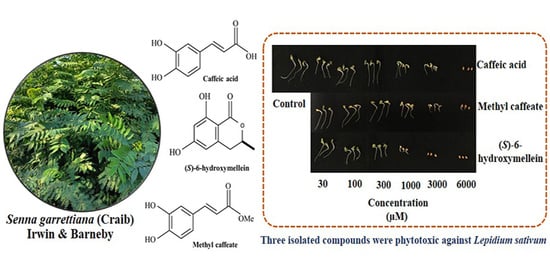
Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Procedure
2.2. Bioassay Procedure
2.3. Bioassay-Guided Fractionation and Purification of the Active Substances
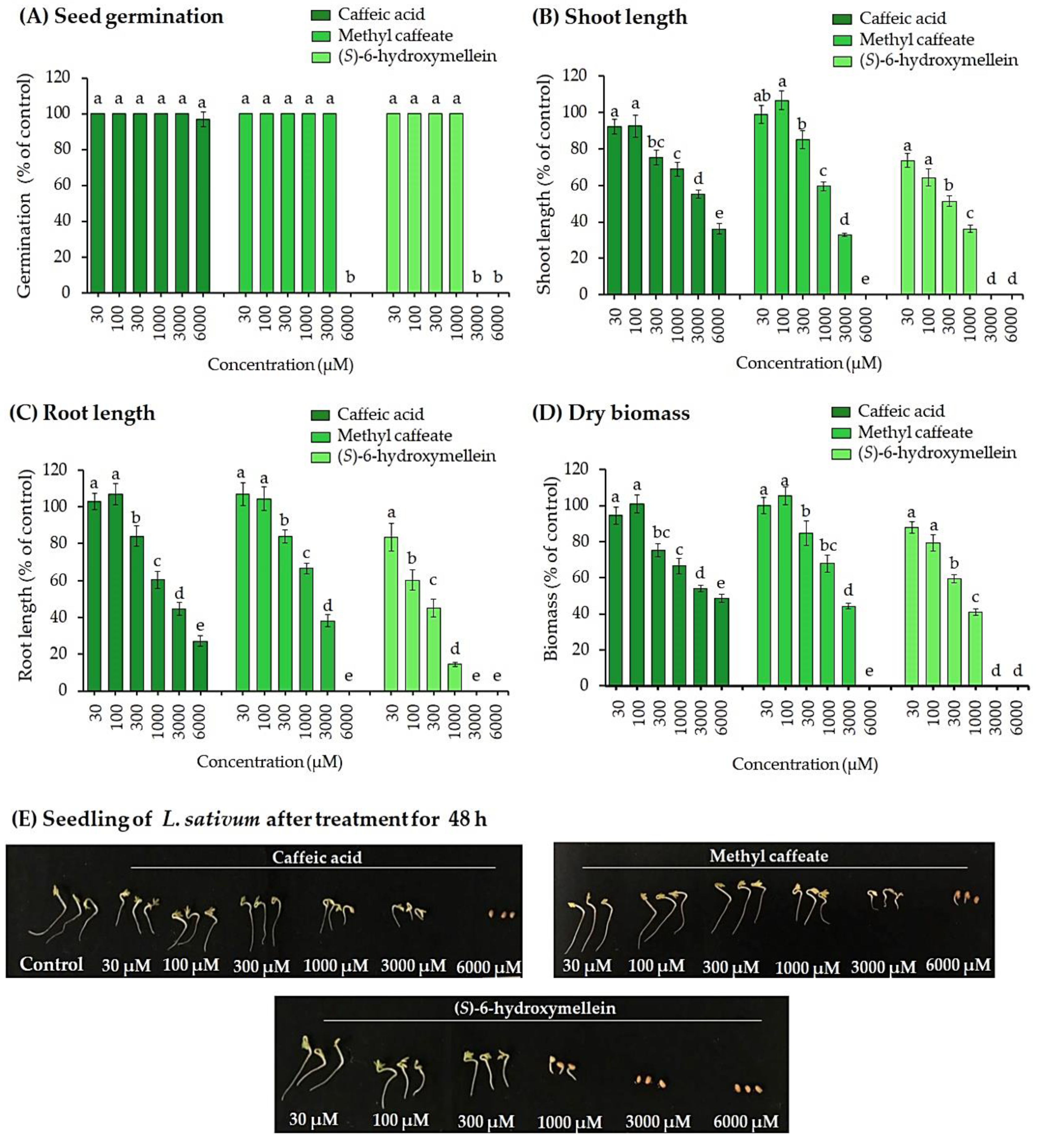
2.4. Bioassays of the Active Substances
2.5. Statistical Analysis
3. Results
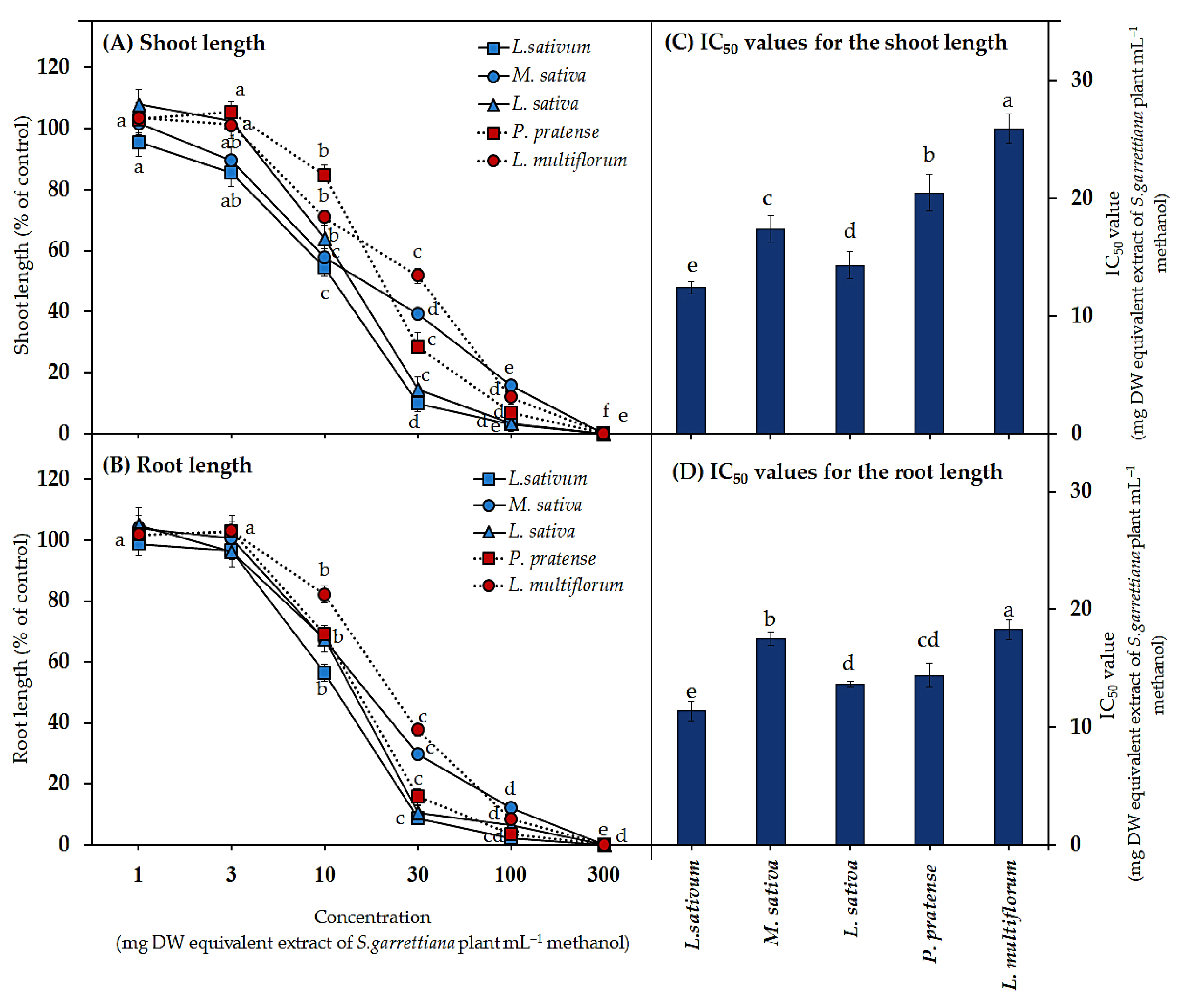
3.1. Biological Activities of the S. garrettiana Leaf Extracts
3.2. Identification of the Active Substances in the S. garrettiana Leaf Extracts
3.3. Biological Activities of Active Substances in the S. garrettiana Leaf Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdisc. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, J.P. Impact of pesticides application in agricultural industry: An Indian scenario. Int. J. Agric. Food Sci. Technol. 2013, 4, 817–822. [Google Scholar]
- He, B.; Wu, F.X.; Yu, L.K.; Wu, L.; Chen, Q.; Hao, G.F.; Yang, W.C.; Lin, H.Y.; Yang, G.F. Discovery of novel pyrazole–quinazoline-2,4-dione hybrids as 4-hydroxyphenylpyruvate dioxygenase inhibitors. Agric. Food. Chem. 2020, 68, 5059–5067. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, J.; Ma, X.; Sun, J.; Tang, F. Laboratory and field evaluation of the phytotoxic activity of Sapindus mukorossi Gaertn pulp extract and identification of a phytotoxic substance. Molecules 2021, 26, 1318. [Google Scholar] [CrossRef] [PubMed]
- Poonpaiboonpipat, T.; Krumsri, R.; Kato-Noguchi, H. Allelopathic and herbicidal effects of crude extract from Chromolaena odorata (L.) RM King and H. Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants 2021, 10, 1609. [Google Scholar] [CrossRef]
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014; pp. 245–266. [Google Scholar]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Zerbe, P.; Hamberger, B.; Yuen, M.M.; Chiang, A.; Sandhu, H.K.; Madilao, L.L.; Nguyen, A.; Hamberger, B.; Bach, S.S.; Bohlmann, J. Gene discovery of modular diterpene metabolism in nonmodel systems. Plant Physiol. 2013, 162, 1073–1091. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Soltys, D.; Rudzińska-Langwald, A.; Kurek, W.; Gniazdowska, A.; Sliwinska, E.; Bogatek, R. Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation. Planta 2011, 234, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, M.R.; Lupini, A.; Oliva, S.; Sorgonà, A. Allelochemical effects on net nitrate uptake and plasma membrane H+-ATPase activity in maize seedlings. Biol. Plant. 2010, 54, 149–153. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.; Galindo, J.C.; Varela, R.M.; Simonet, A.M.; Castellano, D. The use of allelopathic studies in the search for natural herbicides. J. Crop Prod. 2001, 4, 237–255. [Google Scholar] [CrossRef]
- Loiseleur, O. Natural products in the discovery of agrochemicals. Chimia 2017, 71, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.; Varela, R.M.; Galindo, J.C. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Duke, S.O.; Scheffler, B.E.; Dayan, F.E.; Weston, L.A.; Ota, E. Strategies for using transgenes to produce allelopathic crops. Weed Technol. 2001, 15, 826–834. [Google Scholar] [CrossRef]
- Ladhari, A.; Romanucci, V.; De Marco, A.; De Tommaso, G.; Di Marino, C.; Di Fabio, G.; Zarrelli, A. Herbicidal potential of phenolic and cyanogenic glycoside compounds isolated from Mediterranean plants. Ecol. Quest. 2018, 29, 25–34. [Google Scholar] [CrossRef]
- Rob, M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a novel compound involved in allelopathic activity of Garcinia Xanthochymus hook. Plants 2019, 8, 301. [Google Scholar] [CrossRef]
- Soriano, G.; Fernández-Aparicio, M.; Masi, M.; Vilariño-Rodríguez, S.; Cimmino, A. Complex mixture of arvensic acids isolated from Convolvulus arvensis roots identified as inhibitors of radicle growth of broomrape weeds. Agriculture. 2022, 12, 585. [Google Scholar] [CrossRef]
- Soriano, G.; Siciliano, A.; Fernández-Aparicio, M.; Cala Peralta, A.; Masi, M.; Moreno-Robles, A.; Guida, M.; Cimmino, A. Iridoid glycosides isolated from Bellardia trixago identified as inhibitors of Orobanche cumana radicle growth. Toxins. 2022, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathy of the medicinal plant Dregea volubilis (Lf) Benth. ex Hook. f. and its phytotoxic substances with allelopathic activity. Agronomy 2022, 12, 303. [Google Scholar] [CrossRef]
- Rial, C.; Novaes, P.; Varela, R.M.; Molinillo, J.M.G.; Macias, F.A. Phytotoxicity of cardoon (Cynara cardunculus) allelochemicals on standard target species and weeds. J. Agric. Food Chem. 2014, 62, 6699–6706. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Gandara, F.; Torres, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Varela, R.; Martínez de la Ossa-Fernández, E.J.; Macias, F.A. Selective fractionation and isolation of allelopathic compounds from Helianthus annuus L. leaves by means of high-pressure techniques. J. Supercrit. Fluids 2019, 143, 32–41. [Google Scholar] [CrossRef]
- Monkheang, P.; Sudmoon, R.; Tanee, T.; Noikotr, K.; Bletter, N.; Chaveerach, A. Species diversity, usages, molecular markers and barcode of medicinal Senna species (Fabaceae, Caesalpinioideae) in Thailand. J. Med. Plant Res. 2011, 5, 6173–6181. [Google Scholar] [CrossRef]
- Pattarapongdilok, N.; Malichim, P.; Simmee, N.; Sichaem, J. Senna flower extract as an indicator for acid-base titration. Rasayan J. Chem. 2021, 14, 1402–1407. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic actions of anthrone-C-glucoside, cassialoin isolated from Cassia garrettiana heartwood in colon 26-bearing mice. Cancer Sci. 2008, 99, 2336–2348. [Google Scholar] [CrossRef]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Surapanthanakorn, S.; Phadoongsombut, N.; Wattanapiromsakul, C.; Reanmongkol, W. In vivo evaluation of analgesic and antipyretic activities of piceatannol-rich extract from Senna garrettiana heartwood. Songklanakarin J. Sci. Technol. 2016, 39, 589–599. [Google Scholar]
- Surapanthanakorn, S.; Wattanapiromsakul, C.; Reanmongkol, W. Assessment of the anti-inflammatory activity of piceatan-nol-rich extract from Senna garrettiana heartwood. Chiang Mai J. Sci. 2018, 45, 2691–2702. [Google Scholar]
- Tewtrakul, S.; Subhadhirasakul, S.; Rattanasuwan, P.; Puripattanavong, J. HIV-1 protease inhibitory substances from Cassia garrettiana. Songklanakarin J. Sci. Technol. 2007, 29, 145–149. [Google Scholar]
- Yuenyongsawad, S.; Bunluepuech, K.; Wattanapiromsakul, C.; Tewtrakul, S. Anti-cancer activity of compounds from Cassia garrettiana heartwood. Songklanakarin J. Sci. Technol. 2014, 36, 189–194. [Google Scholar]
- Sakunpak, A.; Saingam, W. Screening and compound isolation from selected Thai herbal medicine for anti-hyaluronidase and anti-elastase activities. In Proceedings of the RSU International Research Conference, Bangkok, Thailand, 1 May 2020; pp. 305–311. [Google Scholar]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Senna garrettiana leaves and identification of potent phytotoxic substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Pereira, J.C.; Paulino, C.L.A.; Endres, L.; Santana, A.E.G.; Pereira, F.R.S.; Souza, R.C. Allelopathic potential of ethanolic extract and phytochemical analysis of Paspalum maritimum Trind. Planta Daninha 2019, 37, 1–12. [Google Scholar] [CrossRef]
- Martins, S.A.; Dos Santos, R.C.; De Rezende Ramos, A.; Figueiredo, P.L.B.; Da Silva, C.R.C.; Da Silva, J.K.R. Allelopathic potential and phytochemical screening of Piper divaricatum extracts on germination and growth of indicator plant (Lactuca sativa). S. Afr. J. Bot. 2021, 138, 495–499. [Google Scholar] [CrossRef]
- Shixing, Z.; Xunzhi, Z.; Kai, S.; Caixia, H.; Kuchkarova, N.; Chi, Z. Chemical composition and allelopathic potential of the invasive plant Solanum rostratum Dunal essential oil. Flora 2021, 274, 151730. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suzuki, M.; Noguchi, K.; Ohno, O.; Suenaga, K.; Laosinwattana, C. A potent phytotoxic substance in Aglaia odorata Lour. Chem. Biodivers. 2016, 13, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot. 2017, 70, 1720. [Google Scholar] [CrossRef]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic activity of leaf and stem extracts and identification of a phytotoxic substance from Caesalpinia mimosoides Lamk. Theor. Exp. Plant Physiol. 2018, 30, 129–139. [Google Scholar] [CrossRef]
- Rob, M.; Hossen, K.; Khatun, M.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and application of bioactive compounds from Garcinia xanthochymus Hook. for weed management. Appl. Sci. 2021, 11, 2264. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, S.; Singh, H.P.; Batish, D.R. Alterations in phytotoxicity and allelochemistry in response to intraspecific variation in Parthenium hysterophorus. Ecol. Complex. 2022, 50, 100999. [Google Scholar] [CrossRef]
- Teng, R.W.; Wang, D.Z.; Wu, Y.S.; Lu, Y.; Zheng, Q.T.; Yang, C.R. NMR assignments and single-crystal X-ray diffraction analysis of deoxyloganic acid. Magn. Reson. Chem. 2005, 43, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, L.X.; Zhao, Y.; Huang, G.D. Unusual sesquiterpene lactones with a new carbon skeleton and new acetylenes from Ajania przewalskii. Food Chem. 2010, 118, 228–238. [Google Scholar] [CrossRef]
- Prevost, M.S.; Delarue-Cochin, S.; Marteaux, J.; Colas, C.; Van Renterghem, C.; Blondel, A.; Malliavin, T.; Corringer, P.J.; Joseph, D. Identification of cinnamic acid derivatives as novel antagonists of the prokaryotic proton-gated ion channel GLIC. J. Med. Chem. 2013, 56, 4619–4630. [Google Scholar] [CrossRef]
- Schlingmann, G.; Milne, L.; Carter, G.T. Isolation and identification of antifungal polyesters from the marine fungus Hypoxylon oceanicum LL-15G256. Tetrahedron 2002, 58, 6825–6835. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity of Clerodendrum indicum (L.) Kuntze and its potential phytotoxic substance. Emir. J. Food Agric. 2021, 33, 884–892. [Google Scholar] [CrossRef]
- Inderjit; Duke, S.O. Ecophysiological aspects of allélopathie. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef]
- De Armas-Ricard, M.; Ruiz-Reyes, E.; Ramírez-Rodríguez, O. Caffeates and caffeamides: Synthetic methodologies and their antioxidant properties. Int. J. Med. Chem. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Qi, Y.; Kim, R.; Song, J.; Kim, H.; Kim, H.Y.; Jang, D.S.; Kang, K.S. Methyl caffeate isolated from the flowers of Prunus persica (L.) Batsch enhances glucose-stimulated insulin secretion. Biomolecules 2021, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, M.; Jiang, Q.; Xu, Y.; Zhou, X.; Zhang, N.; Sun, D.; Li, H.; Chen, L. Chemical components of the fruits of Morus nigra Linn.: Methyl caffeate as a potential anticancer agent by targeting 3-phosphoglycerate dehydrogenase. J. Agric. Food Chem. 2021, 69, 12433–12444. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, F.; Nishi, A. A methyltransferase for synthesis of the phytoalexin 6-methoxymellein in carrot cells. FEBS Lett. 1988, 227, 183–186. [Google Scholar] [CrossRef]
- Schlingmann, G.; Roll, D.M. Absolute stereochemistry of unusual biopolymers from Ascomycete culture LL-W1278: Examples that derivatives of (S)-6-hydroxymellein are also natural fungal metabolites. Chirality 2005, 17, S48–S51. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, L.; Wang, M.; Wei, L.; Qu, H.; Ma, J.; Ju, J.; Han, Z. Chemical constituents from marine derived fungus Talaromyces cellulolyticus SHJ-3 and its chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 100, 104377. [Google Scholar] [CrossRef]
- Bubna, G.A.; Lima, R.B.; Zanardo, D.Y.L.; Dos Santos, W.D.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef]
- Fási, L.; Latif, A.D.; Zupkó, I.; Lévai, S.; Dékány, M.; Béni, Z.; Könczöl, Á.; Balogh, G.T.; Hunyadi, A. AAPH or peroxynitrite-induced biorelevant oxidation of methyl caffeate yields a potent antitumor metabolite. Biomolecules 2020, 10, 1537. [Google Scholar] [CrossRef]
- Balachandran, C.; Emi, N.; Arun, Y.; Yamamoto, Y.; Ahilan, B.; Sangeetha, B.; Duraipandiyan, V.; Inaguma, Y.; Okamoto, A.; Ignacimuthu, S.; et al. In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. fruit. Chem. Biol. Interact. 2015, 242, 81–90. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. An efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds. J. Nat. Prod. 2010, 73, 1193–1195. [Google Scholar] [CrossRef]
- Sakamoto, C.; Suzuki, M.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of allelopathic competency of Lamium amplexicaule and identification of its allelopathic active substance. Emir. J. Food Agric. 2019, 13, 76–80. [Google Scholar] [CrossRef]
- Ripardo Filho, H.D.S.; Pacheco, L.C.; Andrade, E.S.; Ribeiro, W.D.S.; Guilhon, G.M.; Corrêa, M.J.; Romero, O.A.S.; Santos, L.S.; Souza Filho, A.P.D.S. Phytotoxic activity of compounds from Moutabea guianensis aubl. on Amazonian invasive species. Eclet. Quim. 2015, 40, 71–76. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.J.; Yin, Y.; Ge, M. A novel chromene with anti-tumor activities from fungus Phomopsis sp. China J. Chin. Mater. Med. 2015, 40, 667–671. [Google Scholar] [CrossRef]
- Stabler, M.; Anke, H.; Sterner, O. New metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceum (Karst.) Kars. IV. Structural elucidation of novel isocoumarin derivatives. J. Antibiot. Res. 1995, 48, 267–270. [Google Scholar] [CrossRef]
- Shimada, A.; Kusano, M.; Takeuchi, S.; Fujioka, S.; Inokuchi, T.; Kimura, Y. Aspterric acid and 6-hydroxymellein, inhibitors of pollen development in Arabidopsis thaliana, produced by Aspergillus terreus. Z. Naturforsch. C. J. Biosci. 2002, 57, 459–464. [Google Scholar] [CrossRef]
- Islam, M.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of three potential phytotoxic compounds from Chrysopogon aciculatus (Retz.) Trin. Acta Physiol. Plant. 2021, 43, 56. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; Cui, H.; Zhang, D.; Sun, Y.; Jin, H.; Li, X.; Yang, X.; Guo, H.; He, X.; et al. Phytotoxicity mechanisms of two coumarin allelochemicals from Stellera chamaejasme in lettuce seedlings. Acta Physiol. Plant. 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Sunohara, Y.; Nakano, K.; Matsuyama, S.; Oka, T.; Matsumoto, H. Cuminaldehyde, a cumin seed volatile component, induces growth inhibition, overproduction of reactive oxygen species and cell cycle arrest in onion roots. Sci. Hortic. 2021, 289, 110493. [Google Scholar] [CrossRef]
- Tkalec, M.; Malarić, K.; Pavlica, M.; Pevalek-Kozlina, B.; Vidaković-Cifrek, Ž. Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 672, 76–81. [Google Scholar] [CrossRef]
- Ladhari, A.; Omezzine, F.; Haouala, R. The impact of Tunisian Capparidaceae species on cytological, physiological and biochemical mechanisms in lettuce. S. Afr. J. Bot. 2014, 93, 222–230. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Goda, A.M. Antifungal and cytotoxic constituents from the endophytic fungus Penicillium sp. Bull. Fac. Pharm. Cairo. Univ. 2017, 55, 85–89. [Google Scholar] [CrossRef]
- Zhang, W.H.; Xiao, Y.W.; Liang, W.Z.; Wang, Y.; Gao, B.L.; Zhu, D. Isolation of secondary metabolites from endophytic fungus Chaetomium globosum S108 and its antimelanoma activity. Nat. Prod. Res. Dev. 2021, 33, 1854. [Google Scholar]
- Shimada, A.; Inokuchi, T.; Kusano, M.; Takeuchi, S.; Inoue, R.; Tanita, M.; Fujioka, S. 4-Hydroxykigelin and 6-demethylkigelin, root growth promoters, produced by Aspergillus terreus. Z. Naturforsch. C. J. Biosci. 2004, 59, 218–222. [Google Scholar] [CrossRef] [PubMed]
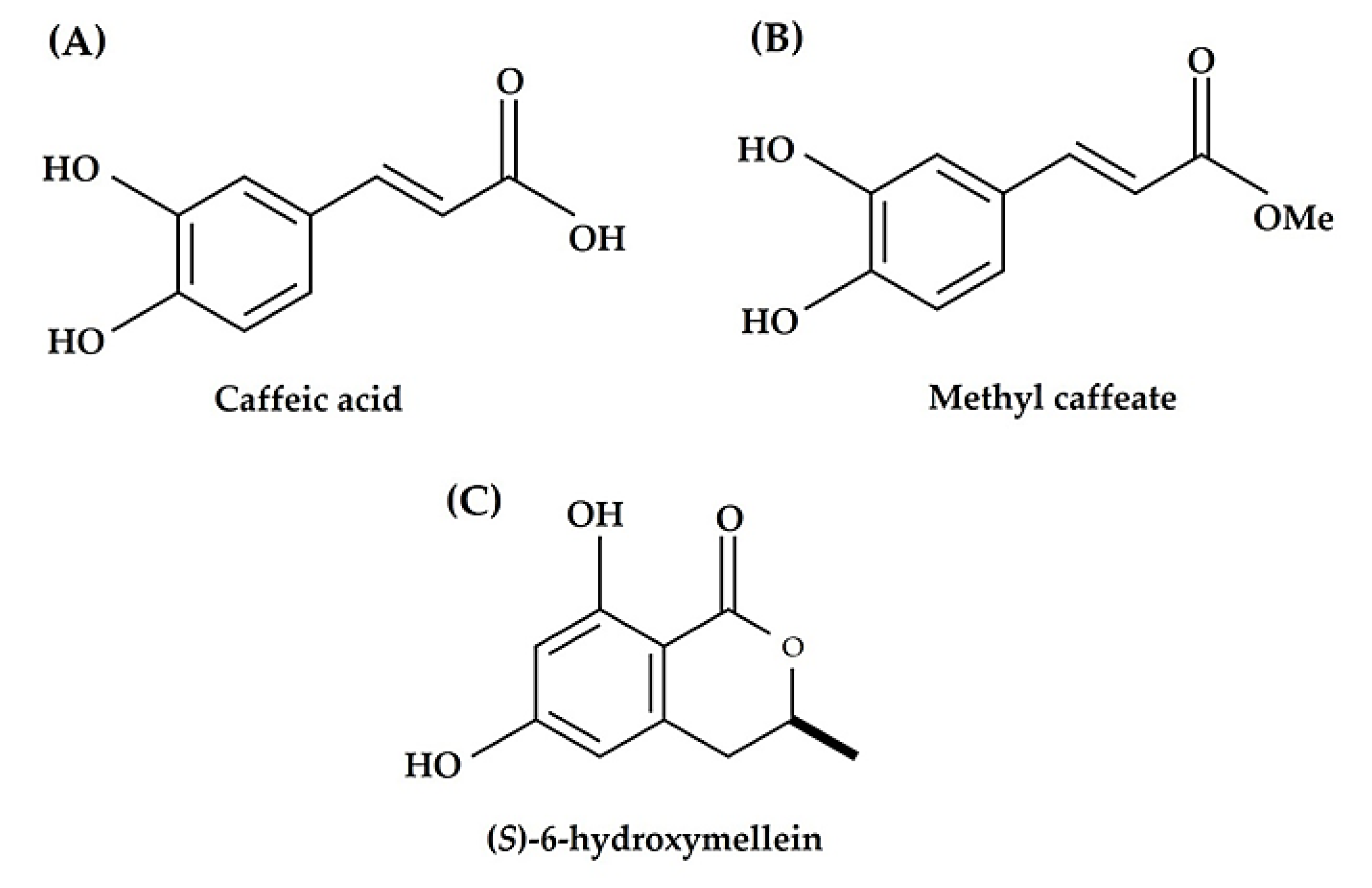

| Compound | L. sativum Growth Parameter | IC50 Value (µM) | R2 a | Probability b |
|---|---|---|---|---|
| Caffeic acid | Germination | not converged | 0.42 | >0.05 |
| Shoot length | 4253 | 0.98 | 0.001 *** | |
| Root length | 2627 | 0.95 | 0.001 *** | |
| Biomass | 5820 | 0.94 | 0.001 *** | |
| Methyl caffeate | Germination | 5120 | 0.43 | 0.046 * |
| Shoot length | 1361 | 0.86 | 0.001 *** | |
| Root length | 1586 | 0.94 | 0.001 *** | |
| Biomass | 2780 | 0.92 | 0.043 * | |
| (S)-6-hydroxymellein | Germination | 2740 | 0.58 | 0.001 *** |
| Shoot length | 475 | 0.94 | 0.001 *** | |
| Root length | 383 | 0.95 | 0.001 *** | |
| Biomass | 750 | 0.95 | 0.004 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides. Agriculture 2022, 12, 1338. https://doi.org/10.3390/agriculture12091338
Krumsri R, Iwasaki A, Suenaga K, Kato-Noguchi H. Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides. Agriculture. 2022; 12(9):1338. https://doi.org/10.3390/agriculture12091338
Chicago/Turabian StyleKrumsri, Ramida, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2022. "Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides" Agriculture 12, no. 9: 1338. https://doi.org/10.3390/agriculture12091338
APA StyleKrumsri, R., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2022). Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides. Agriculture, 12(9), 1338. https://doi.org/10.3390/agriculture12091338