Screening of 258 Pesticide Residues in Silage Using Modified QuEChERS with Liquid- and Gas Chromatography-Quadrupole/Orbitrap Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. Chromatographic and MS Conditions of LC-Q-Orbitrap/MS
2.1.2. Chromatographic and MS Conditions of GC-Q-Orbitrap/MS
2.2. Reagents and Materials
2.3. Preparation of Standard Solutions
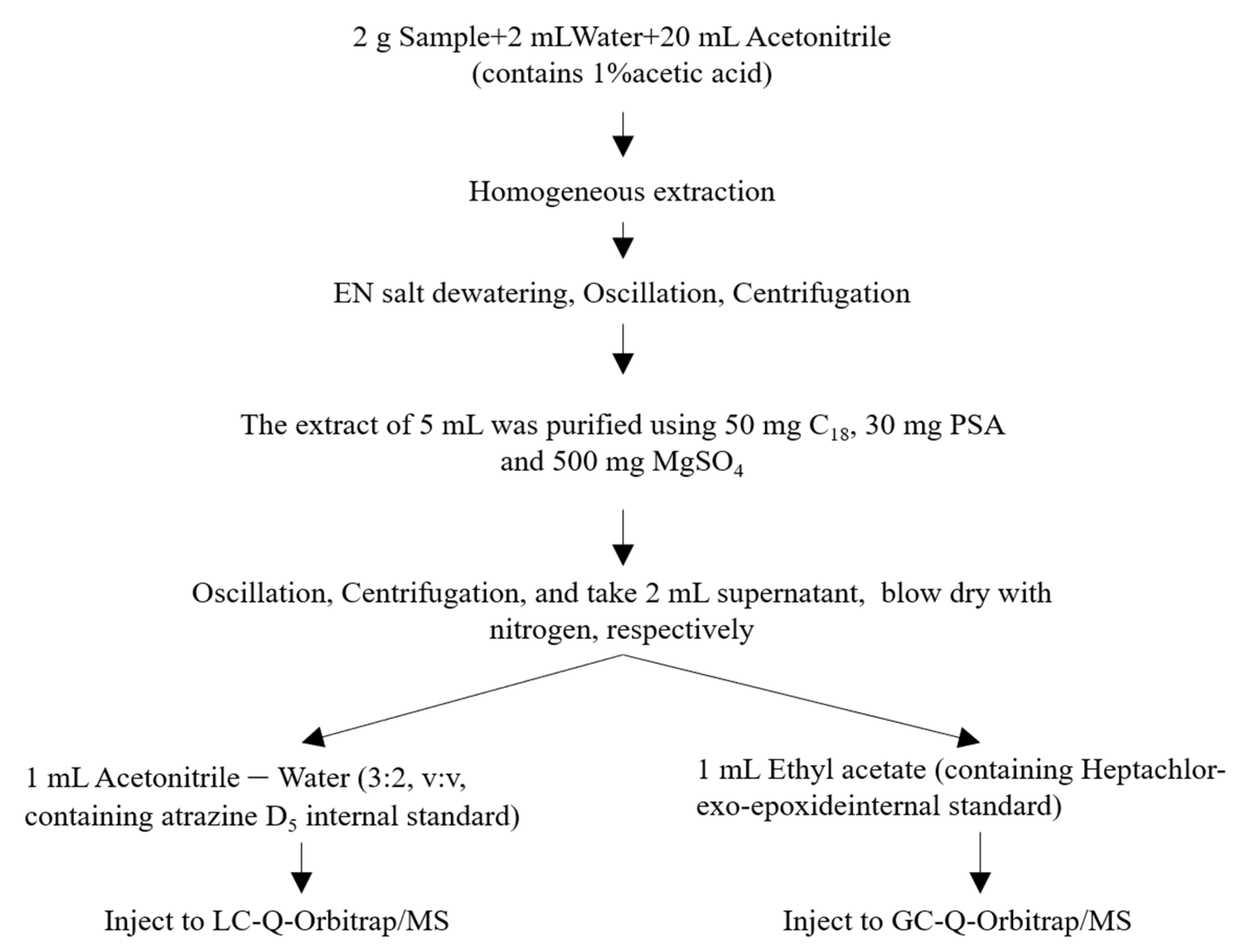
2.4. Sample Preparation
2.4.1. Extraction
2.4.2. Clean-up
2.5. Validation of the Method
3. Results and Discussion
3.1. Sample Extraction and Clean-Up
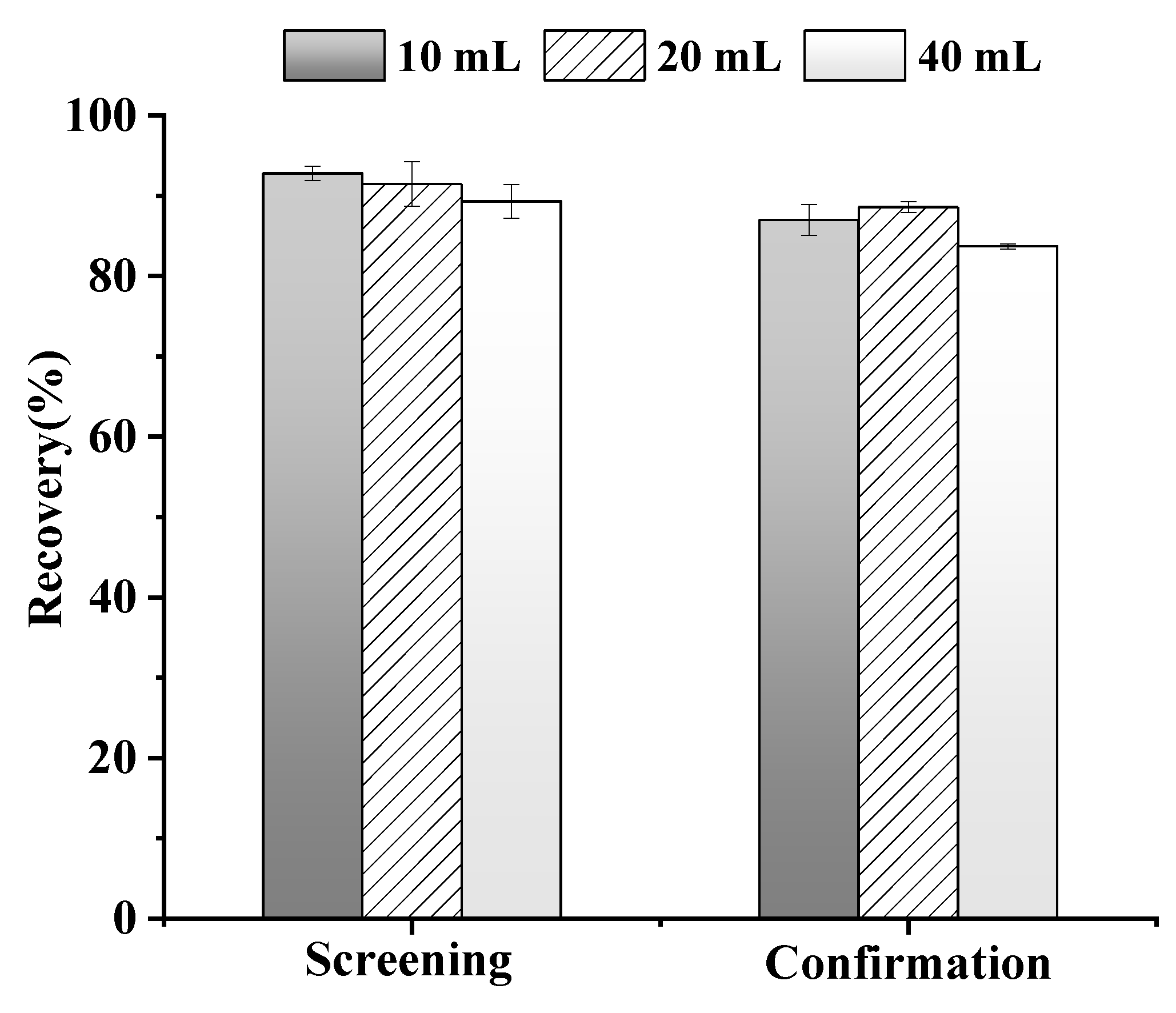
3.1.1. Optimization of Extraction Solvent Volume
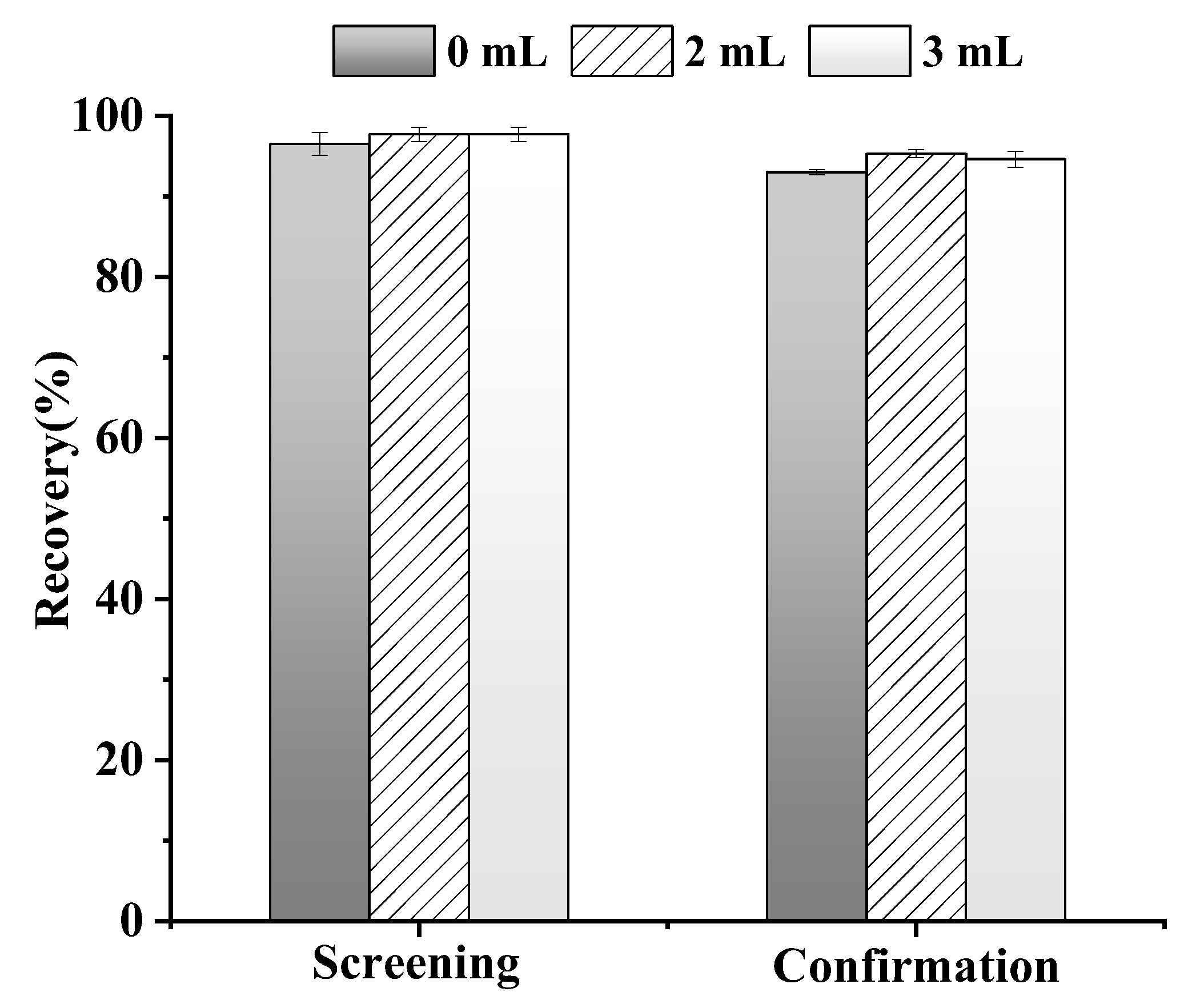
3.1.2. Optimization of Hydration Volume
3.1.3. Optimization of the Type of Extraction Salt
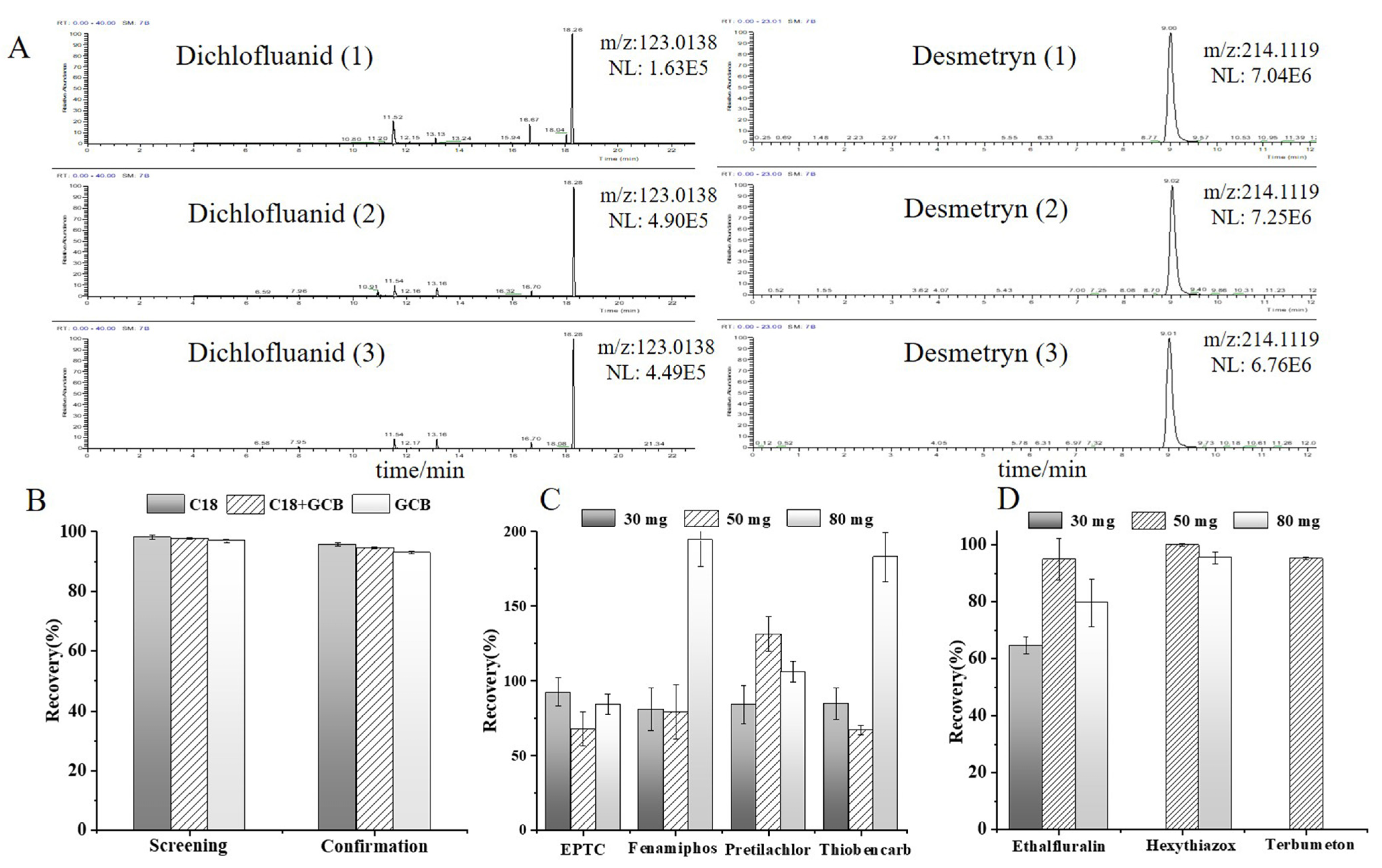
3.1.4. Optimization of the Adsorbent
3.1.5. Optimization of Purification Volume
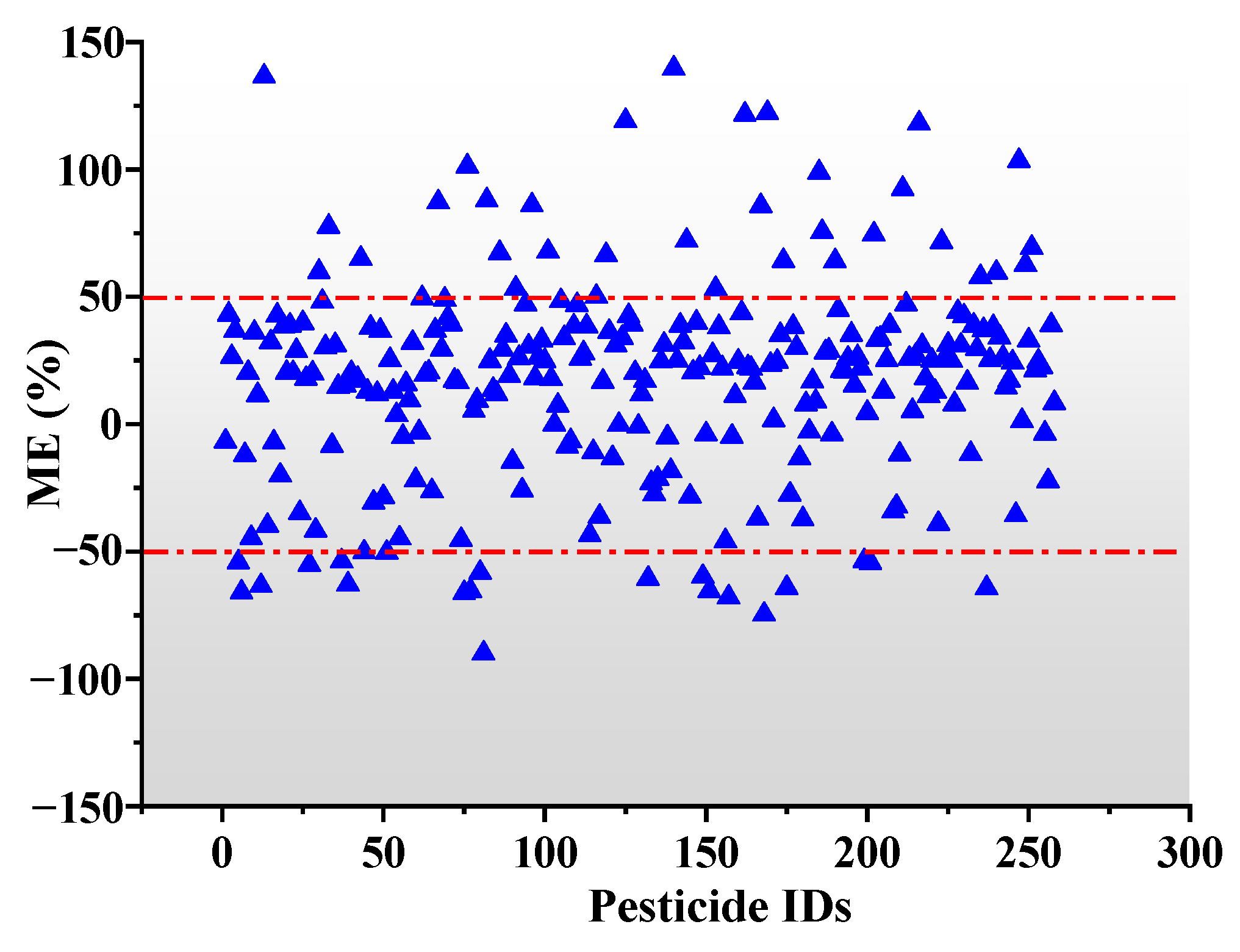
3.2. Matrix Effect
- |ME|, lower than 20% shows a weak matrix effect;
- |ME|, between 20% and 50% shows a medium matrix effect;
- |ME|, higher than 50% shows a strong matrix effect.
3.3. Method Validation
3.3.1. Linear Range, SDL, and LOQ
3.3.2. Recovery and Precision
3.4. Comparison of LC/GC-Q-Orbitrap/MS
3.5. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmio, A.; Sairanen, A.; Kuoppala, K.; Rinne, M. Milk production potential of whole crop faba bean silage compared with grass silage and rapeseed meal. Livest. Sci. 2022, 259, 104881. [Google Scholar] [CrossRef]
- Álvarez, C.; Nielsen, N.I.; Weisbjerg, M.R.; Volden, H.; Eknæs, M.; Prestløkken, E. High-digestible silages allow low concentrate supply without affecting milk production or methane emissions. J. Dairy Sci. 2022, 105, 3633–3647. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q. Main points of production technology of whole plant corn silage. Anim. Breed. Feed. 2021, 20, 55–56. [Google Scholar]
- Chen, A.Q.; Wang, T.G. Technical points of high-quality silage production. Agric. Knowl. 2021, 23, 27–29. [Google Scholar]
- Zhu, G.H. Main technical points and quality evaluation of corn silage production. Mod. Anim. Husb. 2020, 7, 47. [Google Scholar]
- Lotfi, S.; Rouzbehan, Y.; Fazaeli, H.; Feyzbakhsh, M.T.; Rezaeia, J. The nutritional value and yields of amaranth (Amaranthus hypochondriacus) cultivar silages compared to silage from corn (Zea mays) harvested at the milk stage grown in a hot-humid climate. Anim. Feed Sci. Tech. 2022, 289, 115336. [Google Scholar] [CrossRef]
- Weiby, K.V.; Krizsan, S.J.; Eknæs, M.; Schwarm, A.; Whist, A.C.; Schei, I.; Steinshamn, H.; Lund, P.; Beauchemin, K.A.; Dønnem, I. Associations among nutrient concentration, silage fermentation products, in vivo organic matter digestibility, rumen fermentation and in vitro methane yield in 78 grass silages. Anim. Feed Sci. Tech. 2022, 285, 115249. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Li, J.G.; Yao, T.; Yang, J.; Li, J. Research progress of pesticide residues in ruminant animal feed. J. Food Saf. Qual. 2019, 10, 3225–3230. [Google Scholar]
- Wu, Z.H.; Hua, W.Y.; Luo, L.G.; Tanaka, K. Technical Efficiency of Maize Production and Its Influencing Factors in the World’s Largest Groundwater Drop Funnel Area, China. Agriculture 2022, 12, 649. [Google Scholar] [CrossRef]
- Aioanei, N.M.; Pop, I.M. Heavy metal and pesticide contamination of maize silage derived from two different production systems (conventional and organic). Curr. Opin. Biotech. 2013, 24, S86. [Google Scholar] [CrossRef]
- Zhang, S.L.; Hua, Z.L.; Yao, W.X.; Lu, T.; Chen, Y.P.; Fang, Z.; Zhao, H.T. Use of corn straw-derived biochar for magnetic solid-phase microextraction of organophosphorus pesticides from environmental samples. J. Chromatogr. A 2021, 1660, 462673. [Google Scholar] [CrossRef]
- Du Preez, L.H.; Van Rensburg, P.J.J.; Jooste, A.M.; Carr, J.A.; Giesy, J.P.; Gross, T.S.; Kendall, R.J.; Smith, E.E.; Der Kraak, G.V.; Solomon, K.R. Seasonal exposures to triazine and other pesticides in surface waters in the western Highveld corn-production region in South Africa. Environ. Pollut. 2005, 135, 131–141. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Li, S.J.; Deng, B.; Li, Q.H.; Yang, L.Y.; Mao, H.M. Current status of pesticide residues in different cow feeds. China Feed. 2012, 17, 41–42. [Google Scholar]
- Wang, F.E.; Deng, L.G.; Li, X.; Wang, Z.W.; Wang, L.; Lu, K.; Zhang, S.Q.; Zhang, S.Q. Simultaneous determination of ten pesticide residues in forage grass using QuEChERS-gas chromatography-tandem mass spectrometry. Chin. J. Pestic. Sci. 2017, 19, 68–75. [Google Scholar]
- Nag, S.K.; Raikwar, M.K. Persistent organochlorine pesticide residues in animal feed. Environ. Monit. Assess. 2011, 174, 327–335. [Google Scholar] [CrossRef]
- Hall, M.J.; Dang, V.; Bradbury, S.P.; Coats, J.R. Optimization of QuEChERS Method for Simultaneous Determination of Neonicotinoid Residues in Pollinator Forage. Molecules 2020, 25, 2732. [Google Scholar] [CrossRef]
- Viera, M.S.; Rizzetti, T.M.; de Souza, M.P.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Multiresidue determination of pesticides in crop plants by the quick, easy, cheap, effective, rugged, and safe method and ultra-high-performance liquid chromatography tandem mass spectrometry using a calibration based on a single level standard addition in the sample. J. Chromatogr. A 2017, 1526, 119–127. [Google Scholar]
- Liang, Z.; Abdelshafy, A.M.; Luo, Z.S.; Belwal, T.; Lin, X.Y.; Xu, Y.Q.; Wang, L.; Yang, M.Y.; Qi, M.; Dong, Y.Y.; et al. Occurrence, detection, and dissipation of pesticide residue in plant-derived foodstuff: A state-of-the-art review. Food Chem. 2022, 384, 132494. [Google Scholar] [CrossRef]
- Cara, I.G.; Topa, D.; Raus, L.; Calistru, A.E.; Filipov, F.; Jitareanu, G. Selective and Sensitive Quantification of Acetochlor and S-Metolachlor in Maize and Soybean Plant Samples by Gas Chromatography-Tandem Mass Spectrometry. Agriculture 2021, 11, 283. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Estimation of pesticide residues in selected products of plant origin from Poland with the use of the HPLC-MS/MS technique. Agriculture 2020, 10, 192. [Google Scholar] [CrossRef]
- Paul, A.; Khan, Z.; Bhattacharyya, A.; Majumder, S.; Banerjee, K. Multiclass pesticide residue analysis in tobacco (Nicotiana tabacum) using high performance liquid chromatography-high resolution (Orbitrap) mass spectrometry: A simultaneous screening and quantitative method. J. Chromatogr. A 2021, 1468, 462208. [Google Scholar] [CrossRef]
- Dong, H.; Xian, Y.P.; Li, H.X.; Wu, Y.L.; Bai, W.D.; Zeng, X.F. Analysis of heterocyclic aromatic amine profiles in Chinese traditional bacon and sausage based on ultrahigh-performance liquid chromatography-quadrupole-Orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap-HRMS). Food Chem. 2020, 310, 125937. [Google Scholar] [CrossRef]
- Suo, D.C.; Fan, X.; Xiao, Z.M.; Li, Y.; Wang, P.L. Simultaneous determination of eight carbapenems in milk by modified QuEChERS and ultra high performance liquid chromatography coupled with high-field quadrupole-orbitrap high-resolution mass spectrometry. J. Chromatogr. A 2022, 1670, 462979. [Google Scholar]
- Liu, J.M.; Miao, S.; Li, W.T.; Pan, H.Q.; Ji, S. Application of high resolution mass spectrometry in the detection of pesticide residues. Chin. J. Anal. Lab. 2020, 39, 116–124. [Google Scholar]
- SANTE/12682/2021; Analytical Quality Control and Method Validation Procedures for Pesticides Residues and Analysis in Food and Feed. Directorate General for Health and Food Safety. European Union: Maastricht, The Netherlands, 2022.
- Wu, X.Q.; Li, T.M.; Feng, H.L.; Xie, Y.J.; Liu, F.Y.; Tong, K.X.; Fan, C.L.; Liu, Y.T.; Chen, H. Multi-residue analysis of 206 pesticides in grass forage by the one-step quick, easy, cheap, effective, rugged, and safe method combined with ultrahigh-performance liquid chromatography quadrupole orbitrap mass spectrometry. J. Sep. Sci. 2022, 45, 2520–2528. [Google Scholar] [CrossRef]
- Wang, J.; Leung, D.; Chow, W.; Wong, J.W.; Chang, J. UHPLC/ESI Q-Orbitrap quantitation of 655 pesticide residues in fruits and vegetables—A companion to an nDATA working flow. J. AOAC Int. 2020, 103, 1547–1559. [Google Scholar] [CrossRef]
- Pang, G.F.; Fan, C.L.; Chang, Q.Y.; Li, J.X.; Kang, J.; Lu, M.L. Screening of 485 pesticide residues in fruits and vegetables by liquid chromatography-quadrupole-time-of-flight mass spectrometry based on TOF accurate mass database and QTOF spectrum library. J. AOAC Int. 2018, 101, 1156–1182. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.H.; Zhang, L.; Wang, Y.J.; Yan, Y.H.; Tang, S.N.; Liu, R.; Zhao, H.T. Determination of four kinds of pesticide residues in silage corn by gas chromatography. Feed Res. 2021, 44, 90–92. [Google Scholar]
- Lucht, K.; Loose, D.; Ruschmeier, M.; Strotkötter, V.; Dyker, G.; Morgenstern, K. Hydrophilicity and Microsolvation of an Organic Molecule Resolved on the Sub-molecular Level by Scanning Tunneling Microscopy. Angew. Chem. Int. Ed. 2018, 57, 1266–1270. [Google Scholar] [CrossRef]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Nikita, T.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]

| No. | Compound | Category | RT/Min | MS1 ion (m/z) | MS2 ion (m/z) | R2 | SDL (µg kg−1) | LOQ (µg kg−1) | 1-LOQ | 2-LOQ | 10-LOQ | Instrumentation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REC/% | RSD/% | REC/% | RSD/% | REC/% | RSD/% | ||||||||||
| 1 | 1-(2-chloro-4-(4-chlorophenoxy)phenyl)-2-(1H-1,2,4-triazol-1-yl)ethanol | Fungicides | 17.45 | 350.0453 | 118.0412 | 0.9994 | 50 | 50 | 85.7 | 6.7 | 88.1 | 6.2 | 86.2 | 1 | LC-Q-Orbitrap/MS |
| 2 | 1-(2-Chloro-pyridin-5-yl-methyl)-2-imino-imidazolidine hydrochloride | Insecticides | 3.03 | 211.0744 | 126.0105 | 0.9983 | 10 | 10 | 85.5 | 5.3 | 90.4 | 7.7 | 87.5 | 6 | LC-Q-Orbitrap/MS |
| 3 | 1-methyl-3-(tetrahydro-3-furylmethyl) urea | Insecticides | 2.8 | 159.1127 | 102.0914 | 0.9995 | 20 | 20 | 87.8 | 6.8 | 83.1 | 1.9 | 83.4 | 3 | LC-Q-Orbitrap/MS |
| 4 | 2,4-D butylate | Herbicides | 17.03 | 185 | 186.997 | 0.9991 | 2 | 2 | 71.2 | 8.7 | 104.7 | 6.4 | 85.3 | 5 | GC-Q-Orbitrap/MS |
| 5 | 2,4′-DDD | Insecticides | 22.62 | 235.0076 | 165.0699 | 0.9995 | 2 | 5 | 90.7 | 9.2 | 97.7 | 2.1 | 97.8 | 2 | GC-Q-Orbitrap/MS |
| 6 | 2,4′-DDE | Insecticides | 21.17 | 245.9999 | 176.062 | 0.9991 | 1 | 1 | 99.2 | 9.1 | 81 | 6.5 | 100.9 | 2 | GC-Q-Orbitrap/MS |
| 7 | 3-(Trifluoromethyl)-1-methyl-1H-pyrazole-4-carboxamide | / | 3.37 | 194.0536 | 134.0349 | 0.9986 | 5 | 5 | 95.2 | 6 | 96.5 | 5.7 | 97.6 | 2 | LC-Q-Orbitrap/MS |
| 8 | 4,4′-DDD | Insecticides | 23.91 | 235.0077 | 165.0699 | 0.999 | 1 | 2 | 87.5 | 10 | 89.1 | 6.5 | 88.2 | 2 | GC-Q-Orbitrap/MS |
| 9 | 5-hydroxy Imidacloprid | Insecticides | 4.02 | 272.0541 | 225.0539 | 0.9988 | 50 | 50 | 86.1 | 5.1 | 88.3 | 4.4 | 75.4 | 4 | LC-Q-Orbitrap/MS |
| 10 | Acetamiprid | Insecticides | 5.46 | 223.0744 | 126.0106 | 0.9999 | 5 | 5 | 99.3 | 3.8 | 94.3 | 4.2 | 79.1 | 5 | LC-Q-Orbitrap/MS |
| 11 | Acetamiprid-N-desmethyl | Insecticides | 5.17 | 209.059 | 126.0105 | 0.9997 | 5 | 5 | 94.7 | 7.7 | 97 | 6.2 | 81 | 3 | LC-Q-Orbitrap/MS |
| 12 | Acetochlor | Herbicides | 16.9 | 146.0965 | 162.0914 | 0.9996 | 2 | 5 | 106.7 | 4.4 | 86.6 | 6.5 | 94.2 | 3 | GC-Q-Orbitrap/MS |
| 13 | Acrinathrin | Insecticides | 29.35 | 181.0647 | 180.081 | 0.999 | 5 | 5 | 89 | 6 | 99 | 6.3 | 99.7 | 5 | GC-Q-Orbitrap/MS |
| 14 | Alachlor | Herbicides | 17.22 | 188.107 | 146.0965 | 0.9996 | 5 | 5 | 98.2 | 8.2 | 113.5 | 3.4 | 95.1 | 2 | GC-Q-Orbitrap/MS |
| 15 | Aldicarb-sulfone | Insecticides | 3.44 | 240.101 | 86.06002 | 0.9997 | 50 | 50 | 93.5 | 4.2 | 94 | 4 | 77.9 | 3 | LC-Q-Orbitrap/MS |
| 16 | Allethrin | Insecticides | 19.33 | 303.195 | 135.0805 | 0.9994 | 10 | 10 | 95.8 | 6 | 92.4 | 6.4 | 94.9 | 8 | LC-Q-Orbitrap/MS |
| 17 | Allidochlor | Herbicides | 6.9 | 174.0678 | 98.09644 | 0.9998 | 0.5 | 1 | 102.1 | 5.6 | 102.5 | 4.4 | 99.8 | 4 | LC-Q-Orbitrap/MS |
| 18 | alpha-HCH | Insecticides | 13.53 | 180.9373 | 218.911 | 0.9996 | 5 | 10 | 96.4 | 4.4 | 89.7 | 2.3 | 109.1 | 12 | GC-Q-Orbitrap/MS |
| 19 | Ametryn | Herbicides | 17.72 | 227.1199 | 170.0493 | 0.9983 | 5 | 5 | 100.7 | 1.9 | 98.4 | 1.3 | 84.5 | 4 | GC-Q-Orbitrap/MS |
| 20 | Atrazine | Herbicides | 11.73 | 216.101 | 174.0543 | 0.9996 | 2 | 2 | 107.8 | 12.8 | 107.3 | 3.7 | 86.6 | 2 | LC-Q-Orbitrap/MS |
| 21 | Azoxystrobin | Fungicides | 15.39 | 404.1237 | 344.1031 | 0.9996 | 5 | 5 | 101.8 | 3.3 | 93.6 | 3.8 | 89.6 | 7 | LC-Q-Orbitrap/MS |
| 22 | Benalaxyl | Fungicides | 18.04 | 326.1747 | 148.1122 | 0.9998 | 2 | 2 | 98.2 | 5.1 | 98.7 | 3.1 | 94.9 | 4 | LC-Q-Orbitrap/MS |
| 23 | Bendiocarb | Insecticides | 8.38 | 224.0914 | 109.0285 | 0.9998 | 10 | 10 | 104.3 | 7.3 | 90.1 | 4.8 | 96.3 | 3 | LC-Q-Orbitrap/MS |
| 24 | Benfluralin | Herbicides | 13 | 292.0541 | 276.0589 | 0.9979 | 10 | 10 | 101.3 | 14 | 96.5 | 9 | 117.1 | 14 | GC-Q-Orbitrap/MS |
| 25 | Benoxacor | Herbicides | 13.66 | 260.0236 | 149.0836 | 0.9998 | 20 | 20 | 89.5 | 4.3 | 89.7 | 3.1 | 94.3 | 6 | LC-Q-Orbitrap/MS |
| 26 | Benzovindiflupyr | / | 32.37 | 159.0365 | 238.9715 | 0.9996 | 5 | 5 | 93.6 | 5.2 | 103.1 | 3.3 | 91.7 | 7 | GC-Q-Orbitrap/MS |
| 27 | beta-HCH | Insecticides | 14.52 | 180.9371 | 218.911 | 0.9975 | 10 | 10 | 108.8 | 4.9 | 93.1 | 5 | 113.3 | 11 | GC-Q-Orbitrap/MS |
| 28 | Bifenox | Herbicides | 27.67 | 340.985 | 173.0154 | 0.9991 | 20 | 20 | 84.6 | 17.8 | 93.6 | 12.9 | 92.6 | 1 | GC-Q-Orbitrap/MS |
| 29 | Bifenthrin | Insecticides | 27.12 | 181.1011 | 166.0776 | 0.9997 | 2 | 2 | 103.9 | 12.8 | 93 | 5.3 | 86.3 | 4 | GC-Q-Orbitrap/MS |
| 30 | Bitertanol | Fungicides | 30.1 | 170.0726 | 141.0699 | 0.9987 | 2 | 2 | 103.1 | 10.2 | 84 | 5.4 | 87 | 6 | GC-Q-Orbitrap/MS |
| 31 | Boscalid | Fungicides | 31.51 | 139.9898 | 111.9949 | 0.9996 | 5 | 5 | 94.3 | 8.8 | 104.4 | 2.2 | 93 | 2 | GC-Q-Orbitrap/MS |
| 32 | Bromobutide | Herbicides | 16.96 | 312.0951 | 119.0857 | 0.9993 | 20 | 20 | 90.8 | 6.3 | 92.9 | 3.5 | 94.1 | 5 | LC-Q-Orbitrap/MS |
| 33 | Bromophos-methyl | Insecticides | 19.46 | 328.8798 | 332.8746 | 0.9998 | 5 | 5 | 105.4 | 9.2 | 113.2 | 5.9 | 102.7 | 2 | GC-Q-Orbitrap/MS |
| 34 | Bromopropylate | Insecticides | 27.1 | 182.944 | 340.8993 | 0.9998 | 2 | 5 | 87.9 | 3.7 | 97.3 | 2.5 | 95 | 2 | GC-Q-Orbitrap/MS |
| 35 | Bupirimate | Fungicides | 17 | 317.1636 | 108.0114 | 0.9997 | 2 | 2 | 101.2 | 5 | 96.4 | 2.9 | 88.5 | 4 | LC-Q-Orbitrap/MS |
| 36 | Buprofezin | Insecticides | 18.93 | 306.1629 | 106.0652 | 0.9996 | 2 | 2 | 97.9 | 5.3 | 99.4 | 2.9 | 95.9 | 5 | LC-Q-Orbitrap/MS |
| 37 | Butachlor | Herbicides | 21.44 | 176.107 | 188.107 | 0.9997 | 5 | 5 | 90.7 | 2.8 | 99.8 | 2.3 | 97.1 | 3 | GC-Q-Orbitrap/MS |
| 38 | Butamifos | Herbicides | 18.33 | 333.1028 | 95.96676 | 0.9997 | 10 | 10 | 102.3 | 14.7 | 96.5 | 7 | 93 | 4 | LC-Q-Orbitrap/MS |
| 39 | Butylate | Herbicides | 18.99 | 218.157 | 156.1385 | 0.9995 | 1 | 2 | 73.1 | 15.5 | 75.3 | 9.4 | 81.4 | 6 | LC-Q-Orbitrap/MS |
| 40 | Cadusafos | Insecticides | 18.61 | 271.0946 | 158.9699 | 0.9999 | 2 | 2 | 90.7 | 9.8 | 114.9 | 11.4 | 94.4 | 11 | LC-Q-Orbitrap/MS |
| 41 | Carbaryl | Insecticides | 17.38 | 144.057 | 116.0621 | 0.9998 | 2 | 2 | 70.2 | 11.3 | 100 | 5.3 | 91.4 | 4 | GC-Q-Orbitrap/MS |
| 42 | Carbendazim | Fungicides | 4.04 | 192.0767 | 160.0506 | 0.9993 | 5 | 5 | 97.6 | 3.3 | 95.7 | 4.3 | 108.9 | 8 | LC-Q-Orbitrap/MS |
| 43 | Carbofuran | Insecticides | 8.47 | 222.1122 | 123.0441 | 0.9989 | 1 | 1 | 94.3 | 7.6 | 88.5 | 11 | 85.5 | 4 | LC-Q-Orbitrap/MS |
| 44 | Carbofuran-3-hydroxy | Insecticides | 4.94 | 238.107 | 163.0752 | 0.9973 | 0.5 | 10 | 95.1 | 0.9 | 100.5 | 2.4 | 99.6 | 4 | LC-Q-Orbitrap/MS |
| 45 | Carfentrazone-ethyl | Herbicides | 17.69 | 429.07 | 345.9957 | 0.9984 | 10 | 10 | 95.1 | 6.9 | 92.9 | 9 | 99.6 | 6 | LC-Q-Orbitrap/MS |
| 46 | Chlorantraniliprole | Insecticides | 14.15 | 481.9779 | 283.9221 | 0.9996 | 50 | 50 | 82.7 | 5.3 | 94.3 | 7.6 | 80.1 | 4 | LC-Q-Orbitrap/MS |
| 47 | Chlorfenapyr | Insecticides | 23.06 | 247.0479 | 363.9409 | 0.9988 | 50 | 50 | 94.3 | 13 | 104.7 | 12.2 | 89.6 | 2 | GC-Q-Orbitrap/MS |
| 48 | Chlorfenvinphos | Insecticides | 20.37 | 266.9379 | 323.0002 | 0.9998 | 5 | 5 | 92.3 | 9.6 | 94.5 | 2.8 | 96.3 | 9 | GC-Q-Orbitrap/MS |
| 49 | Chloridazon | Herbicides | 5.26 | 222.0427 | 104.0495 | 0.9997 | 5 | 5 | 108.8 | 2 | 107.1 | 2.1 | 87.3 | 5 | LC-Q-Orbitrap/MS |
| 50 | Chlormequat | Plant growth regulators | 0.91 | 122.073 | 62.9999 | 0.9984 | 50 | 50 | 76.6 | 11.3 | 75.9 | 5.2 | 79.6 | 6 | LC-Q-Orbitrap/MS |
| 51 | Chloroneb | Fungicides | 10.04 | 190.9663 | 205.9897 | 0.9996 | 2 | 2 | 78.6 | 10.6 | 93.1 | 7.2 | 84.6 | 2 | GC-Q-Orbitrap/MS |
| 52 | Chlorotoluron | Herbicides | 11.32 | 213.0788 | 72.04449 | 0.9999 | 5 | 20 | 88.4 | 3.2 | 85.5 | 4.4 | 82.6 | 2 | LC-Q-Orbitrap/MS |
| 53 | Chlorpropham | Herbicides | 12.76 | 213.055 | 152.9976 | 0.9996 | 20 | 20 | 70.9 | 11.6 | 96.3 | 6.3 | 94.6 | 4 | GC-Q-Orbitrap/MS |
| 54 | Chlorpyrifos-methyl | Insecticides | 16.91 | 285.9257 | 124.9822 | 0.9979 | 2 | 2 | 100.8 | 11.2 | 91 | 12.4 | 91.9 | 6 | GC-Q-Orbitrap/MS |
| 55 | Chlozolinate | Fungicides | 20.24 | 186.9586 | 258.9798 | 0.998 | 10 | 10 | 96.3 | 13.4 | 94.8 | 5.6 | 113.9 | 11 | GC-Q-Orbitrap/MS |
| 56 | Cis-Chlordane (alpha) | Insecticides | 21.49 | 370.8284 | 374.8226 | 0.9993 | 10 | 20 | 83.7 | 11.8 | 106.1 | 4.7 | 89.7 | 3 | GC-Q-Orbitrap/MS |
| 57 | Clodinafop-propargyl | Herbicides | 17.73 | 350.0586 | 266.038 | 0.9997 | 5 | 5 | 102.2 | 4.1 | 95 | 2.9 | 91.8 | 6 | LC-Q-Orbitrap/MS |
| 58 | Clofentezine | Insecticides | 18.61 | 303.0193 | 138.0106 | 0.9996 | 10 | 10 | 101.9 | 5.7 | 87.5 | 7.5 | 99 | 8 | LC-Q-Orbitrap/MS |
| 59 | Clomazone | Herbicides | 14.57 | 204.1019 | 127.0124 | 0.9998 | 5 | 5 | 105 | 3.3 | 100.1 | 0.9 | 87.8 | 4 | GC-Q-Orbitrap/MS |
| 60 | Clothianidin | Insecticides | 4.7 | 250.0157 | 131.967 | 0.9997 | 50 | 50 | 87.8 | 5 | 86.4 | 4.4 | 80.8 | 2 | LC-Q-Orbitrap/MS |
| 61 | Cyanazine | Herbicides | 7.63 | 241.096 | 214.0856 | 0.9998 | 5 | 5 | 110.5 | 2.2 | 100.9 | 1.6 | 86.3 | 5 | LC-Q-Orbitrap/MS |
| 62 | Cyanofenphos | Insecticides | 24.93 | 156.9872 | 169.0413 | 0.996 | 2 | 2 | 83.3 | 4.4 | 91.9 | 3.4 | 88.1 | 2 | GC-Q-Orbitrap/MS |
| 63 | Cyanophos | Insecticides | 14.94 | 243.0116 | 109.005 | 0.9997 | 5 | 10 | 88.4 | 7.6 | 96.6 | 2.5 | 114.9 | 11 | GC-Q-Orbitrap/MS |
| 64 | Cycloate | Herbicides | 18.6 | 216.1415 | 83.08556 | 0.9999 | 2 | 2 | 97.1 | 10.8 | 89.9 | 4.4 | 97.7 | 2 | LC-Q-Orbitrap/MS |
| 65 | Cycloxydim | Herbicides | 18.78 | 326.1779 | 180.1013 | 0.9981 | 10 | 10 | 83.3 | 7.4 | 75.2 | 8 | 72.5 | 7 | LC-Q-Orbitrap/MS |
| 66 | Cyfluthrin | Insecticides | 31.18 | 206.0601 | 163.0075 | 0.9993 | 20 | 20 | 98.9 | 13.6 | 91.9 | 2.3 | 93.7 | 1 | GC-Q-Orbitrap/MS |
| 67 | Cypermethrin | Insecticides | 31.69 | 181.0647 | 127.031 | 0.9973 | 50 | 50 | 84.5 | 9.5 | 96.6 | 13.9 | 83.6 | 3 | GC-Q-Orbitrap/MS |
| 68 | Cyprodinil | Fungicides | 20.03 | 224.1182 | 210.1027 | 0.9999 | 5 | 5 | 98.8 | 3.1 | 99.5 | 2.7 | 93 | 5 | GC-Q-Orbitrap/MS |
| 69 | delta-HCH | Insecticides | 15.82 | 180.9374 | 218.911 | 0.9993 | 5 | 5 | 92.5 | 11.2 | 104.6 | 4.6 | 95.2 | 4 | GC-Q-Orbitrap/MS |
| 70 | Deltamethrin | Insecticides | 33.53 | 181.0647 | 252.9047 | 0.9944 | 20 | 20 | 96.9 | 7 | 99.5 | 8.3 | 97.4 | 5 | GC-Q-Orbitrap/MS |
| 71 | Desmetryn | Herbicides | 9.51 | 214.1119 | 172.0653 | 0.9999 | 2 | 2 | 94.8 | 5.2 | 101 | 2.3 | 99.4 | 2 | LC-Q-Orbitrap/MS |
| 72 | Diallate | Herbicides | 18.79 | 270.0477 | 86.06006 | 0.9983 | 10 | 10 | 94.3 | 4.8 | 88.7 | 5.3 | 89.6 | 4 | LC-Q-Orbitrap/MS |
| 73 | Diazinon | Insecticides | 15.26 | 179.1178 | 199.0631 | 0.9999 | 2 | 2 | 100.1 | 4.8 | 96.5 | 3.6 | 100.9 | 4 | GC-Q-Orbitrap/MS |
| 74 | Dichlofenthion | Insecticides | 16.71 | 222.938 | 224.935 | 0.9993 | 1 | 1 | 78.1 | 10.7 | 83.5 | 9.4 | 99.7 | 3 | GC-Q-Orbitrap/MS |
| 75 | Dichlofluanid | Fungicides | 18.3 | 123.0138 | 223.95 | 0.9983 | 20 | 20 | 85.5 | 4.7 | 89.9 | 5.1 | 93.9 | 7 | GC-Q-Orbitrap/MS |
| 76 | Dichlorvos | Insecticides | 6.41 | 184.9764 | 78.99435 | 0.9997 | 1 | 1 | 73.1 | 6.3 | 93.7 | 10.5 | 79.6 | 6 | GC-Q-Orbitrap/MS |
| 77 | Dieldrin | Insecticides | 22.47 | 260.8595 | 79.05431 | 0.9945 | 50 | 50 | 86.7 | 15.3 | 99.5 | 10.8 | 81.5 | 5 | GC-Q-Orbitrap/MS |
| 78 | Difenoconazole | Fungicides | 18.66 | 406.0715 | 251.0026 | 0.9992 | 10 | 10 | 94.5 | 4.4 | 94.8 | 6.7 | 93.8 | 7 | LC-Q-Orbitrap/MS |
| 79 | Diflubenzuron | Insecticides | 17.45 | 311.0389 | 141.0148 | 0.9998 | 10 | 10 | 99.6 | 5.1 | 87.9 | 6.3 | 91.9 | 6 | LC-Q-Orbitrap/MS |
| 80 | Dimethenamid | Herbicides | 15.03 | 276.0816 | 168.0842 | 0.9997 | 2 | 2 | 84.2 | 5.8 | 89.4 | 1.6 | 84.3 | 2 | LC-Q-Orbitrap/MS |
| 81 | Dimethoate | Insecticides | 14.03 | 124.9822 | 142.9927 | 0.9954 | 5 | 5 | 88.1 | 3.6 | 101.9 | 5.1 | 93.7 | 3 | GC-Q-Orbitrap/MS |
| 82 | Dimethylvinphos (E) | Insecticides | 18.29 | 294.9688 | 127.0155 | 0.9987 | 2 | 5 | 93.4 | 6.3 | 107.2 | 4.2 | 95.3 | 3 | GC-Q-Orbitrap/MS |
| 83 | Dimethylvinphos (Z) | Insecticides | 16.61 | 330.9452 | 127.0156 | 0.9996 | 10 | 10 | 96.9 | 4.5 | 92.5 | 5.1 | 91.3 | 3 | LC-Q-Orbitrap/MS |
| 84 | Diniconazole | Fungicides | 23.79 | 268.0041 | 232.0273 | 0.9974 | 10 | 10 | 88.7 | 12.5 | 91.3 | 4.8 | 111.6 | 8 | GC-Q-Orbitrap/MS |
| 85 | Dinotefuran | Insecticides | 3.23 | 203.1138 | 129.0897 | 0.9983 | 50 | 50 | 88.1 | 11.4 | 91.2 | 5.7 | 77.9 | 3 | LC-Q-Orbitrap/MS |
| 86 | Dioxabenzofos | Insecticides | 12.94 | 216.0005 | 200.977 | 0.9995 | 5 | 5 | 101.5 | 9.6 | 103.6 | 7.7 | 88.9 | 4 | GC-Q-Orbitrap/MS |
| 87 | Dipropetryn | Herbicides | 17.21 | 256.1586 | 186.0811 | 0.9996 | 2 | 2 | 94.1 | 4.4 | 100.7 | 2.2 | 98.9 | 3 | LC-Q-Orbitrap/MS |
| 88 | Diuron | Herbicides | 12.8 | 233.0241 | 72.04452 | 0.9996 | 5 | 50 | 85.5 | 5.1 | 92.7 | 5.9 | 81.9 | 1 | LC-Q-Orbitrap/MS |
| 89 | Edifenphos | Fungicides | 24.95 | 109.0108 | 172.9821 | 0.9996 | 2 | 2 | 98.1 | 3.2 | 99.9 | 2.6 | 101.4 | 3 | GC-Q-Orbitrap/MS |
| 90 | Endosulfan-sulfate | Insecticides | 25.03 | 269.8128 | 236.8407 | 0.9975 | 20 | 20 | 95.3 | 19.2 | 111.4 | 7 | 94.2 | 4 | GC-Q-Orbitrap/MS |
| 91 | EPN | Insecticides | 27.04 | 156.9872 | 169.0413 | 0.9998 | 2 | 2 | 87.3 | 16.7 | 79.1 | 8.2 | 86.4 | 4 | GC-Q-Orbitrap/MS |
| 92 | EPTC | Herbicides | 7.75 | 128.1071 | 104.0531 | 0.9997 | 5 | 5 | 86.4 | 11.1 | 80.1 | 8.2 | 76.1 | 4 | GC-Q-Orbitrap/MS |
| 93 | Ethalfluralin | Herbicides | 12.59 | 276.0595 | 292.0538 | 0.9998 | 20 | 50 | 114.2 | 16.5 | 93.4 | 11.4 | 81.1 | 1 | GC-Q-Orbitrap/MS |
| 94 | Ethion | Insecticides | 23.94 | 230.9732 | 96.95084 | 0.9998 | 2 | 2 | 79.2 | 17.2 | 91.5 | 4 | 91.3 | 3 | GC-Q-Orbitrap/MS |
| 95 | Ethoprophos | Insecticides | 17.05 | 243.0633 | 130.9386 | 0.9997 | 2 | 2 | 93.1 | 4 | 99.6 | 2.5 | 98.8 | 2 | LC-Q-Orbitrap/MS |
| 96 | Etofenprox | Insecticides | 31.87 | 163.1117 | 107.0492 | 0.9997 | 5 | 5 | 89.7 | 14.9 | 101.7 | 7.8 | 96.9 | 4 | GC-Q-Orbitrap/MS |
| 97 | Etrimfos | Insecticides | 17.79 | 293.0717 | 142.9928 | 0.9997 | 10 | 10 | 104.1 | 5.6 | 89.8 | 5.1 | 88.8 | 4 | LC-Q-Orbitrap/MS |
| 98 | Fenamidone | Fungicides | 27.44 | 268.0905 | 237.1023 | 0.9998 | 5 | 5 | 100.4 | 2 | 99.3 | 3 | 90.3 | 6 | GC-Q-Orbitrap/MS |
| 99 | Fenamiphos | Insecticides | 17.52 | 304.1125 | 217.0083 | 0.9989 | 2 | 2 | 92.7 | 9.1 | 86.7 | 4.9 | 87.7 | 6 | LC-Q-Orbitrap/MS |
| 100 | Fenamiphos-sulfone | Insecticides | 10.34 | 336.1026 | 139.0213 | 0.9998 | 5 | 5 | 95.8 | 4.8 | 104.4 | 2.7 | 86.9 | 5 | LC-Q-Orbitrap/MS |
| 101 | Fenamiphos-sulfoxide | Insecticides | 9.78 | 320.1076 | 171.0476 | 0.9973 | 10 | 10 | 119.7 | 4.1 | 112 | 11.8 | 99.9 | 4 | LC-Q-Orbitrap/MS |
| 102 | Fenarimol | Fungicides | 29.16 | 138.9946 | 251.0028 | 0.9999 | 5 | 5 | 75.9 | 6.4 | 95.5 | 4.7 | 90.2 | 3 | GC-Q-Orbitrap/MS |
| 103 | Fenbuconazole | Fungicides | 17.43 | 337.1209 | 125.0153 | 0.9998 | 5 | 5 | 103 | 5.6 | 94.3 | 2.4 | 88.3 | 5 | LC-Q-Orbitrap/MS |
| 104 | Fenchlorphos | Insecticides | 17.55 | 284.9304 | 124.9822 | 0.9974 | 2 | 2 | 102.2 | 8.5 | 102.4 | 3.7 | 88.3 | 3 | GC-Q-Orbitrap/MS |
| 105 | Fenitrothion | Insecticides | 18.08 | 277.017 | 124.9822 | 0.9987 | 20 | 20 | 73.5 | 13.1 | 115.4 | 7.8 | 90.2 | 4 | GC-Q-Orbitrap/MS |
| 106 | Fenobucarb | Insecticides | 11.79 | 121.0649 | 122.0682 | 0.9995 | 5 | 5 | 117.6 | 9.7 | 98.3 | 5.2 | 91.5 | 4 | GC-Q-Orbitrap/MS |
| 107 | Fenpropathrin | Insecticides | 27.45 | 181.0647 | 97.10129 | 0.9997 | 5 | 5 | 89.5 | 7.6 | 99.3 | 3.5 | 98.7 | 4 | GC-Q-Orbitrap/MS |
| 108 | Fenpropimorph | Fungicides | 19.13 | 128.1069 | 117.0697 | 0.9993 | 5 | 5 | 100.4 | 8.8 | 98.6 | 7.5 | 102 | 4 | GC-Q-Orbitrap/MS |
| 109 | Fensulfothion | Insecticides | 13.29 | 309.0375 | 157.0318 | 0.9996 | 5 | 5 | 99.7 | 2 | 98.4 | 3.1 | 83.9 | 5 | LC-Q-Orbitrap/MS |
| 110 | Fenthion | Insecticides | 18.84 | 278.0195 | 245.0398 | 0.9981 | 10 | 10 | 111.5 | 11.9 | 102.7 | 2.9 | 103.6 | 3 | GC-Q-Orbitrap/MS |
| 111 | Fenthion-sulfone | Insecticides | 10.93 | 311.0166 | 142.9928 | 0.9998 | 10 | 10 | 97.6 | 3.8 | 96.6 | 3.6 | 92.7 | 6 | LC-Q-Orbitrap/MS |
| 112 | Fenthion-sulfoxide | Insecticides | 10.21 | 295.022 | 127.0156 | 0.9997 | 5 | 5 | 106.1 | 3.3 | 102.7 | 3.6 | 107.2 | 11 | LC-Q-Orbitrap/MS |
| 113 | Fipronil | Insecticides | 20.24 | 366.9429 | 212.9481 | 0.9999 | 5 | 5 | 109.4 | 19.9 | 102.1 | 7.9 | 95.7 | 4 | GC-Q-Orbitrap/MS |
| 114 | Fipronil Desulfinyl | Insecticides | 17.2 | 332.9961 | 389.9683 | 0.9985 | 5 | 5 | 96.1 | 18.5 | 106.3 | 5.7 | 97.5 | 2 | GC-Q-Orbitrap/MS |
| 115 | Fipronil-sulfide | Insecticides | 19.86 | 350.9479 | 254.9699 | 0.9999 | 5 | 5 | 112.3 | 15 | 107.6 | 8.2 | 98.6 | 4 | GC-Q-Orbitrap/MS |
| 116 | Fipronil-sulfone | Insecticides | 22.51 | 382.9377 | 212.9481 | 0.9998 | 20 | 20 | 66.5 | 5.5 | 102.2 | 10.1 | 89.8 | 3 | GC-Q-Orbitrap/MS |
| 117 | Fluacrypyrim | Insecticides | 24.38 | 145.0649 | 204.0781 | 0.9998 | 10 | 10 | 79 | 4.4 | 90.7 | 8.5 | 102.4 | 10 | GC-Q-Orbitrap/MS |
| 118 | Fluazifop-butyl | Herbicides | 23.41 | 282.0736 | 268.0582 | 0.9993 | 2 | 2 | 94.1 | 8.9 | 87.1 | 1.5 | 92.6 | 3 | GC-Q-Orbitrap/MS |
| 119 | Flucythrinate | Insecticides | 31.7 | 157.046 | 181.0647 | 0.9999 | 5 | 5 | 103.2 | 14.8 | 99.5 | 7.8 | 90.3 | 4 | GC-Q-Orbitrap/MS |
| 120 | Fluopicolide | Fungicides | 15.97 | 382.9722 | 172.9555 | 0.9999 | 10 | 10 | 99 | 4.1 | 88.8 | 5.5 | 92.7 | 4 | LC-Q-Orbitrap/MS |
| 121 | Fluquinconazole | Fungicides | 30.38 | 340.0395 | 341.0428 | 0.9953 | 10 | 10 | 100.8 | 6.8 | 81.4 | 2.8 | 106.8 | 12 | GC-Q-Orbitrap/MS |
| 122 | Fluridone | Herbicides | 14.6 | 330.1095 | 259.0989 | 0.9999 | 2 | 2 | 95 | 2.6 | 100.3 | 2.3 | 94.9 | 2 | LC-Q-Orbitrap/MS |
| 123 | Flusilazole | Fungicides | 17.63 | 316.1072 | 165.0701 | 0.9998 | 2 | 10 | 97.7 | 4.1 | 85.9 | 5.4 | 94.9 | 6 | LC-Q-Orbitrap/MS |
| 124 | Flutriafol | Fungicides | 12.4 | 302.1095 | 70.04012 | 0.9996 | 5 | 5 | 98.5 | 4.7 | 96.9 | 4 | 80.1 | 6 | LC-Q-Orbitrap/MS |
| 125 | Fluxapyroxad | Fungicides | 27.04 | 159.0364 | 139.0302 | 0.9998 | 1 | 2 | 91.8 | 7.3 | 91.1 | 3.6 | 84.4 | 5 | GC-Q-Orbitrap/MS |
| 126 | Fonofos | Insecticides | 15.05 | 137.0187 | 246.0297 | 0.9999 | 2 | 2 | 93.5 | 12.5 | 102.4 | 3 | 91 | 3 | GC-Q-Orbitrap/MS |
| 127 | Fosthiazate | Insecticides | 11.16 | 284.0536 | 104.0165 | 0.9999 | 10 | 10 | 102.2 | 7.5 | 80.4 | 7.5 | 90.2 | 6 | LC-Q-Orbitrap/MS |
| 128 | Furathiocarb | Insecticides | 27.97 | 163.0753 | 194.0396 | 0.9993 | 2 | 2 | 84.1 | 7.3 | 92.8 | 3.4 | 97.5 | 3 | GC-Q-Orbitrap/MS |
| 129 | Haloxyfop | Herbicides | 17.72 | 362.0396 | 91.05431 | 0.9981 | 20 | 20 | 78.3 | 6.4 | 97.8 | 4.4 | 97.7 | 6 | LC-Q-Orbitrap/MS |
| 130 | Haloxyfop-2-ethoxyethyl | Herbicides | 26.22 | 302.019 | 316.0345 | 0.9997 | 5 | 5 | 95.9 | 7 | 94.8 | 2.1 | 90.4 | 7 | GC-Q-Orbitrap/MS |
| 131 | Haloxyfop-methyl | Herbicides | 21.21 | 288.0035 | 375.0478 | 0.9999 | 5 | 5 | 95.4 | 4.1 | 90.3 | 3.7 | 92.8 | 7 | GC-Q-Orbitrap/MS |
| 132 | Heptachlor | Insecticides | 17.36 | 269.813 | 273.8069 | 0.9964 | 50 | 50 | 92.1 | 6.2 | 112.7 | 13.6 | 84.4 | 4 | GC-Q-Orbitrap/MS |
| 133 | Hexachlorobenzene | Fungicides | 13.67 | 281.8127 | 285.8068 | 0.9988 | 2 | 5 | 93.8 | 13.4 | 89.4 | 7.4 | 85.7 | 3 | GC-Q-Orbitrap/MS |
| 134 | Hexaconazole | Fungicides | 18.17 | 314.0817 | 70.04015 | 0.9995 | 5 | 10 | 96.1 | 3.1 | 84.5 | 5.2 | 90.3 | 7 | LC-Q-Orbitrap/MS |
| 135 | Hexythiazox | Insecticides | 19.53 | 353.1079 | 168.0576 | 0.9995 | 10 | 10 | 115.9 | 7.7 | 105.9 | 9.7 | 88.3 | 6 | LC-Q-Orbitrap/MS |
| 136 | Imazalil | Fungicides | 11.55 | 297.0553 | 158.9764 | 0.9991 | 10 | 10 | 99.3 | 1.1 | 86.1 | 3.8 | 86.9 | 6 | LC-Q-Orbitrap/MS |
| 137 | Imazapyr | Herbicides | 4.63 | 262.1184 | 217.0972 | 0.9995 | 5 | 5 | 102 | 4.9 | 99.1 | 2.9 | 88.1 | 4 | LC-Q-Orbitrap/MS |
| 138 | Imidacloprid | Insecticides | 4.76 | 256.0592 | 209.0589 | 0.9997 | 10 | 10 | 100.2 | 4.7 | 89.2 | 3.7 | 90.8 | 4 | LC-Q-Orbitrap/MS |
| 139 | Imidaclothiz | Insecticides | 5.06 | 262.0157 | 181.0542 | 0.9977 | 20 | 20 | 79.3 | 3.3 | 84.6 | 4.4 | 82.3 | 2 | LC-Q-Orbitrap/MS |
| 140 | Ipconazole | Fungicides | 29.05 | 125.0154 | 127.0124 | 0.9999 | 2 | 5 | 89.1 | 8.4 | 85.5 | 2.8 | 93.7 | 4 | GC-Q-Orbitrap/MS |
| 141 | Iprobenfos | Fungicides | 17.76 | 289.1018 | 91.0543 | 0.9993 | 20 | 20 | 85.5 | 7.3 | 93 | 3.7 | 93.9 | 3 | LC-Q-Orbitrap/MS |
| 142 | Iprovalicarb | Fungicides | 16.88 | 321.2167 | 119.0856 | 0.9996 | 10 | 10 | 73.2 | 8.3 | 89.5 | 4 | 100.4 | 5 | LC-Q-Orbitrap/MS |
| 143 | Isazofos | Insecticides | 15.66 | 118.9883 | 162.0429 | 0.9999 | 5 | 5 | 102.3 | 2.5 | 95.7 | 4.6 | 91.3 | 6 | GC-Q-Orbitrap/MS |
| 144 | Isocarbophos | Insecticides | 19.14 | 135.9976 | 120.0205 | 0.9998 | 2 | 5 | 72.4 | 10 | 87.9 | 9.8 | 95.7 | 4 | GC-Q-Orbitrap/MS |
| 145 | Isofenphos | Insecticides | 20.27 | 213.0311 | 121.0285 | 0.9998 | 2 | 2 | 96.5 | 19.4 | 97.9 | 7.1 | 86.3 | 2 | GC-Q-Orbitrap/MS |
| 146 | Isoprocarb | Insecticides | 11.76 | 194.1176 | 95.0492 | 0.9996 | 5 | 5 | 113.4 | 9.8 | 99.2 | 9.8 | 87.7 | 5 | LC-Q-Orbitrap/MS |
| 147 | Isoproturon | Herbicides | 12.5 | 207.149 | 72.0445 | 0.9994 | 2 | 10 | 97.2 | 2.4 | 89.3 | 3.8 | 93 | 5 | LC-Q-Orbitrap/MS |
| 148 | Isopyrazam | / | 18.64 | 360.1877 | 244.0882 | 0.9999 | 2 | 2 | 92.8 | 6.6 | 102.8 | 3.9 | 94.4 | 3 | LC-Q-Orbitrap/MS |
| 149 | Kresoxim-methyl | Fungicides | 22.79 | 116.0496 | 206.0812 | 0.9998 | 5 | 5 | 92.9 | 8.1 | 95.8 | 5.6 | 99.8 | 2 | GC-Q-Orbitrap/MS |
| 150 | Lactofen | Herbicides | 19.2 | 479.0826 | 222.977 | 0.9992 | 10 | 10 | 98.9 | 5.6 | 89.2 | 8.4 | 86.5 | 7 | LC-Q-Orbitrap/MS |
| 151 | Lindane | Insecticides | 14.52 | 180.9371 | 218.911 | 0.9933 | 10 | 20 | 90.4 | 7.7 | 94.7 | 6 | 92.8 | 6 | GC-Q-Orbitrap/MS |
| 152 | Linuron | Herbicides | 14.67 | 249.0189 | 159.9717 | 0.9996 | 10 | 10 | 102.1 | 2.7 | 97.5 | 5.4 | 97.3 | 5 | LC-Q-Orbitrap/MS |
| 153 | Malaoxon | Insecticides | 17.15 | 127.0156 | 194.9876 | 0.9994 | 5 | 5 | 98.1 | 6.3 | 109.6 | 4.4 | 86.4 | 4 | GC-Q-Orbitrap/MS |
| 154 | Malathion | Insecticides | 15.94 | 331.0428 | 99.00771 | 0.9999 | 10 | 10 | 99.9 | 6.7 | 91.6 | 3.9 | 94.4 | 3 | LC-Q-Orbitrap/MS |
| 155 | Mepanipyrim | Fungicides | 16.87 | 224.118 | 106.0652 | 0.9997 | 2 | 2 | 99.7 | 4.1 | 94.9 | 1.9 | 100.7 | 4 | LC-Q-Orbitrap/MS |
| 156 | Metaflumizone | Insecticides | 19.28 | 507.1238 | 178.0475 | 0.9994 | 50 | 50 | 83.4 | 9.1 | 89.7 | 6.4 | 80.7 | 6 | LC-Q-Orbitrap/MS |
| 157 | Metalaxyl | Fungicides | 17.22 | 160.1121 | 132.0809 | 0.9992 | 10 | 10 | 97.8 | 4.7 | 82.4 | 5.7 | 107.2 | 10 | GC-Q-Orbitrap/MS |
| 158 | Metconazole | Fungicides | 27.69 | 125.0154 | 138.0664 | 0.9997 | 5 | 5 | 97.7 | 6.6 | 100.9 | 4.5 | 95.3 | 7 | GC-Q-Orbitrap/MS |
| 159 | Methidathion | Insecticides | 20.98 | 145.0067 | 124.9822 | 0.9998 | 5 | 5 | 92.6 | 6 | 108.4 | 5.6 | 95.4 | 3 | GC-Q-Orbitrap/MS |
| 160 | Methiocarb | Insecticides | 14.95 | 226.0894 | 121.0648 | 0.9996 | 10 | 10 | 96.6 | 2.6 | 86.5 | 3.9 | 92.8 | 4 | LC-Q-Orbitrap/MS |
| 161 | Methiocarb-sulfone | Insecticides | 5.65 | 275.1055 | 122.0727 | 0.9995 | 50 | 50 | 90.4 | 4.1 | 100.5 | 4.4 | 83.1 | 2 | LC-Q-Orbitrap/MS |
| 162 | Methiocarb-sulfoxide | Insecticides | 5.16 | 242.0841 | 185.0631 | 0.9996 | 10 | 10 | 106.8 | 3.6 | 88.7 | 3.7 | 90.9 | 5 | LC-Q-Orbitrap/MS |
| 163 | Metolachlor | Herbicides | 17.15 | 284.1408 | 148.1121 | 0.9998 | 10 | 10 | 97.2 | 2.7 | 94.8 | 4.8 | 92 | 4 | LC-Q-Orbitrap/MS |
| 164 | Metolcarb | Insecticides | 9.31 | 108.057 | 79.05431 | 0.9997 | 5 | 5 | 112.9 | 9.1 | 105.9 | 9.6 | 90.7 | 12 | GC-Q-Orbitrap/MS |
| 165 | Metrafenone | Fungicides | 18.42 | 409.064 | 226.9703 | 0.9998 | 10 | 10 | 79.3 | 6.7 | 95.3 | 5.7 | 92.4 | 5 | LC-Q-Orbitrap/MS |
| 166 | Metribuzin | Herbicides | 16.93 | 198.0696 | 144.0465 | 0.9975 | 10 | 10 | 93 | 8.7 | 75.7 | 7.5 | 103.1 | 11 | GC-Q-Orbitrap/MS |
| 167 | Mevinphos | Insecticides | 6.23 | 225.052 | 127.0155 | 0.9995 | 2 | 2 | 75.8 | 5.9 | 89.6 | 3.7 | 84.8 | 3 | LC-Q-Orbitrap/MS |
| 168 | Mirex | Insecticides | 28.83 | 269.8128 | 273.8067 | 0.9991 | 10 | 10 | 81.7 | 10.6 | 75.3 | 5.4 | 111.7 | 9 | GC-Q-Orbitrap/MS |
| 169 | Monocrotophos | Insecticides | 4.24 | 224.068 | 127.0155 | 0.9997 | 2 | 10 | 95 | 5.6 | 78.4 | 5.2 | 101.1 | 2 | LC-Q-Orbitrap/MS |
| 170 | Monolinuron | Herbicides | 10.17 | 215.058 | 126.0107 | 0.9999 | 5 | 5 | 104.1 | 2.2 | 103 | 2.6 | 90.4 | 4 | LC-Q-Orbitrap/MS |
| 171 | Myclobutanil | Fungicides | 22.65 | 179.0244 | 181.0214 | 0.9993 | 5 | 5 | 104.4 | 2.7 | 96.8 | 4.3 | 86.8 | 5 | GC-Q-Orbitrap/MS |
| 172 | Napropamide | Herbicides | 17.17 | 272.1641 | 171.0806 | 0.9999 | 2 | 2 | 98.9 | 2.6 | 96.7 | 3.1 | 99 | 2 | LC-Q-Orbitrap/MS |
| 173 | Norflurazon | Herbicides | 13.21 | 304.0454 | 284.0396 | 0.9999 | 5 | 10 | 100.7 | 1.9 | 90.6 | 3.9 | 94.1 | 7 | LC-Q-Orbitrap/MS |
| 174 | Omethoate | Insecticides | 3.05 | 214.0296 | 142.9927 | 0.9996 | 10 | 10 | 97.8 | 4.9 | 91.4 | 10.6 | 113.7 | 8 | LC-Q-Orbitrap/MS |
| 175 | Oxadiazon | Herbicides | 22.5 | 174.9587 | 258.0323 | 0.9994 | 2 | 5 | 100.1 | 9.9 | 103.1 | 2.2 | 98 | 2 | GC-Q-Orbitrap/MS |
| 176 | Oxadixyl | Fungicides | 23.87 | 132.0809 | 233.0922 | 0.9966 | 1 | 1 | 86.6 | 8.7 | 76.8 | 12.2 | 81.6 | 10 | GC-Q-Orbitrap/MS |
| 177 | Oxyfluorfen | Herbicides | 22.72 | 252.0395 | 317.006 | 0.9993 | 10 | 10 | 92.7 | 14.8 | 92.6 | 4 | 112.4 | 13 | GC-Q-Orbitrap/MS |
| 178 | Paclobutrazol | Plant growth regulators | 15.83 | 294.1364 | 70.04013 | 0.9994 | 5 | 10 | 98.3 | 2.7 | 87.1 | 4.3 | 90.5 | 5 | LC-Q-Orbitrap/MS |
| 179 | Parathion | Insecticides | 18.98 | 291.0326 | 155.0036 | 0.9987 | 50 | 50 | 84.1 | 9.1 | 82.7 | 10.2 | 84 | 5 | GC-Q-Orbitrap/MS |
| 180 | Pendimethalin | Herbicides | 19.92 | 252.098 | 191.0688 | 0.9991 | 5 | 5 | 78.7 | 9.3 | 92.1 | 6.8 | 88.9 | 3 | GC-Q-Orbitrap/MS |
| 181 | Pentachloroaniline | / | 16.36 | 262.8627 | 266.8568 | 0.9975 | 2 | 2 | 107.5 | 14.2 | 91 | 9.5 | 85.4 | 6 | GC-Q-Orbitrap/MS |
| 182 | Pentachloroanisole | / | 13.87 | 262.8389 | 236.8409 | 0.9993 | 5 | 5 | 85 | 10.9 | 99.8 | 9.9 | 90.7 | 3 | GC-Q-Orbitrap/MS |
| 183 | Penthiopyrad | Fungicides | 17.95 | 360.1346 | 177.0271 | 0.9993 | 2 | 2 | 90 | 8.8 | 90.9 | 2.3 | 90.2 | 4 | LC-Q-Orbitrap/MS |
| 184 | Phenothrin | Insecticides | 28.19 | 183.0804 | 81.06996 | 0.9998 | 5 | 5 | 94.3 | 9.3 | 102 | 13.9 | 94.4 | 3 | GC-Q-Orbitrap/MS |
| 185 | Phenthoate | Insecticides | 20.48 | 273.9883 | 245.9933 | 0.9997 | 5 | 5 | 114.6 | 12.8 | 100.4 | 9.2 | 91.6 | 4 | GC-Q-Orbitrap/MS |
| 186 | Phorate | Insecticides | 18.23 | 261.0201 | 75.02644 | 0.9918 | 10 | 20 | 116.2 | 9.8 | 97.3 | 5.9 | 95.4 | 2 | LC-Q-Orbitrap/MS |
| 187 | Phorate-Sulfone | Insecticides | 11.8 | 293.0096 | 171.024 | 0.9995 | 20 | 20 | 90.8 | 2.3 | 87.3 | 4.6 | 94.4 | 5 | LC-Q-Orbitrap/MS |
| 188 | Phorate-Sulfoxide | Insecticides | 11.48 | 277.0147 | 114.9614 | 0.9998 | 10 | 10 | 106.2 | 2.2 | 105.4 | 10.4 | 98.7 | 5 | LC-Q-Orbitrap/MS |
| 189 | Phosalone | Insecticides | 28.22 | 182.0003 | 121.0414 | 0.9996 | 5 | 5 | 104.8 | 11.6 | 97.8 | 6.7 | 97.6 | 6 | GC-Q-Orbitrap/MS |
| 190 | Phosmet | Insecticides | 14.19 | 318.0018 | 160.0394 | 0.9994 | 20 | 20 | 78 | 15.2 | 95.6 | 10.2 | 95.3 | 2 | LC-Q-Orbitrap/MS |
| 191 | Phosphamidon | Insecticides | 7.68 | 300.076 | 127.0156 | 0.999 | 10 | 10 | 71.9 | 2.7 | 81.3 | 13.5 | 89 | 11 | LC-Q-Orbitrap/MS |
| 192 | Phoxim | Insecticides | 18.28 | 299.061 | 129.0448 | 0.9997 | 10 | 10 | 105.6 | 5.8 | 90.8 | 4.4 | 90 | 5 | LC-Q-Orbitrap/MS |
| 193 | Picoxystrobin | Fungicides | 17.68 | 368.11 | 145.065 | 0.9969 | 20 | 20 | 80 | 3.3 | 99 | 6.3 | 101.2 | 3 | LC-Q-Orbitrap/MS |
| 194 | Piperonyl Butoxide | Insecticides | 26.16 | 176.0832 | 119.0856 | 0.9997 | 5 | 5 | 96.3 | 7.3 | 112.6 | 7.2 | 88.1 | 5 | GC-Q-Orbitrap/MS |
| 195 | Pirimicarb | Insecticides | 7.76 | 239.1493 | 72.04455 | 0.9999 | 5 | 5 | 102.2 | 2.6 | 95.6 | 1 | 87.1 | 4 | LC-Q-Orbitrap/MS |
| 196 | Pirimiphos-methyl | Insecticides | 18.02 | 306.1032 | 108.0557 | 0.9999 | 2 | 2 | 103.2 | 6 | 96.2 | 2.9 | 94.6 | 4 | LC-Q-Orbitrap/MS |
| 197 | Pretilachlor | Herbicides | 18.84 | 312.1719 | 252.115 | 0.9994 | 5 | 5 | 113 | 5.4 | 96.6 | 3.7 | 89.5 | 3 | LC-Q-Orbitrap/MS |
| 198 | Prochloraz | Fungicides | 18.09 | 376.0377 | 70.02892 | 0.9997 | 10 | 10 | 105.1 | 3.7 | 90.6 | 6.2 | 92.3 | 6 | LC-Q-Orbitrap/MS |
| 199 | Procymidone | Fungicides | 20.64 | 283.0162 | 285.0132 | 0.9989 | 5 | 10 | 110.3 | 7.1 | 81.7 | 3.4 | 119.8 | 11 | GC-Q-Orbitrap/MS |
| 200 | Profenofos | Insecticides | 19.07 | 372.9418 | 302.8643 | 0.9997 | 10 | 10 | 98.7 | 2.1 | 95.3 | 5.3 | 88 | 6 | LC-Q-Orbitrap/MS |
| 201 | Prometryn | Herbicides | 17.85 | 241.1357 | 226.1122 | 0.9983 | 5 | 5 | 93 | 7.8 | 93.8 | 9 | 96.1 | 3 | GC-Q-Orbitrap/MS |
| 202 | Propamocarb | Fungicides | 3 | 189.1596 | 102.055 | 0.9999 | 10 | 10 | 79.8 | 3.9 | 98.9 | 13.5 | 86.8 | 12 | LC-Q-Orbitrap/MS |
| 203 | Propanil | Herbicides | 16.85 | 160.9793 | 217.0055 | 0.9996 | 1 | 2 | 83.4 | 6.3 | 90.7 | 3.1 | 85.6 | 5 | GC-Q-Orbitrap/MS |
| 204 | Propaphos | Insecticides | 17.98 | 305.0968 | 221.0033 | 0.9996 | 2 | 2 | 81.6 | 8.6 | 88.8 | 4.7 | 71 | 19 | LC-Q-Orbitrap/MS |
| 205 | Propargite | Insecticides | 19.69 | 368.1884 | 81.06996 | 0.9997 | 5 | 5 | 117.5 | 5.7 | 98.9 | 4 | 92.5 | 4 | LC-Q-Orbitrap/MS |
| 206 | Propazine | Herbicides | 14.54 | 230.1164 | 146.0229 | 0.9999 | 1 | 1 | 106.2 | 2.1 | 102 | 1.8 | 99.7 | 2 | LC-Q-Orbitrap/MS |
| 207 | Propham | Herbicides | 9.26 | 179.094 | 93.0574 | 0.9928 | 2 | 5 | 91.6 | 8.7 | 96.2 | 6.2 | 94.4 | 2 | GC-Q-Orbitrap/MS |
| 208 | Propiconazole | Fungicides | 25.27 | 172.9556 | 174.9526 | 0.9992 | 5 | 5 | 90.7 | 8.5 | 92.8 | 9.5 | 91.8 | 2 | GC-Q-Orbitrap/MS |
| 209 | Propyzamide | Herbicides | 15.14 | 172.9556 | 254.0135 | 0.999 | 2 | 2 | 78.4 | 11.9 | 98.8 | 4.5 | 91.5 | 2 | GC-Q-Orbitrap/MS |
| 210 | Prothioconazole-desthio | Fungicides | 17.14 | 312.066 | 70.04014 | 0.9991 | 5 | 5 | 103.4 | 2 | 102.8 | 2.9 | 93.5 | 6 | LC-Q-Orbitrap/MS |
| 211 | Prothiofos | Insecticides | 20.39 | 344.9699 | 258.9149 | 0.9993 | 50 | 50 | 84.9 | 10.6 | 72.9 | 4.5 | 80.2 | 4 | LC-Q-Orbitrap/MS |
| 212 | Pymetrozine | Insecticides | 2.76 | 218.1034 | 105.0448 | 0.9963 | 50 | 50 | 71.4 | 10.6 | 93.4 | 11.4 | 89 | 10 | LC-Q-Orbitrap/MS |
| 213 | Pyraclostrobin | Fungicides | 18.31 | 388.1054 | 163.0629 | 0.9995 | 2 | 5 | 93.8 | 7.5 | 103.6 | 5.6 | 99.7 | 5 | LC-Q-Orbitrap/MS |
| 214 | Pyridaben | Insecticides | 20.22 | 365.1444 | 147.1169 | 0.9998 | 5 | 5 | 101.9 | 3.8 | 99.3 | 4.3 | 91.1 | 5 | LC-Q-Orbitrap/MS |
| 215 | Pyridaphenthion | Insecticides | 16.58 | 341.0715 | 189.066 | 0.9997 | 2 | 2 | 99.2 | 4 | 101.8 | 3.3 | 89.9 | 5 | LC-Q-Orbitrap/MS |
| 216 | Pyrimethanil | Fungicides | 15.51 | 198.1026 | 183.0791 | 0.9975 | 2 | 2 | 72.5 | 8.8 | 97.7 | 7.2 | 79.4 | 5 | GC-Q-Orbitrap/MS |
| 217 | Pyriproxyfen | Insecticides | 19.44 | 322.1433 | 96.0444 | 0.9998 | 1 | 1 | 90.9 | 5.4 | 102 | 4.1 | 97.9 | 4 | LC-Q-Orbitrap/MS |
| 218 | Quinalphos | Insecticides | 20.51 | 146.0475 | 157.076 | 0.9998 | 2 | 2 | 104.1 | 6.7 | 101.8 | 2.9 | 95.3 | 4 | GC-Q-Orbitrap/MS |
| 219 | Quinoxyfen | Fungicides | 25.06 | 237.0585 | 306.9964 | 0.9999 | 2 | 2 | 85.5 | 6.1 | 100.8 | 3.2 | 99.7 | 3 | GC-Q-Orbitrap/MS |
| 220 | Quintozene | Fungicides | 14.57 | 234.8438 | 238.8379 | 0.9995 | 10 | 10 | 74.6 | 10.6 | 74.2 | 11.9 | 112.3 | 14 | GC-Q-Orbitrap/MS |
| 221 | Quizalofop-ethyl | Herbicides | 19.03 | 373.0945 | 299.0581 | 0.9999 | 5 | 5 | 104.8 | 4 | 105.3 | 2 | 92.7 | 7 | LC-Q-Orbitrap/MS |
| 222 | Resmethrin | Insecticides | 20.38 | 339.1951 | 128.0621 | 0.9985 | 5 | 5 | 74.1 | 3.5 | 72.8 | 5.6 | 80.9 | 4 | LC-Q-Orbitrap/MS |
| 223 | Sedaxane | Fungicides | 28.88 | 159.0365 | 130.0652 | 0.9998 | 10 | 10 | 87 | 6.2 | 85.7 | 5.9 | 104.7 | 12 | GC-Q-Orbitrap/MS |
| 224 | Simazine | Herbicides | 8.35 | 202.0851 | 132.0324 | 0.9998 | 10 | 10 | 98.8 | 1.6 | 88.4 | 2.5 | 92.4 | 5 | LC-Q-Orbitrap/MS |
| 225 | Spinosyn A | Insecticides | 18.35 | 732.4695 | 142.1228 | 0.9997 | 20 | 50 | 94.3 | 6.9 | 86 | 7.7 | 85.5 | 4 | LC-Q-Orbitrap/MS |
| 226 | Spinosyn D | Insecticides | 18.73 | 746.4852 | 142.1228 | 0.9955 | 50 | 50 | 80.7 | 17.7 | 97.5 | 6 | 80.7 | 3 | LC-Q-Orbitrap/MS |
| 227 | Spirodiclofen | Insecticides | 19.93 | 411.1121 | 71.08568 | 0.9995 | 10 | 10 | 92.4 | 4.7 | 91 | 6.4 | 96.4 | 5 | LC-Q-Orbitrap/MS |
| 228 | Spirotetramat | Insecticides | 17 | 374.1957 | 216.102 | 0.9997 | 20 | 20 | 87.7 | 9.4 | 91.5 | 9.4 | 89.4 | 3 | LC-Q-Orbitrap/MS |
| 229 | Spirotetramat-enol | Insecticides | 11.3 | 302.1748 | 216.102 | 0.9996 | 5 | 5 | 102.1 | 3 | 100.6 | 2.2 | 86.6 | 5 | LC-Q-Orbitrap/MS |
| 230 | Spiroxamine | Fungicides | 15.41 | 298.2737 | 144.1384 | 0.9997 | 2 | 2 | 87.6 | 8.5 | 99.5 | 5.3 | 87.7 | 5 | LC-Q-Orbitrap/MS |
| 231 | Sulfotep | Insecticides | 17.84 | 323.0296 | 114.9614 | 0.9999 | 2 | 2 | 97.6 | 6.6 | 96.1 | 3 | 95.3 | 5 | LC-Q-Orbitrap/MS |
| 232 | Sulprofos | Insecticides | 19.63 | 323.035 | 218.9701 | 0.9967 | 10 | 10 | 79.9 | 9.8 | 82 | 5.5 | 81.4 | 10 | LC-Q-Orbitrap/MS |
| 233 | Tebuconazole | Fungicides | 17.87 | 308.152 | 125.0153 | 0.9995 | 5 | 5 | 76.2 | 8.5 | 95.8 | 5 | 96 | 2 | LC-Q-Orbitrap/MS |
| 234 | Terbufos | Insecticides | 14.94 | 230.9733 | 174.9106 | 0.992 | 10 | 10 | 91.6 | 8.5 | 117.6 | 4.6 | 104 | 14 | GC-Q-Orbitrap/MS |
| 235 | Terbufos-sulfone | Insecticides | 20.08 | 199.001 | 170.9698 | 0.9999 | 5 | 5 | 117.6 | 7.8 | 116.8 | 7.2 | 93.2 | 3 | GC-Q-Orbitrap/MS |
| 236 | Terbufos-Sulfoxide | Insecticides | 14.77 | 305.0459 | 130.9386 | 0.9981 | 20 | 50 | 98.9 | 7.6 | 93.6 | 2.7 | 84.6 | 3 | LC-Q-Orbitrap/MS |
| 237 | Terbumeton | Herbicides | 11.1 | 226.1661 | 170.1039 | 0.9952 | 20 | 20 | 78.1 | 8 | 95.3 | 2.5 | 83.5 | 7 | LC-Q-Orbitrap/MS |
| 238 | Terbuthylazine | Herbicides | 15.23 | 230.1164 | 174.0543 | 0.9997 | 2 | 2 | 97.9 | 4.7 | 97.2 | 1.4 | 102.2 | 2 | LC-Q-Orbitrap/MS |
| 239 | Tetradifon | Insecticides | 28 | 158.9666 | 226.8887 | 0.9997 | 5 | 5 | 90.2 | 3.7 | 93.3 | 3.8 | 93.9 | 3 | GC-Q-Orbitrap/MS |
| 240 | Tetramethrin | Insecticides | 19.12 | 332.1852 | 164.0707 | 0.9998 | 1 | 1 | 96.1 | 13.7 | 93.6 | 5.8 | 96.4 | 4 | LC-Q-Orbitrap/MS |
| 241 | Thiabendazole | Fungicides | 4.78 | 202.0432 | 175.0325 | 0.9995 | 5 | 5 | 92.9 | 3.8 | 95.5 | 4.2 | 82.8 | 6 | LC-Q-Orbitrap/MS |
| 242 | Thiacloprid | Insecticides | 6.22 | 253.0307 | 126.0106 | 0.9997 | 5 | 5 | 106.6 | 4.6 | 98.6 | 2.6 | 83.9 | 5 | LC-Q-Orbitrap/MS |
| 243 | Thiamethoxam | Insecticides | 4.04 | 292.0263 | 131.967 | 0.9984 | 20 | 20 | 89.1 | 5.5 | 94.8 | 4.7 | 91.9 | 5 | LC-Q-Orbitrap/MS |
| 244 | Thiobencarb | Herbicides | 18.47 | 258.0711 | 125.0154 | 0.9997 | 5 | 5 | 98.5 | 3.4 | 98.2 | 6.4 | 92.3 | 5 | LC-Q-Orbitrap/MS |
| 245 | Thiophanate-methyl | Fungicides | 8.05 | 343.0526 | 151.0326 | 0.9904 | 50 | 50 | 71.8 | 8.3 | 71.5 | 6.2 | 79.8 | 9 | LC-Q-Orbitrap/MS |
| 246 | Tolfenpyrad | Insecticides | 19.34 | 384.147 | 197.0962 | 0.9996 | 5 | 5 | 96.8 | 6.2 | 97 | 4.7 | 89.5 | 6 | LC-Q-Orbitrap/MS |
| 247 | Tolylfluanid | Fungicides | 17.88 | 346.9848 | 137.0295 | 0.9982 | 50 | 50 | 100 | 8.1 | 93.1 | 7.3 | 82.4 | 3 | LC-Q-Orbitrap/MS |
| 248 | Triadimefon | Fungicides | 16.2 | 294.1 | 197.0729 | 0.9993 | 5 | 5 | 101.8 | 4 | 97.3 | 2.8 | 90.9 | 7 | LC-Q-Orbitrap/MS |
| 249 | Triadimenol | Fungicides | 16.5 | 296.1156 | 70.04016 | 0.9999 | 5 | 5 | 84 | 13.1 | 90.7 | 2.6 | 92.8 | 2 | LC-Q-Orbitrap/MS |
| 250 | Triazophos | Insecticides | 16.73 | 314.0718 | 162.0662 | 0.9995 | 2 | 2 | 95.6 | 1.9 | 105.8 | 4.3 | 96.4 | 5 | LC-Q-Orbitrap/MS |
| 251 | Trichlorfon | Insecticides | 4.93 | 256.9295 | 127.0155 | 0.9987 | 20 | 20 | 88.3 | 5.1 | 93.8 | 3.8 | 92.5 | 3 | LC-Q-Orbitrap/MS |
| 252 | Trifloxystrobin | Fungicides | 18.77 | 409.1364 | 186.0525 | 0.9984 | 5 | 5 | 98.2 | 5.5 | 102 | 2.6 | 92.2 | 5 | LC-Q-Orbitrap/MS |
| 253 | Triflumizole | Fungicides | 18.8 | 346.0923 | 278.0554 | 0.9999 | 10 | 10 | 76.7 | 12.2 | 81.7 | 8.3 | 85.1 | 7 | LC-Q-Orbitrap/MS |
| 254 | Trinexapac-ethyl | Plant growth regulators | 12.72 | 253.1067 | 69.03366 | 0.9964 | 20 | 20 | 85.2 | 9.5 | 96.2 | 11.9 | 114 | 6 | LC-Q-Orbitrap/MS |
| 255 | Uniconazole | Fungicides | 22.49 | 234.0429 | 165.0102 | 0.9997 | 5 | 5 | 106.2 | 3.5 | 97 | 2.5 | 89.5 | 6 | GC-Q-Orbitrap/MS |
| 256 | Vinclozolin | Fungicides | 17.09 | 212.0029 | 197.9872 | 0.9994 | 5 | 5 | 97.8 | 7.3 | 105.8 | 3.5 | 90.8 | 6 | GC-Q-Orbitrap/MS |
| 257 | Warfarin | / | 15.51 | 309.1118 | 163.0391 | 0.9999 | 2 | 2 | 100.8 | 5.7 | 102.7 | 3.9 | 91.1 | 3 | LC-Q-Orbitrap/MS |
| 258 | Zoxamide | Fungicides | 17.89 | 336.0315 | 186.971 | 0.9999 | 5 | 5 | 91.3 | 6.2 | 103.8 | 3.1 | 97 | 7 | LC-Q-Orbitrap/MS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Wu, X.; Song, Y.; Sun, Y.; Tong, K.; Yu, X.; Fan, C.; Chen, H. Screening of 258 Pesticide Residues in Silage Using Modified QuEChERS with Liquid- and Gas Chromatography-Quadrupole/Orbitrap Mass Spectrometry. Agriculture 2022, 12, 1231. https://doi.org/10.3390/agriculture12081231
Xie Y, Wu X, Song Y, Sun Y, Tong K, Yu X, Fan C, Chen H. Screening of 258 Pesticide Residues in Silage Using Modified QuEChERS with Liquid- and Gas Chromatography-Quadrupole/Orbitrap Mass Spectrometry. Agriculture. 2022; 12(8):1231. https://doi.org/10.3390/agriculture12081231
Chicago/Turabian StyleXie, Yujie, Xingqiang Wu, Yanling Song, Yini Sun, Kaixuan Tong, Xiaoxuan Yu, Chunlin Fan, and Hui Chen. 2022. "Screening of 258 Pesticide Residues in Silage Using Modified QuEChERS with Liquid- and Gas Chromatography-Quadrupole/Orbitrap Mass Spectrometry" Agriculture 12, no. 8: 1231. https://doi.org/10.3390/agriculture12081231






