Evaluating Physicochemical and Sensory Properties of Functional Yogurt Supplemented with Glycyrrhiza Polysaccharide as Potential Replacement for Gelatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GPs Extraction
2.3. Yogurt Fermentation
2.4. Determination of Viable Cell Count of Yogurt
2.5. Physical and Chemical Analyses
2.6. Water-Holding Capacity (WHC)
2.7. Textural Characteristics
2.8. Low-Field 1H NMR
2.9. Flow Behaviour Analysis
2.10. Microstructure of Yogurt
2.11. Sensory Analysis
2.12. Data Analysis
3. Results and Discussion
3.1. Viable Cell Count of Yogurt Samples
3.2. Physicochemical Characteristics of Yogurt Samples
3.3. Texture Analysis
3.4. Low-Field 1H NMR
3.5. Flow Behaviour Analysis
3.6. Microstructure
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V.; Akbarian-Moghari, A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mishra, H.N. Mango soy fortified set yoghurt: Effect of stabilizer addition on physicochemical, sensory and textural properties. Food Chem. 2004, 87, 501–507. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Yang, H.; Prakash, S.; Bansal, N. Evaluation of tilapia skin gelatin as a mammalian gelatin replacer in acid milk gels and low-fat stirred yoghurt. J. Dairy Sci. 2017, 100, 3436–3447. [Google Scholar] [CrossRef] [PubMed]
- Macit, E.; Bakirci, I. Effect of different stablizers on quality characteristics of the set-type yogurt. Afr. J. Biotechnol. 2017, 16, 2142–2151. [Google Scholar]
- Huang, T.; Tu, Z.C.; Shangguan, X.C.; Sha, X.M.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Lu, N.; Liu, D.; Regenstein, J.M.; Zhou, P. Effects of hydrocolloids on the rheological and microstructural properties of semisolid whey protein-rich systems. Food Biosci. 2019, 30, 100424. [Google Scholar] [CrossRef]
- Ng, S.B.X.; Nguyen, P.T.; Bhandari, B.; Prakash, S. Influence of different functional ingredients on physical properties, rheology, tribology, and oral perceptions of no fat stirred yoghurt. J. Texture Stu. 2018, 49, 274–285. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Ain, N.U.; Wu, S.; Li, X.; Li, D.; Zhang, Z. Isolation, Characterization, Pharmacology and Biopolymer Applications of Licorice Polysaccharides: Review. Materials 2022, 15, 3654. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Abd Elaziz, A.I.; Hassan, S.A.; Shalaby, M.A.; Mohdaly, A.A.A. Chemical Composition, Antioxidant, Antimicrobial and Anticancer (Glycyrrhiza glabra L.) Root and Its Application in Functional Yoghurt. J. Food Nutr. Res. 2020, 8, 707–715. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macrmol. 2020, 144, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Rozi, P.; Abuduwaili, A.; Ma, S.; Bao, X.; Xu, H.; Zhu, J.; Yadikar, N.; Wang, J.; Yang, X.; Abulimiti, Y.L. Isolations, characterizations and bioactivities of polysaccharides from the seeds of three species Glycyrrhiza. Int. J. Biol. Macromol. 2020, 145, 364–371. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.W.; Ma, S.J.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouth feel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of pectin-rich orange fibre on gel characteristics and sensory properties in lactic acid fermented yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Korkmaz, I.O.; Bilici, C.; Korkmaz, S. Sensory, pH, synaeresis, water-holding capacity, and microbiological changes in homemade yogurt prepared with maca (Lepidium meyenii) powder and propolis extract. Int. J. Gastron. Food Sci. 2021, 23, 100291. [Google Scholar] [CrossRef]
- Sahan, N.; Yasar, K.; Hayaloglu, A.A. Physical, chemical and flavour quality of non-fat yogurt as affected by a beta-glucan hydrocolloidal composite during storage. Food Hydrocoll. 2008, 22, 1291–1297. [Google Scholar] [CrossRef]
- Santillán-Urquiza, E.; Méndez-Rojas, M.Á.; Vélez-Ruiz, J.F. Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT-Food Sci. Technol. 2017, 80, 462–469. [Google Scholar] [CrossRef]
- Zeynep, G.; Tuba, E.-K.; Mustafa, S. Evaluation of physicochemical, microbiological, texture and microstructure characteristics of set-style yoghurt supplemented with quince seed mucilage powder as a novel natural stabiliser. Int. Dairy J. 2021, 114, 104938. [Google Scholar]
- Salomonsen, T.; Sejersen, M.T.; Viereck, N.; Ipsen, R.; Engelsen, S.B. Water mobility in acidified milk drinks studied by low-field 1H NMR. Int. Dairy J. 2007, 17, 294–301. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.M.; Du, J.H.; Zhang, Z.H. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yu, B.; Dong, D.; Hou, Z.; Cui, B. Rheological, thermal and microstructural properties of whey protein isolate-modified cassava starch mixed gels at different pH values. Int. J. Food Sci. Technol. 2017, 52, 2445–2454. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Physicochemical and macromolecule properties of RG-I enriched pectin from citrus wastes by manosonication extraction. Int. J. Biol. Macromol. 2021, 176, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Laiho, S.; Williams, R.P.W.; Poelman, A.; Appelqvist, I.; Logan, A. Effect of whey protein phase volume on the tribology, rheology and sensory properties of fat-free stirred yoghurts. Food Hydrocoll. 2017, 67, 166–177. [Google Scholar] [CrossRef]
- Basiri, S.; Haidary, N.; Shekarforoush, S.S.; Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr. Polym. 2018, 187, 59–65. [Google Scholar] [CrossRef]
- Hao, J.; Yang, C.; Ding, X.; Wu, X.Y.; Zhang, Z.; Chen, Y.Y. Effect of Polysaccharides from Pleurotus eryngii on Fermentation of Lactic Acid Bacteria and Quality of Yogurt. Food Industry. 2020, 41, 182–185. [Google Scholar]
- Pang, Z.; Deeth, H.; Bansal, N. Effect of polysaccharides with different ionic charge on the rheological microstructural and textural properties of acid milk gels. Food Res. Int. 2015, 72, 62–73. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci. Technol. 2016, 56, 61–76. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Formation and physical properties of yogurt. Asian Austral. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Tan, P.Y.; Tan, T.B.; Chang, H.W.; Tey, B.T.; Chan, E.S.; Lai, O.M. Effects of storage and yogurt matrix on the stability of tocotrienols encapsulated in chitosan-alginate microcapsules. Food Chem. 2018, 241, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.K.S.; Bhandari, B.; Prakash, S.; Liu, D.S.; Zhou, P.; Bansal, N. Modifying textural and microstructural properties of low fat Cheddar cheese using sodium alginate. Food Hydrocoll. 2018, 83, 97–108. [Google Scholar] [CrossRef]
- Chetachukwu, A.S.; Thongraung, C.; Yupanqui, C.T. Effect of short-chain inulin on the rheological and sensory characteristics of reduced fat set coconut milk yoghurt. J. Texture Stu. 2018, 49, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Deeth, H.; Prakash, S.; Bansal, N. Development of rheological and sensory properties of combinations of milk proteins and gelling polysaccharides as potential gelatin replacements in the manufacture of stirred acid milk gels and yogurt. J. Food Eng. 2016, 169, 27–37. [Google Scholar] [CrossRef]
- Andiç, S.; Boran, G.; Tunçtürk, Y. Effects of carboxyl methyl cellulose and edible cow gelatin on physico-chemical, textural and sensory properties of yoghurt. Int. J. Agric. Biol. 2013, 15, 245–251. [Google Scholar]
- Willemsen, K.L.D.D.; Panozzo, A.; Moelants, K.; Debon, S.J.J.; Desmet, C.; Cardinaels, R. Physico-chemical and viscoelastic properties of high pressure homogenized lemon peel fiber fraction suspensions obtained after sequential pectin extraction. Food Hydrocoll. 2017, 72, 358–371. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Schmitt, C.; Sanchez, C. Protein-polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- Zhang, S.; Hsieh, F.H.; Vardhanabhuti, B. Acid-induced gelation properties of heated whey protein-pectin soluble complex (part I): Effect of initial pH. Food Hydrocoll. 2014, 36, 76–84. [Google Scholar] [CrossRef]
- Ryan, J.; Hutchings, S.C.; Fang, Z.X.; Bandara, N.; Gamlath, S.; Ajlouni, S.; Ranadheera, C.S. Microbial, physico-chemical and sensory characteristics of mango juice-enriched probiotic dairy drinks. Int. J. Dairy Technol. 2020, 73, 182–190. [Google Scholar] [CrossRef]
- Fakhreddin, S. Quality, physicochemical, and textural properties of dairy products containing fruits and vegetables: A review. Food Sci. Nutr. 2021, 9, 4666–4686. [Google Scholar]

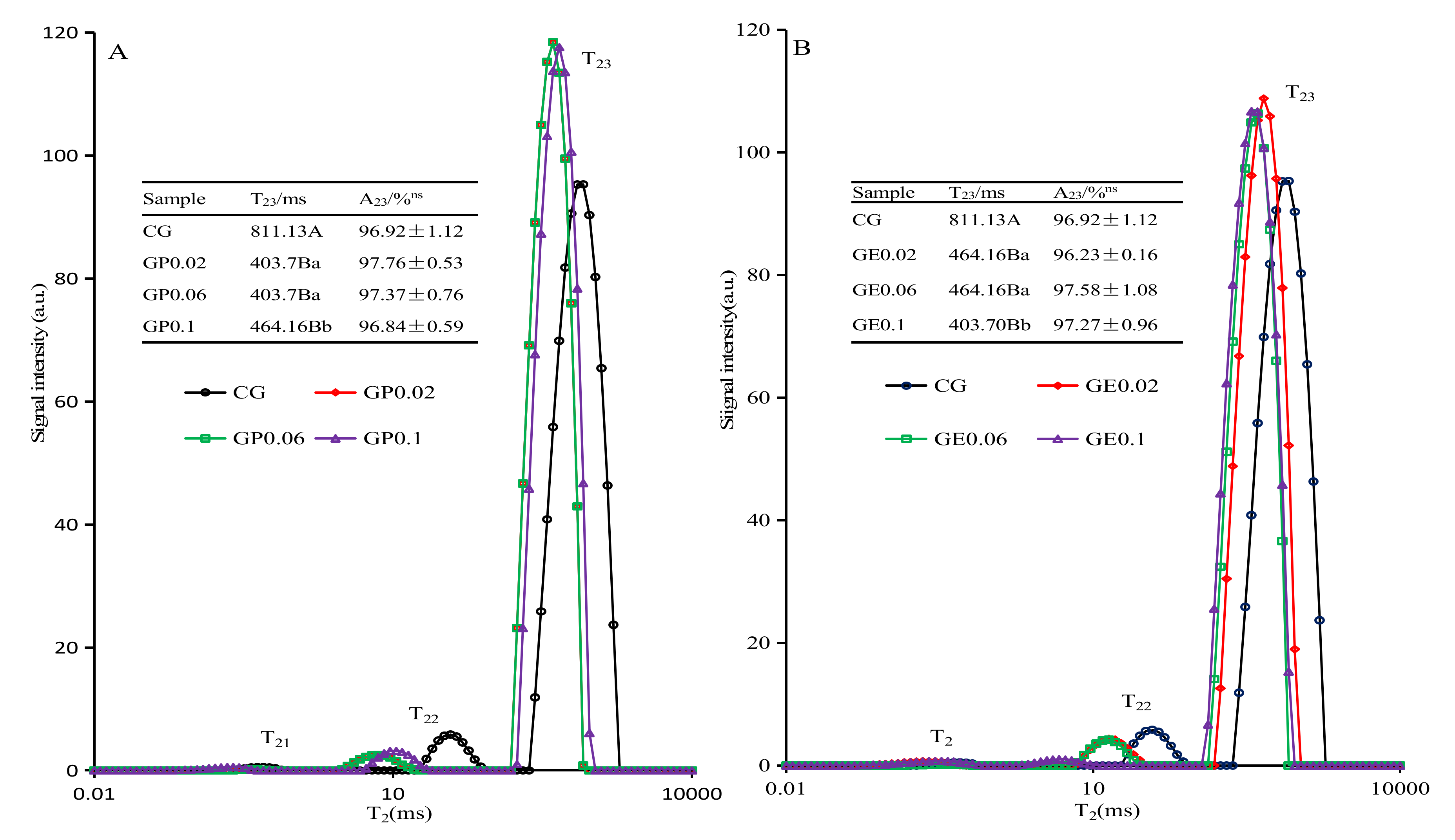

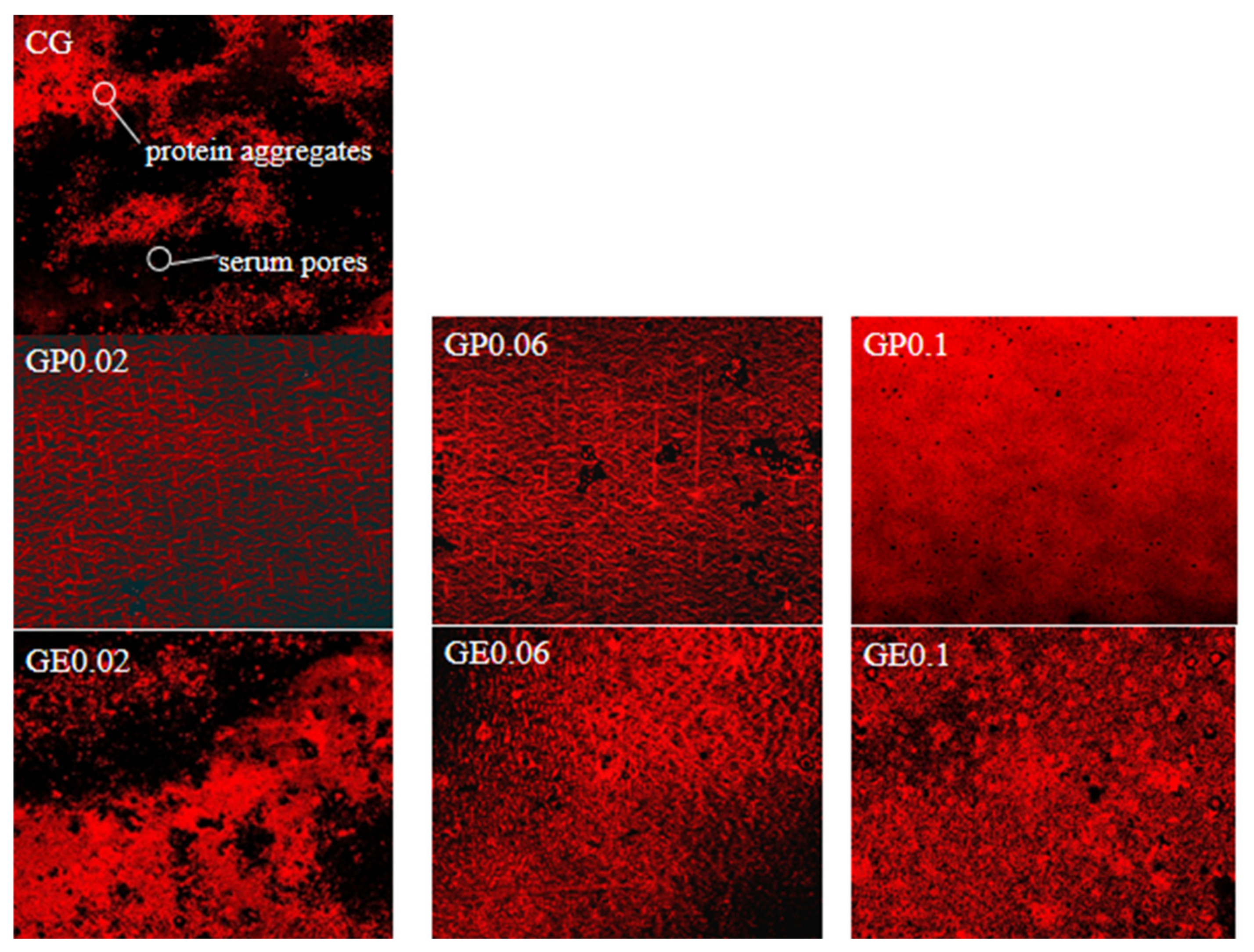
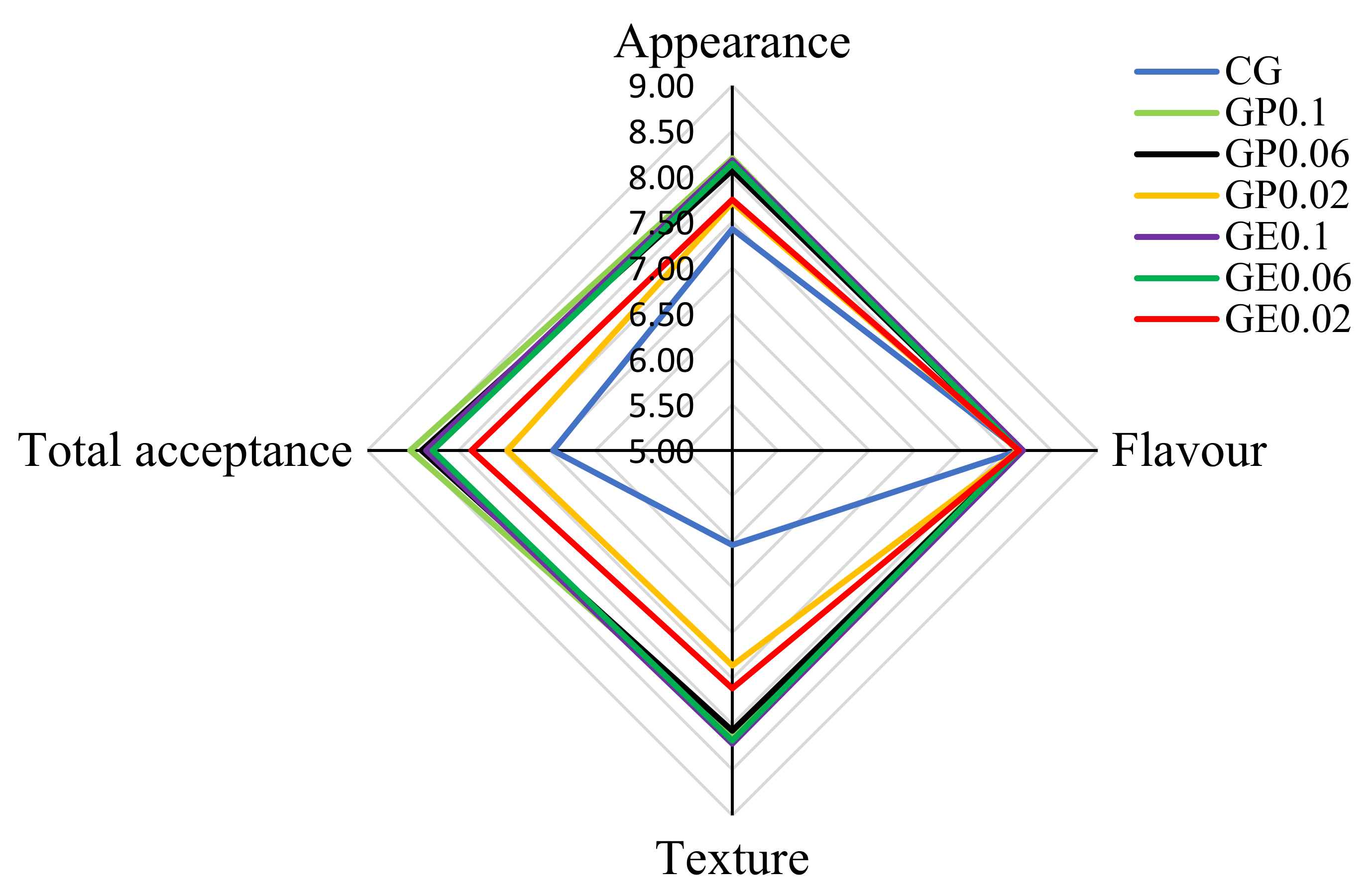
| Samples | Titratable Acidtity (°T) ns | pH ns | TSS (°Brix) | TS (%) | WHC (%) |
|---|---|---|---|---|---|
| CG | 94.33 ± 3.06 | 4.43 ± 0.05 | 6.27 ± 0.15A | 12.69 ± 1.14A | 80.69 ± 0.02A |
| GP0.1 | 94.00 ± 3.60 | 4.23 ± 0.03 | 6.87 ± 0.15Ba | 13.64 ± 0.79Ba | 83.89 ± 0.02Ba |
| GP0.06 | 94.33 ± 4.04 | 4.13 ± 0.02 | 6.77 ± 0.15Ba | 13.06 ± 0.76ABb | 82.79 ± 0.01Ba |
| GP0.02 | 95.00 ± 3.60 | 4.06 ± 0.04 | 6.50 ± 0.20Ba | 12.99 ± 0.83ABbc | 82.66 ± 0.01Bb |
| GE0.1 | 93.33 ± 2.51 | 4.40 ± 0.05 | 6.30 ± 0.265ACa | 12.96 ± 0.92ACa | 85.35 ± 0.02Ca |
| GE0.06 | 93.67 ± 4.16 | 4.37 ± 0.03 | 6.33 ± 0.35ACa | 12.84 ± 0.11ACb | 84.44 ± 0.02Cb |
| GE0.02 | 93.00 ± 1.00 | 4.38 ± 0.04 | 6.23 ± 0.64ACa | 12.70 ± 0.99ACc | 84.85 ± 0.01Cc |
| Samples | Firmness (gf) | Adhesiveness (Nm) | Cohesiveness | Springiness | Resilience |
|---|---|---|---|---|---|
| (mm) | |||||
| CG | 120.02 ± 5.00A | 38.81 ± 1.50A | 0.47 ± 0.02A | 0.37 ± 0.01A | 0.52 ± 0.01A |
| GP0.02 | 124.02 ± 3.40Ba | 79.26 ± 3.40Ba | 0.35 ± 0.01Ba | 0.87 ± 0.02Ba | 1.03 ± 0.02Ba |
| GP0.06 | 130.02 ± 6.50Bb | 82.87 ± 4.32Bb | 0.39 ± 0.01Bb | 0.67 ± 0.05Bb | 0.20 ± 0.01Bb |
| GP0.1 | 134.02 ± 8.01Bc | 44.68 ± 0.56Bc | 0.60 ± 0.03Bc | 0.32 ± 0.02Bc | 0.46 ± 0.02Bc |
| GE0.02 | 138.02 ± 4.10Ca | 95.23 ± 2.50Ca | 0.36 ± 0.02Ca | 1.79 ± 0.04Ca | 2.26 ± 0.01Ca |
| GE0.06 | 144.02 ± 5.30Cb | 109.33 ± 3.50Cb | 0.30 ± 0.01Cb | 0.97 ± 0.01Cb | 0.35 ± 0.01Cb |
| GE0.1 | 158.02 ± 7.35Cc | 90.18 ± 1.80Cc | 0.31 ± 0.15Cb | 1.17 ± 0.01Cc | 1.2 ± 0.02Cc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Yin, X.; Cheng, H.; Ye, X.; Chen, J. Evaluating Physicochemical and Sensory Properties of Functional Yogurt Supplemented with Glycyrrhiza Polysaccharide as Potential Replacement for Gelatin. Agriculture 2022, 12, 1289. https://doi.org/10.3390/agriculture12091289
Guo D, Yin X, Cheng H, Ye X, Chen J. Evaluating Physicochemical and Sensory Properties of Functional Yogurt Supplemented with Glycyrrhiza Polysaccharide as Potential Replacement for Gelatin. Agriculture. 2022; 12(9):1289. https://doi.org/10.3390/agriculture12091289
Chicago/Turabian StyleGuo, Dongqi, Xiuxiu Yin, Huan Cheng, Xingqian Ye, and Jianle Chen. 2022. "Evaluating Physicochemical and Sensory Properties of Functional Yogurt Supplemented with Glycyrrhiza Polysaccharide as Potential Replacement for Gelatin" Agriculture 12, no. 9: 1289. https://doi.org/10.3390/agriculture12091289
APA StyleGuo, D., Yin, X., Cheng, H., Ye, X., & Chen, J. (2022). Evaluating Physicochemical and Sensory Properties of Functional Yogurt Supplemented with Glycyrrhiza Polysaccharide as Potential Replacement for Gelatin. Agriculture, 12(9), 1289. https://doi.org/10.3390/agriculture12091289






