Stable Isotope Analysis Supports Omnivory in Bank Voles in Apple Orchards
Abstract
:1. Introduction
2. Materials and Methods
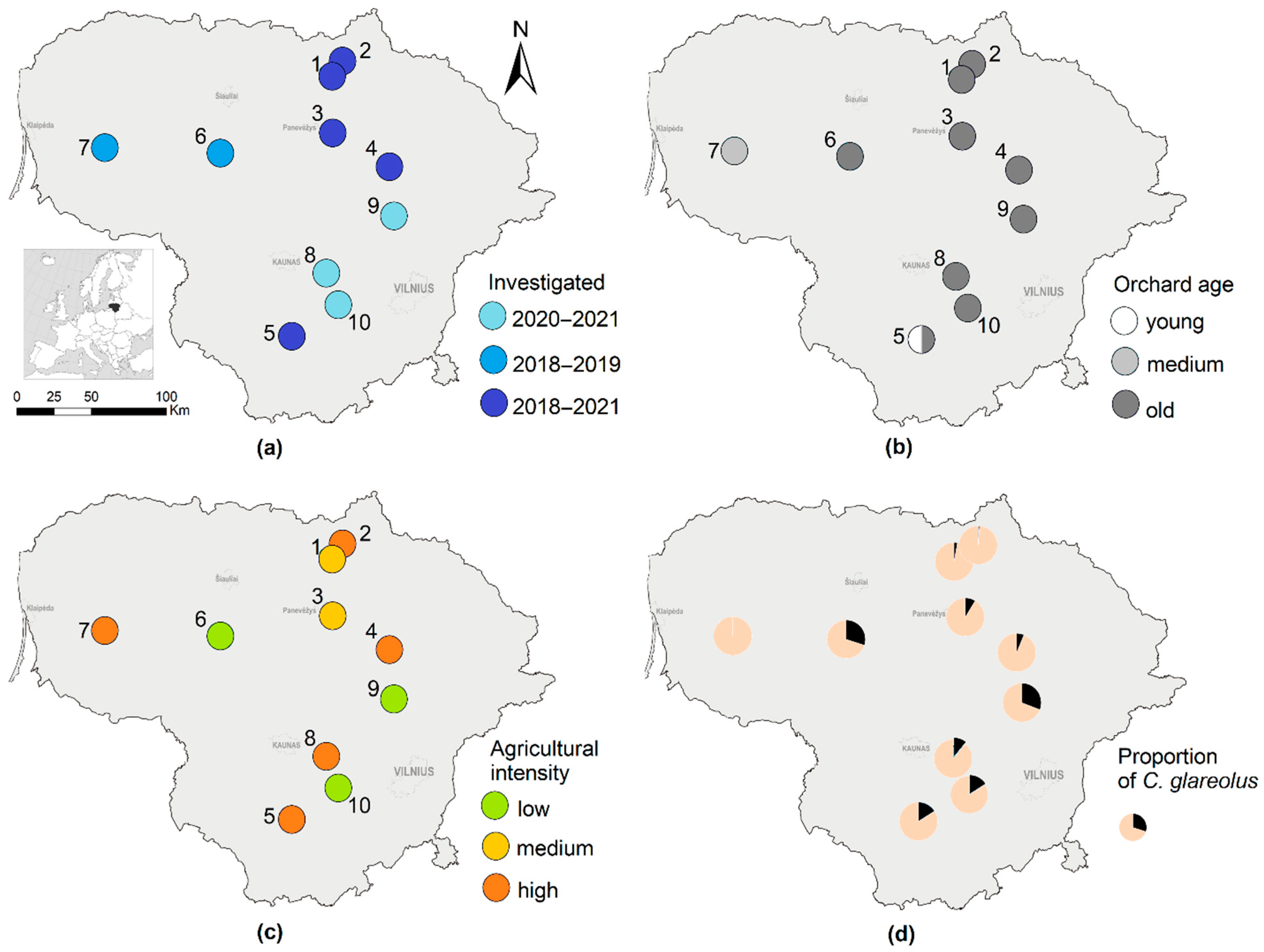
2.1. Study Sites
2.2. Small Mammal Trapping and Size of Bank Vole Samples
2.3. Stable Isotope Analysis of Hair and Basal Resources
2.4. Data Analyses
3. Results
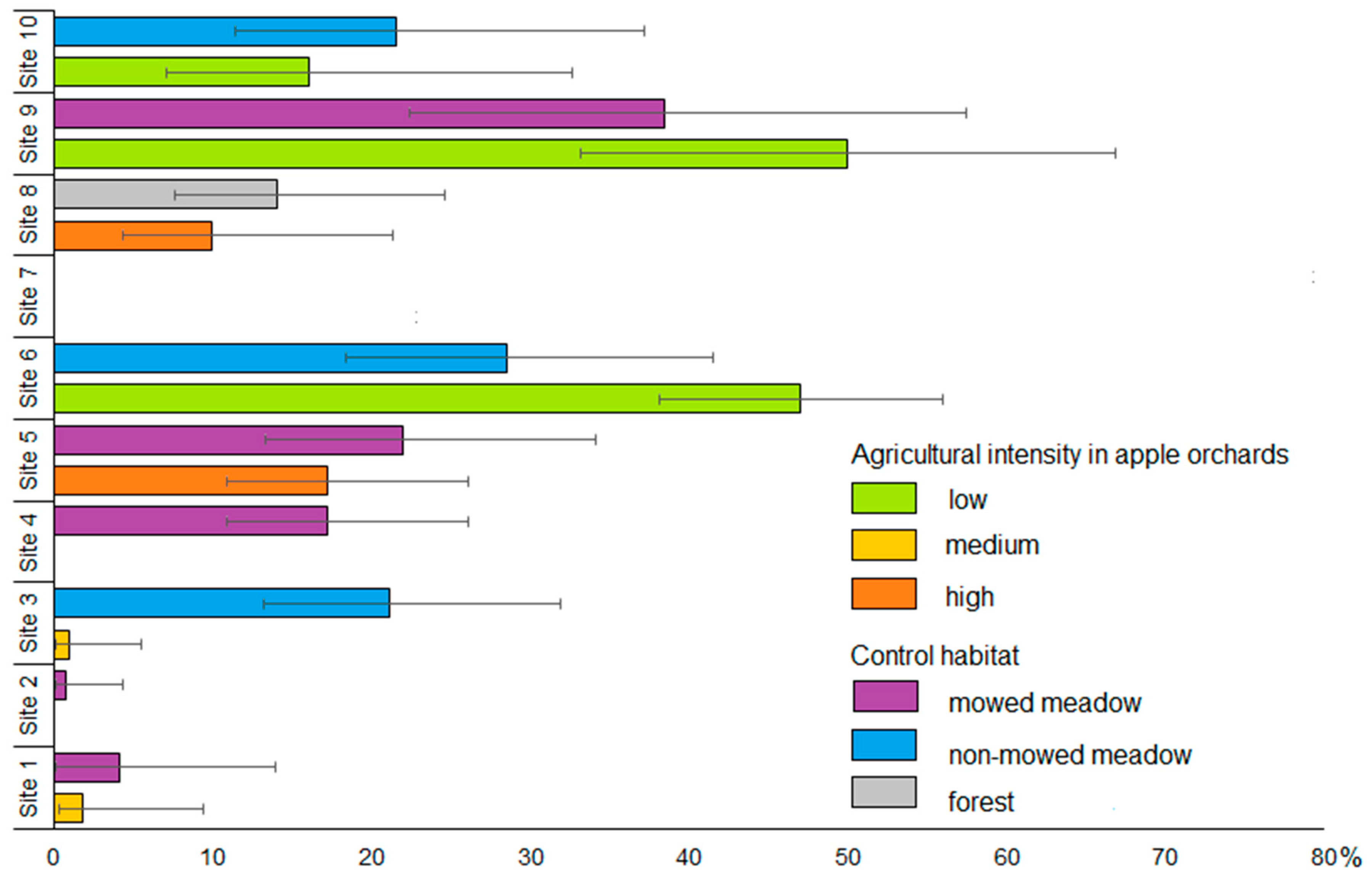
3.1. Proportions of Bank Voles in Small Mammal Communities and Species Abundances in Apple Orchards and Control Habitats
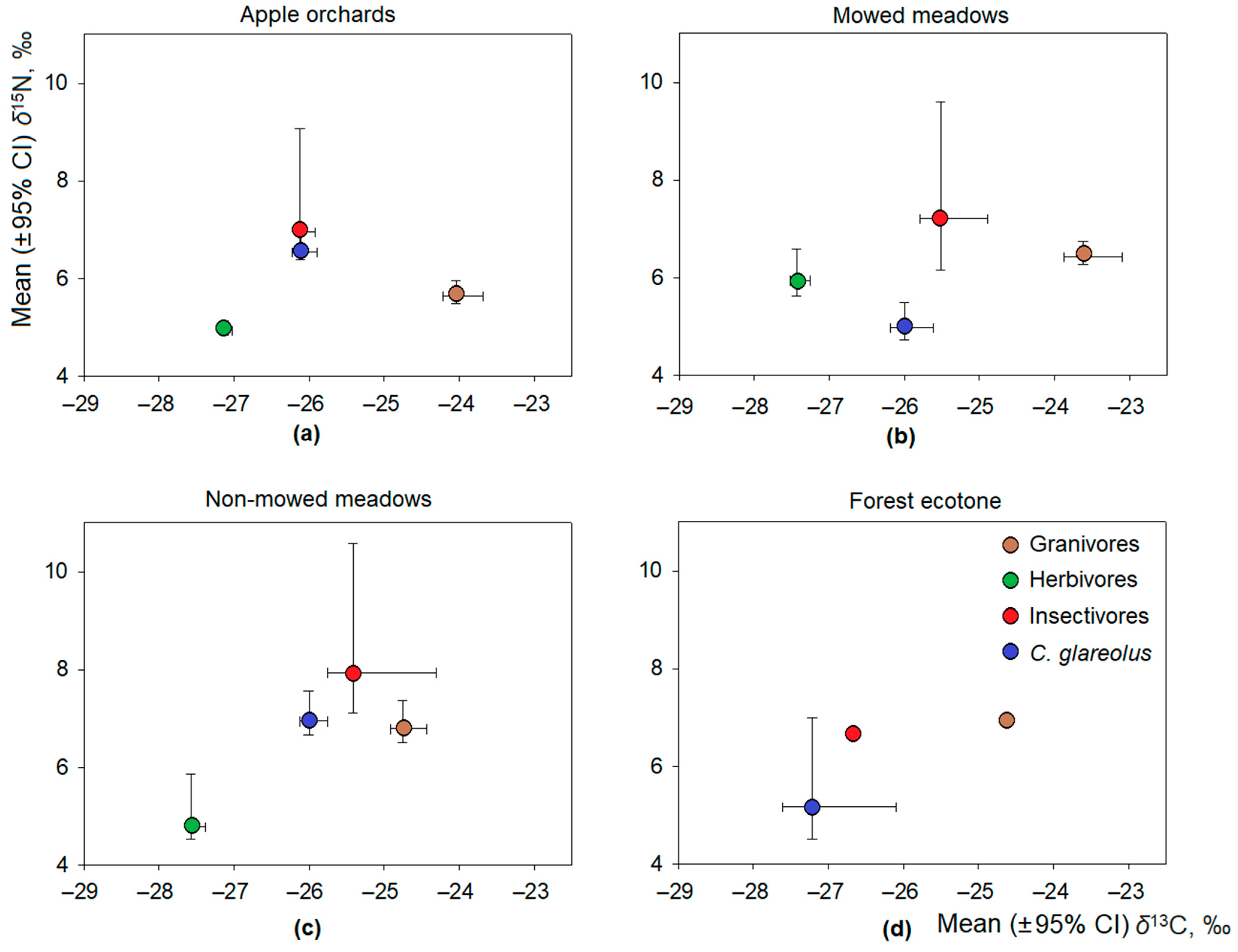
3.2. Stable Isotope Ratios of Omnivorous Bank Voles and Related Factors
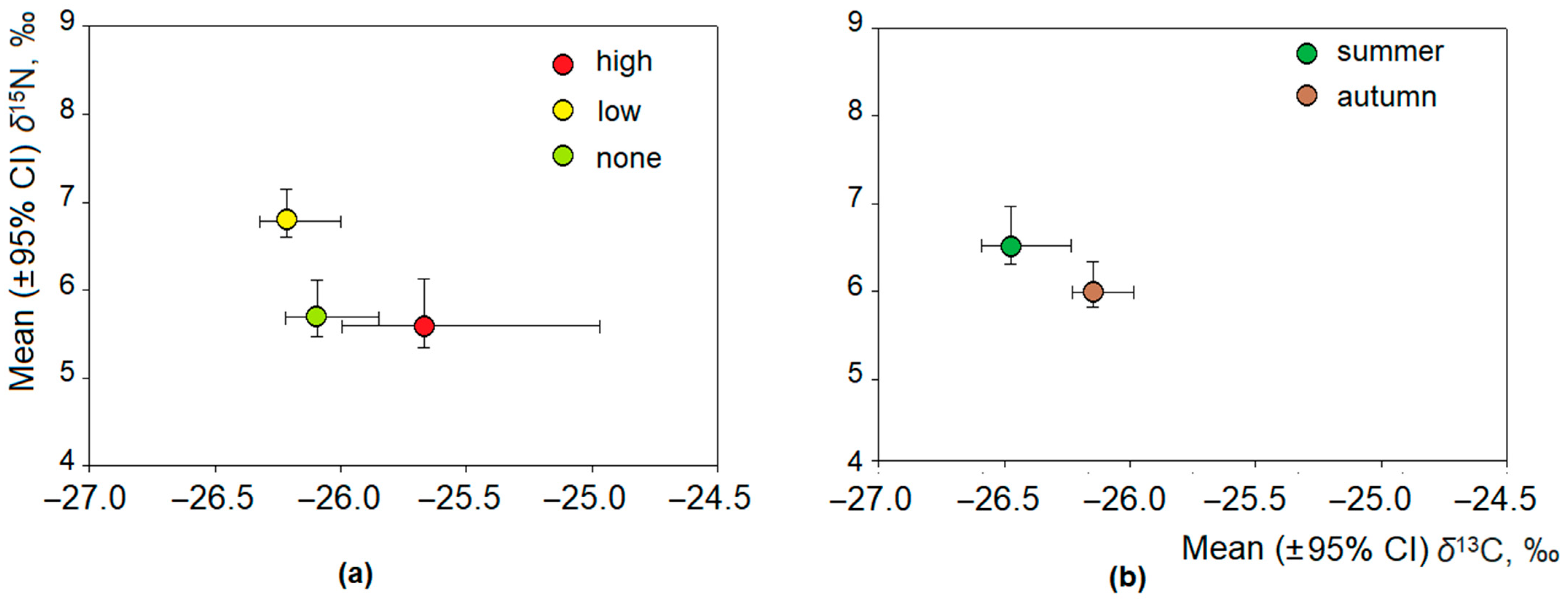
3.3. Intraspecific Differences in Isotopic Ratios in Bank Voles
3.4. Relationship between Bank Vole Breeding Status, Body Mass, Body Condition Index and Their Isotopic Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, D.W. Paradoxical avoidance of enriched habitats: Have we failed to appreciate omnivores? Ecology 2005, 86, 2568–2577. [Google Scholar] [CrossRef]
- Santos, M.J.; Thorne, J.H.; Moritz, C. Synchronicity in elevation range shifts among small mammals and vegetation over the last century is stronger for omnivores. Ecography 2014, 38, 556–568. [Google Scholar] [CrossRef]
- Landry, S.O. The Rodentia as Omnivores. Q. Rev. Biol. 1970, 45, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Schieck, J.O.; Millar, J.S. Alimentary tract measurements as indicators of diets of small mammals. Mammalia 1985, 49, 93–104. [Google Scholar] [CrossRef]
- Braithwaite, R.W.; Cockburn, A.; Lee, A.K. Resource partitioning by small mammals in lowland heath communities of south-eastern Australia. Austral Ecol. 1978, 3, 423–445. [Google Scholar] [CrossRef]
- Kelt, D.A.; Brown, J.H.; Heske, E.J.; Marquet, P.A.; Morton, S.R.; Reid, J.R.; Rogovin, K.A.; Shenbrot, G. Community structure of desert small mammals: Comparisons across four continents. Ecology 1996, 77, 746–761. [Google Scholar] [CrossRef]
- Campos, C.; Ojeda, R.; Monge, S.; Dacar, M. Utilization of food resources by small and medium-sized mammals in the Monte Desert biome, Argentina. Austral Ecol. 2001, 26, 142–149. [Google Scholar] [CrossRef]
- Fox, B.J. Review of small mammal trophic structure in drylands: Resource availability, use, and disturbance. J. Mammal. 2011, 92, 1179–1192. [Google Scholar] [CrossRef]
- Shaner, P.J.L.; Wu, S.H.; Ke, L.; Kao, S.J. Trophic niche divergence reduces survival in an omnivorous rodent. Evol. Ecol. Res. 2013, 15, 933–946. [Google Scholar]
- Ribeiro, J.F.; Guaraldo, A.; Nardoto, G.B.; Santoro, G.; Vieira, E.M. Habitat type and seasonality influence the isotopic trophic niche of small mammals in a neotropical savanna. Hystrix 2019, 30, 30–38. [Google Scholar] [CrossRef]
- Kirkland, G.L. Responses of Small Mammals to the Clearcutting of Northern Appalachian Forests. J. Mammal. 1977, 58, 600–609. [Google Scholar] [CrossRef]
- Dolan, J.D.; Rose, R.K. Depauperate small mammal communities in managed pine plantations in eastern Virginia. Va. J. Sci. 2007, 58, 1. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S. Responses of small-mammal populations to a forest herbicide application in a 20-year-old conifer plantation. J. Appl. Ecol. 1982, 19, 95–106. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals 2019, 9, 334. [Google Scholar] [CrossRef]
- Kryštufek, B.; Tesakov, A.S.; Lebedev, V.S.; Bannikova, A.A.; Abramson, N.I.; Shenbrot, G. Back to the future: The proper name for red-backed voles is Clethrionomys Tilesius and not Myodes Pallas. Mammalia 2020, 84, 214–217. [Google Scholar] [CrossRef]
- Hutterer, R.; Kryštufek, B.; Yigit, N.; Mitsainas, G.; Palomo, L.; Henttonen, H.; Vohralík, V.; Zagorodnyuk, I.; Juškaitis, R.; Meinig, H.; et al. Myodes glareolus (Amended Version of 2016 Assessment). The IUCN Red List of Threatened Species 2021: E.T4973A197520967. 2021. Available online: https://www.iucnredlist.org/species/4973/197520967 (accessed on 18 June 2022).
- Prūsaitė, J. (Comp.) Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; p. 295. [Google Scholar]
- Balčiauskas, L.; Trakimas, G.; Juškaitis, R.; Ulevičius, A.; Balčiauskienė, L. Atlas of Lithuanian Mammals, Amphibians and Reptiles, 2nd ed.; Akstis: Vilnius, Lithuania, 1999; p. 112. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L.; Garbaras, A.; Stirkė, V. Diversity and Diet Differences of Small Mammals in Commensal Habitats. Diversity 2021, 13, 346. [Google Scholar] [CrossRef]
- Suchomel, J.; Šipoš, J.; Ouředníčková, J.; Skalský, M.; Heroldová, M. Bark Gnawing by Rodents in Orchards during the Growing Season—Can We Detect Relation with Forest Damages? Agronomy 2022, 12, 251. [Google Scholar] [CrossRef]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Wrangel, H. Beitrage zur Biologie der Rötelmaus (Clethrionomys glareolus Schr.). Z. Saügetierk. 1940, 14, 52–93. [Google Scholar]
- Miller, R.S. Food habits of the woodmouse and the bank vole in Wytham Woods, Berks. Siiugetierk. Mitt. 1954, 2, 109–113. [Google Scholar]
- Abt, K.F.; Bock, W.F. Seasonal variations of diet composition in farmland field mice Apodemus spp. and bank voles Clethrionomys glareolus. Acta Theriol. 1998, 43, 379–389. [Google Scholar] [CrossRef]
- Martiniaková, M.; Omelka, R.; Stawarz, R.; Formicki, G. Accumulation of Lead, Cadmium, Nickel, Iron, Copper, and Zinc in Bones of Small Mammals from Polluted Areas in Slovakia. Pol. J. Environ. Stud. 2012, 21, 153–158. [Google Scholar]
- Watts, C.H. The foods eaten by wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) in Wytham Woods, Berkshire. J. Anim. Ecol. 1968, 37, 25–41. [Google Scholar] [CrossRef]
- Hämäläinen, A.; Kiljunen, M.; Koskela, E.; Koteja, P.; Mappes, T.; Rajala, M.; Tiainen, K. Artificial selection for predatory behaviour results in dietary niche differentiation in an omnivorous mammal. Proc. R. Soc. B 2022, 289, 20212510. [Google Scholar] [CrossRef] [PubMed]
- Escalante, M.A.; Horníková, M.; Marková, S.; Kotlík, P. Niche differentiation in a postglacial colonizer, the bank vole Clethrionomys glareolus. Ecol. Evol. 2021, 11, 8054–8070. [Google Scholar] [CrossRef] [PubMed]
- Čepelka, L.; Heroldová, M.; Jánová, E.; Suchomel, J. The Dynamics of Nitrogenous Substances in Rodent Diet in a Forest Environment. Mammalia 2014, 78, 327–333. [Google Scholar] [CrossRef]
- Palo, R.T.; Olsson, G.E. Nitrogen and Carbon Concentrations in the Stomach Content of Bank Voles (Myodes glareolus). Does Food Quality Determine Abundance? Open Ecol. J. 2009, 2, 86–90. [Google Scholar] [CrossRef]
- Hughes, K.L.; Whiteman, J.P.; Newsome, S.D. The relationship between dietary protein content, body condition, and Δ15N in a mammalian omnivore. Oecologia 2018, 186, 357–367. [Google Scholar] [CrossRef]
- Chibowski, P.; Brzeziński, M.; Suska-Malawska, M.; Zub, K. Diet/hair and diet/faeces trophic discrimination factors for stable carbon and nitrogen isotopes, and hair regrowth in the yellow-necked mouse and bank vole. Annales Zoologici Fennici 2022, 59, 171–185. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Stable Isotopes Reveal the Dominant Species to Have the Widest Trophic Niche of Three Syntopic Microtus Voles. Animals 2021, 11, 1814. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Isotopic Niche of Syntopic Granivores in Commercial Orchards and Meadows. Animals 2021, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Common vole as a focal small mammal species in orchards of the Northern Zone. Diversity 2021, 13, 134. [Google Scholar] [CrossRef]
- Balčiauskas, L. Methods of Investigation of Terrestrial Ecosystems; Part I. Animal Surveys; VU Leidykla: Vilnius, Lithuania, 2004; p. 183. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L.; Janonytė, A. Reproduction of the root vole (Microtus oeconomus) at the edge of its distribution range. Turk. J. Zool. 2012, 36, 668–675. [Google Scholar] [CrossRef]
- Moors, P.J. Norway rats (Rattus norvegicus) on the Noises and Motukawao islands, Hauraki Gulf, New Zealand. N. Z. J. Ecol. 1985, 8, 37–54. [Google Scholar]
- Sibly, R.M.; Brown, J.H. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl. Acad. Sci. USA 2007, 104, 17707–17712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Spatiotemporal Variation of Small Mammal Communities in Commercial Orchards across the Small Country. Agriculture 2022, 12, 632. [Google Scholar] [CrossRef]
- Brown, L.D.; Cat, T.T.; DasGupta, A. Interval Estimation for a proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available online: http://OpenEpi.com (accessed on 19 January 2021).
- G-Test Calculator. Available online: https://elem.com/~btilly/effective-ab-testing/g-test-calculator.html (accessed on 10 April 2022).
- Hola, M.; Ježek, M.; Kušta, T.; Košatová, M. Trophic discrimination factors of stable carbon and nitrogen isotopes in hair of corn fed wild boar. PLoS ONE 2015, 10, e0125042. [Google Scholar] [CrossRef]
- Ben-David, M.; Flaherty, E.A. Stable isotopes in mammalian research: A beginner’s guide. J. Mammal. 2012, 93, 312–328. [Google Scholar] [CrossRef]
- Lynggaard, C.; Woolsey, I.D.; Al-Sabi, M.N.S.; Bertram, N.; Jensen, P.M. Parasites in Myodes glareolus and their association with diet assessed by stable isotope analysis. Int. J. Parasitol. Parasites Wildl. 2018, 7, 180–186. [Google Scholar] [CrossRef]
- Mazurkiewicz, M.; Rajska, E. Dispersion of young bank voles from their place of birth. Acta Theriol. 1975, 20, 71–81. [Google Scholar] [CrossRef]
- Gliwicz, J.; Ims, R.A. Dispersal in the bank vole. Pol. J. Ecol. 2000, 48, 51–61. [Google Scholar]
- Boratyński, Z.; Szyrmer, M.; Koteja, P. The metabolic performance predicts home range size of bank voles: A support for the behavioral–bioenergetics theory. Oecologia 2020, 193, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Castilla, Á.; Barja, I. Stressful living in lower-quality habitats? Body mass, feeding behavior and physiological stress levels in wild wood mouse populations. Integr. Zool. 2019, 14, 114–126. [Google Scholar] [CrossRef]
- Stephens, R.B.; Ouimette, A.P.; Hobbie, E.A.; Rowe, R.J. Reevaluating trophic discrimination factors (Δδ13C and Δδ15N) for diet reconstruction. Ecol. Monogr. 2022, e1525. [Google Scholar] [CrossRef]
- Crumsey, J.M.; Searle, J.B.; Sparks, J.P. Isotope values of California vole (Microtus californicus) hair relate to historical drought and land use patterns in California, USA. Oecologia 2019, 190, 769–781. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Ecke, F.; Benskin, J.P.; Berglund, Å.M.; de Wit, C.A.; Engström, E.; Plassmann, M.M.; Rodushkin, I.; Sörlin, D.; Hörnfeldt, B. Spatio-temporal variation of metals and organic contaminants in bank voles (Myodes glareolus). Sci. Total Environ. 2020, 713, 136353. [Google Scholar] [CrossRef]
- Schirmer, A.; Hoffmann, J.; Eccard, J.A.; Dammhahn, M. My niche: Individual spatial niche specialization affects within-and between-species interactions. Proc. Royal Soc. B 2020, 287, 20192211. [Google Scholar] [CrossRef]
- Nunes, S.F.; Mota-Ferreira, M.; Sampaio, M.; Andrade, J.; Oliveira, N.; Rebelo, R.; Rocha, R. Trophic niche changes associated with the eradication of invasive mammals in an insular lizard: An assessment using isotopes. Curr. Zool. 2022, 68, 211–219. [Google Scholar] [CrossRef]
- Yu, X.; Van De Voort, F.R.; Li, Z.; Yue, T. Proximate composition of the apple seed and characterization of its oil. Int. J. Food Eng. 2007, 3. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit seeds as sources of bioactive compounds: Sustainable production of high value-added ingredients from by-products within circular economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Mayr, C. Nitrogen and carbon isotope variability in the green-algal lichen X anthoria parietina and their implications on mycobiont–photobiont interactions. Ecol. Evol. 2012, 2, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Nybakken, L.; Helmersen, A.M.; Gauslaa, Y.; Selås, V. Lichen compounds restrain lichen feeding by bank voles (Myodes glareolus). J. Chem. Ecol. 2010, 36, 298–304. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Jasiulionis, M.; Trakimas, G.; Balčiauskienė, L.; Remeikis, V. The impact of Great Cormorants on biogenic pollution of land ecosystems: Stable isotope signatures in small mammals. Sci. Total Environ. 2016, 565, 376–383. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Jasiulionis, M.; Balčiauskienė, L.; Remeikis, V. Immediate increase in isotopic enrichment in small mammals following the expansion of a great cormorant colony. Biogeosciences 2018, 15, 3883–3891. [Google Scholar] [CrossRef] [Green Version]
- Balčiauskas, L.; Skipitytė, R.; Balčiauskienė, L.; Jasiulionis, M. Resource partitioning confirmed by isotopic signatures allows small mammals to share seasonally flooded meadows. Ecol. Evol. 2019, 9, 5479–5489. [Google Scholar] [CrossRef]
- Casula, P.; Luiselli, L.; Amori, G. Which population density affects home ranges of co-occurring rodents? Basic Appl. Ecol. 2019, 34, 46–54. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Shamhart, J.; Sheriff, M.; Ouimette, A.P.; Trappe, M.; Schuur, E.A.G.; Hobbie, J.E.; Boonstra, R.; Barnes, B.M. Stable isotopes and radiocarbon assess variable importance of plants and fungi in diets of arctic ground squirrels. Arctic Antarct. Alp. Res. 2017, 49, 487–500. [Google Scholar] [CrossRef]
- Selva, N.; Hobson, K.A.; Cortés-Avizanda, A.; Zalewski, A.; Donázar, J.A. Mast pulses shape trophic interactions between fluctuating rodent populations in a primeval forest. PLoS ONE 2012, 7, e51267. [Google Scholar] [CrossRef]
- Swan, G.J.; Bearhop, S.; Redpath, S.M.; Silk, M.J.; Goodwin, C.E.; Inger, R.; McDonald, R.A. Evaluating Bayesian stable isotope mixing models of wild animal diet and the effects of trophic discrimination factors and informative priors. Methods Ecol. Evol. 2020, 11, 139–149. [Google Scholar] [CrossRef]
- Fragoso, R.; Santos-Reis, M.; Rosalino, L.M. Drivers of wood mouse body condition in Mediterranean agroforestry landscapes. Eur. J. Wildl. Res. 2020, 66, 13. [Google Scholar] [CrossRef]
- Bednarz, P.A.; Zwolak, R. Body mass and sex, but not breeding condition and season, influence open-field exploration in the yellow-necked mouse. Ecol. Evol. 2022, 12, e8771. [Google Scholar] [CrossRef] [PubMed]
- Gentili, S.; Sigura, M.; Bonesi, L. Decreased small mammals species diversity and increased population abundance along a gradient of agricultural intensification. Hystrix 2014, 25, 39–44. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Kozakiewicz, A.; Łukowski, A.; Gortat, T. Use of space by bank voles (Clethrionomys glareolus) in a Polish farm landscape. Landsc. Ecol. 1993, 8, 19–24. [Google Scholar] [CrossRef]
- Imholt, C.; Reil, D.; Eccard, J.A.; Jacob, D.; Hempelmann, N.; Jacob, J. Quantifying the past and future impact of climate on outbreak patterns of bank voles (Myodes glareolus). Pest Manag. Sci. 2015, 71, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Jeske, K.; Schulz, J.; Tekemen, D.; Balčiauskas, L.; Balčiauskienė, L.; Hiltbrunner, M.; Drewes, S.; Mayer-Scholl, A.; Heckel, G.; Ulrich, R.G. Cocirculation of Leptospira spp. and multiple orthohantaviruses in rodents, Lithuania, Northern Europe. Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef]
- Fitzgibbon, C.D. Small mammals in farm woodlands: The effects of habitat, isolation and surrounding land-use patterns. J. Appl. Ecol. 1997, 34, 530–539. [Google Scholar] [CrossRef]
- Gelling, M.; Macdonald, D.W.; Mathews, F. Are hedgerows the route to increased farmland small mammal density? Use of hedgerows in British pastoral habitats. Landsc. Ecol. 2007, 22, 1019–1032. [Google Scholar] [CrossRef]
- Michel, N.; Burel, F.; Legendre, P.; Butet, A. Role of habitat and landscape in structuring small mammal assemblages in hedgerow networks of contrasted farming landscapes in Brittany, France. Landsc. Ecol. 2007, 22, 1241–1253. [Google Scholar] [CrossRef]
- Janova, E.; Heroldova, M. Response of small mammals to variable agricultural landscapes in central Europe. Mamm. Biol. 2016, 81, 488–493. [Google Scholar] [CrossRef]
| Habitat | N | Males | Females | Adults | Subadults | Juveniles |
|---|---|---|---|---|---|---|
| Apple orchards | 78 | 43 | 35 | 31 | 14 | 33 |
| Mowed meadow | 36 | 19 | 17 | 10 | 13 | 13 |
| Unmowed meadow | 22 | 11 | 11 | 2 | 4 | 16 |
| Forest | 5 | 2 | 3 | 1 | 1 | 3 |
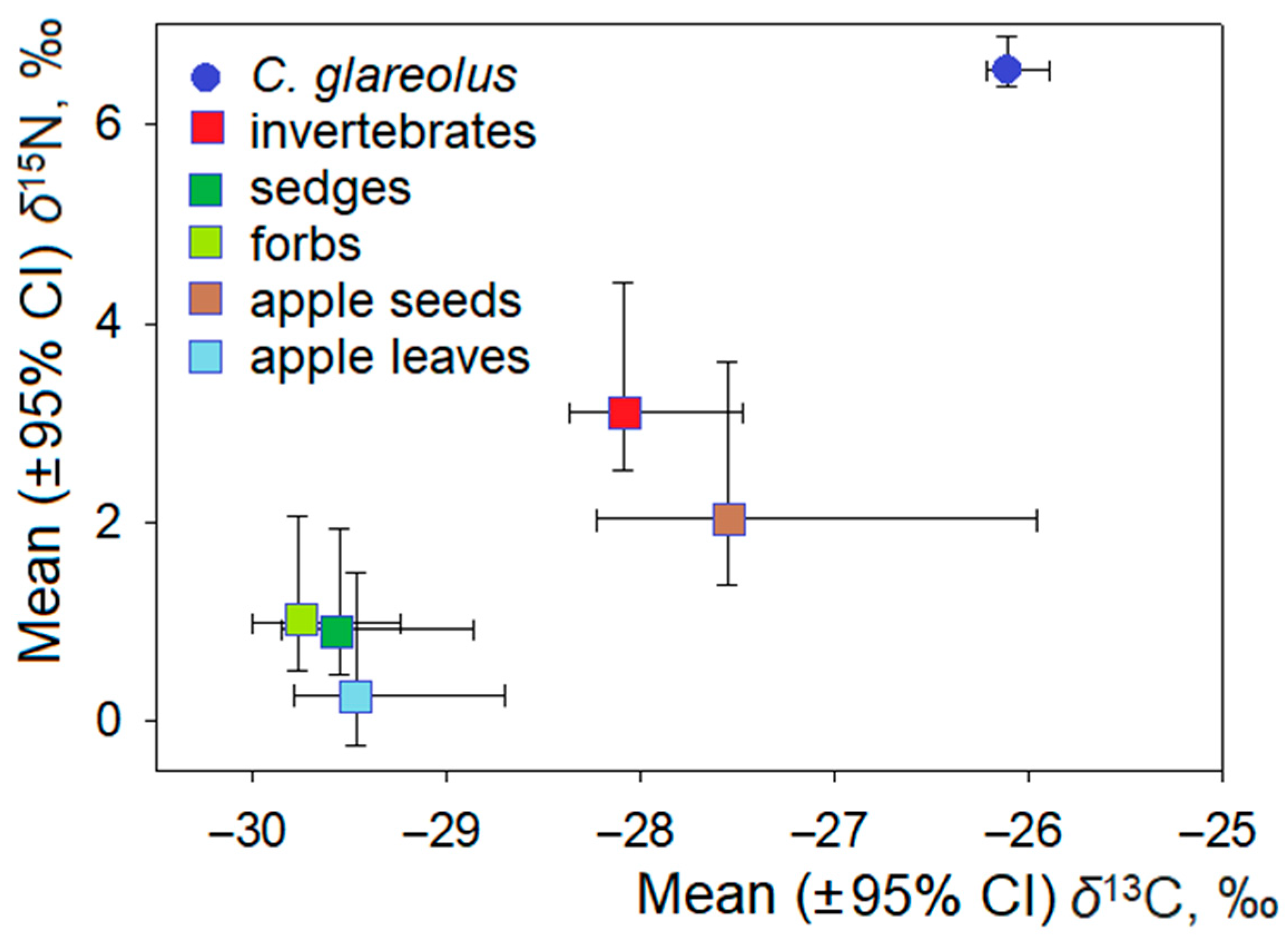
| Resources | n | δ13C, ‰ ± SE | δ13C, ‰ Min–Max | δ15N, ‰ ± SE | δ15N, ‰ Min–Max |
|---|---|---|---|---|---|
| Invertebrates | 10 | −28.09 ± 0.27 | −29.41–−26.77 | 3.10 ± 0.57 | 0.75–6.44 |
| Plants | 50 | −28.69 ± 0.24 | −31.63–−24.95 | 0.76 ± 0.41 | −12.41–5.38 |
| Habitat | Reference | δ13C Values, ‰ | δ15N Values, ‰ | ||
|---|---|---|---|---|---|
| Mean ± SE | Min–Max | Mean ± SE | Min–Max | ||
| Apple orchards | This paper | −26.11 ± 0.11 | −28.28–−25.22 | 6.55 ± 0.16 | 3.03–11.30 |
| Forest | [63] | −27.91 ± 0.17 ** | −29.14–−27.91 | 5.18 ± 0.23 ** | 3.32–7.96 |
| Flooded meadow | [63] | −26.22 ± 0.14 | −27.53–−25.46 | 6.38 ± 0.25 | 4.77–8.21 |
| Forest (colony 1) | [61] | −26.73 ± 0.12 * | −28.51–−24.93 | 8.91 ± 0.55 ** | 3.12–15.80 |
| Forest (colony 2) | [61] | −24.78 ± 0.17 ** | −26.15–−23.12 | 12.42 ± 0.88 ** | 5.04–19.24 |
| Forest (colony 3) | [61] | −25.72 ± 0.17 | −28.88–−24.28 | 17.20 ± 0.56 ** | 5.79–20.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Stirkė, V.; Garbaras, A.; Skipitytė, R.; Balčiauskienė, L. Stable Isotope Analysis Supports Omnivory in Bank Voles in Apple Orchards. Agriculture 2022, 12, 1308. https://doi.org/10.3390/agriculture12091308
Balčiauskas L, Stirkė V, Garbaras A, Skipitytė R, Balčiauskienė L. Stable Isotope Analysis Supports Omnivory in Bank Voles in Apple Orchards. Agriculture. 2022; 12(9):1308. https://doi.org/10.3390/agriculture12091308
Chicago/Turabian StyleBalčiauskas, Linas, Vitalijus Stirkė, Andrius Garbaras, Raminta Skipitytė, and Laima Balčiauskienė. 2022. "Stable Isotope Analysis Supports Omnivory in Bank Voles in Apple Orchards" Agriculture 12, no. 9: 1308. https://doi.org/10.3390/agriculture12091308
APA StyleBalčiauskas, L., Stirkė, V., Garbaras, A., Skipitytė, R., & Balčiauskienė, L. (2022). Stable Isotope Analysis Supports Omnivory in Bank Voles in Apple Orchards. Agriculture, 12(9), 1308. https://doi.org/10.3390/agriculture12091308









