modern Fungicides and Antifungal Compounds VIII: Proceedings of the 18th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 24–28 April 2016; Deising, H.B., Fraaije, B.A., Mehl, A., Oerke, H., Sierotzki, H., Stammler, G., Eds.; DPG Publisher: Braunschweig, Germany, 2017; ISBN 9783941261150. [Google Scholar]
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.A.; Fraaije, B.A. QoI resistance in Mycosphaerella graminicola: What have we learned so far? In Modern Fungicides and Antifungal Compounds. In Modern Fungicides and Antifungal Compounds V: Proceedings of the 15th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 6–10 May 2007; Dehne, H.-W., Deising, H.B., Gisi, U., Kuck, K.H., Russell, P.E., Lyr, H., Eds.; DPG Selbstverlag: Braunschweig, Germany, 2008; pp. 71–78. ISBN 9783941261020. [Google Scholar]
- Schuh, W. Influence of Tillage Systems on Disease Intensity and Spatial Pattern of Septoria Leaf Blotch. Phytopathology 1990, 80, 1337. [Google Scholar] [CrossRef] [Green Version]
- Gladders, P.; Paveley, N.D.; Barrie, I.A.; Hardwick, N.V.; HIMS, M.J.; Langdon, S.; Taylor, M.C. Agronomic and meteorological factors affecting the severity of leaf blotch caused by Mycosphaerella graminicola in commercial wheat crops in England. Ann. Appl. Biol. 2001, 138, 301–311. [Google Scholar] [CrossRef]
- McDonald, B.A.; Mundt, C.C. How Knowledge of Pathogen Population Biology Informs Management of Septoria Tritici Blotch. Phytopathology 2016, 106, 948–955. [Google Scholar] [CrossRef] [Green Version]
- DWD. Nationaler Klimareport. Available online: https://www.dwd.de/DE/leistungen/nationalerklimareport/download_report.pdf;jsessionid=60483CC8697EDE4AEDD9275C20D454AE.live21072?__blob=publicationFile&v=14 (accessed on 18 May 2022).
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 24 May 2022).
- Hunjan, M.S.; Lore, J.S. Climate Change: Impact on Plant Pathogens, Diseases, and Their Management. In Crop. Protection Under Changing Climate, 1st ed.; Springer Nature Switzerland AG, Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 85–100. ISBN 978-3-030-46110-2. [Google Scholar]
- Ohm, H.W.; Shaner, G.E. Three Components of Slow Leaf-Rusting at Different Growth Stages in Wheat. Phytopathology 1976, 66, 1356. [Google Scholar] [CrossRef]
- Sache, I.; Vallavieille-Pope, C. Comparison of the Wheat Brown and Yellow Rusts for Monocyclic Sporulation and Infection Processes, and their Polycyclic Consequences. J. Phytopathol. 1993, 138, 55–65. [Google Scholar] [CrossRef]
- Dachbrodt-Saaydeh, S.; Sellmann, J.; Strassemeyer, J.; Schwarz, J.; Klocke, B.; Krengel, S.; Kehlenbeck, H. Netz Vergleichsbetriebe Pflanzenschutz Zwei-Jahresbericht 2015 und 2016—Analyse der Ergebnisse der Jahre 2007 bis 2016. In Berichte aus dem Julius Kühn-Institut, 194th ed.; Julius Kühn-Institut, Ed.; Saphir Verlag: Ribbesbüttel, Germany, 2018. [Google Scholar]
- BVL. Absatz an Pflanzenschutzmitteln in der Bundesrepublik Deutschland Ergebnisse der Meldungen gemäß § 64 Pflanzenschutzgesetz für das Jahr 2020. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/01_meldungen_par_64/meld_par_64_2020.pdf?__blob=publicationFile&v=5 (accessed on 24 May 2022).


| Location | Coordinates | Crop Rotation | Soil Cultivation | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| Barlt | 54°01′03″ N | 09°01′45″ E | WW-WW-OR | Plough |
| Birkenmoor | 54°26′36″ N | 10°04′18″ E | WW-WB-OR | Reduced tillage |
| Elskop | 53°49′05″ N | 09°30′43″ E | WW-WW-OR | Plough |
| Futterkamp | 54°17′31″ N | 10°38′04″ E | WW-WB-OR | Reduced tillage |
| Kastorf | 53°45′08″ N | 10°33′39″ E | WW-WB-OR | Plough |
| Kluvensiek | 54°19′38″ N | 09°48′25″ E | WW-WB-OR | Reduced tillage |
| Loit | 54°36′19″ N | 09°42′05″ E | WW-WB-OR | Plough |
| SNK 1 | 54°38′01″ N | 08°52′08″ E | WW-WW-OR | Plough |
| Foliar Disease | Observation Period (GS) | Indicating Leaf Layer | IPM—Disease Control Threshold |
|---|---|---|---|
| Septoria tritici blotch | 32–69 | F-6 to F-0 | DI > 50% + 36 h leaf wetness of >98% |
| Glume blotch | 37–39 | F-5 or F-4 | DI > 12% |
| 41–47 | F-4 or F-3 | ||
| 51–69 | F-3 or F-2 | ||
| Tan spot | 32 | F-6 or F-5 | DI > 5% |
| 33–39 | F-5 or F-4 | ||
| 41–49 | F-4 or F-3 | ||
| 51–69 | F-3 or F-2 | ||
| Powdery mildew | 30–69 | F-6 to F-0 * | DI > 70% |
| Leaf rust | 37–69 | F-6 to F-0 | DI > 30% |
| Stripe rust | 30–69 | F-6 to F-0 | DI > 30% |
| Foliar Disease | Effect | df | F | p |
|---|---|---|---|---|
| Septoria tritici blotch | Treatment (T) | 324 | 25.73677 | <0.0001 |
| Year (Y) | 324 | 59.35194 | <0.0001 | |
| T × Y | 324 | 23.68177 | <0.0001 | |
| Glume blotch | Treatment (T) | 324 | 9.288164 | 0.0009 |
| Year (Y) | 324 | 11.220406 | <0.0001 | |
| T × Y | 324 | 3.131020 | 0.2044 | |
| Tan spot | Treatment (T) | 324 | 0.463364 | 0.4965 |
| Year (Y) | 324 | 8.877909 | <0.0001 | |
| T × Y | 324 | 0.441737 | 0.9917 | |
| Powdery mildew | Treatment (T) | 324 | 2.567894 | <0.0001 |
| Year (Y) | 324 | 8.076783 | <0.0001 | |
| T × Y | 324 | 1.810791 | <0.0001 | |
| Stripe rust | Treatment (T) | 324 | 18.671426 | <0.0001 |
| Year (Y) | 324 | 2.065552 | 0.0024 | |
| T × Y | 324 | 1.925722 | 0.0057 | |
| Leaf rust | Treatment (T) | 324 | 18.671426 | <0.0001 |
| Year (Y) | 324 | 2.065552 | 0.0024 | |
| T × Y | 324 | 1.925722 | 0.0057 |
| Effect | df | F | p |
|---|---|---|---|
| Treatment (T) | 2 | 100.2018 | <0.0001 |
| Year (Y) | 25 | 16.0594 | <0.0001 |
| T × Y | 50 | 0.6335 | 0.9764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klink, H.; Prahl, K.C.; Hasler, M.; Verreet, J.-A.; Birr, T. Efficiency and Effectivity of a Biological–Epidemiological Fungal Disease Management System in Wheat—A Study of 26 Years. Agriculture 2022, 12, 1099. https://doi.org/10.3390/agriculture12081099
Klink H, Prahl KC, Hasler M, Verreet J-A, Birr T. Efficiency and Effectivity of a Biological–Epidemiological Fungal Disease Management System in Wheat—A Study of 26 Years. Agriculture. 2022; 12(8):1099. https://doi.org/10.3390/agriculture12081099
Chicago/Turabian StyleKlink, Holger, Ketel Christian Prahl, Mario Hasler, Joseph-Alexander Verreet, and Tim Birr. 2022. "Efficiency and Effectivity of a Biological–Epidemiological Fungal Disease Management System in Wheat—A Study of 26 Years" Agriculture 12, no. 8: 1099. https://doi.org/10.3390/agriculture12081099






