Effects of Nitrogen Addition on Soil Microbial Functional Diversity and Extracellular Enzyme Activities in Greenhouse Cucumber Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil Sampling and Chemical Analysis
2.3. Soil Extracellular-Enzyme-Activity Assays
2.4. Soil Community Level Physiological Profile (CLPP) Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil and Plant Properties
3.2. Soil Enzyme Activities
3.3. Microbial Functional Characteristics (CLPP)
3.4. Links among Soil Extracellular Enzyme Activities, Microbial C Sources Utilization, and Physicochemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gruber, N.; Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Voss, M. The global nitrogen cycle in the twenty-first century. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20130165. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Yadav, R. Targeting nitrogen use efficiency for sustained production of cereal crops. J. Plant Nutr. 2019, 42, 1086–1113. [Google Scholar] [CrossRef]
- Wieder, W.R.; Cleveland, C.C.; Smith, W.K.; Todd-Brown, K. Future productivity and carbon storage limited by terrestrial nutrient availability. Nat. Geosci. 2015, 8, 441–444. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-A critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Alves, L.A.; Denardin, L.G.D.; Martins, A.P.; Anghinoni, I.; Carvalho, P.C.D.; Tiecher, T. Soil acidification and P, K, Ca and Mg budget as affected by sheep grazing and crop rotation in a long-term integrated crop-livestock system in southern Brazil. Geoderma 2019, 351, 197–208. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Zhang, J.; Saleem, M.; He, Y.; Zhong, J.; Ma, R. Seasonality regulates the effects of acid rain on microbial community in a subtropical agricultural soil of Southern China. Ecotoxicol. Environ. Saf. 2021, 224, 112681. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Liu, L.L.; Li, Y.; Qin, S.; Wang, C.J.; Cai, A.D.; Wu, L.; Xu, M.G.; Zhang, W.J. Long-term fertilization leads to specific PLFA finger-prints in Chinese Hapludults soil. J. Integr. Agric. 2020, 19, 1354–1362. [Google Scholar] [CrossRef]
- Högberg, M.N.; Briones, M.J.I.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Midwood, A.J.; Thornton, B.; Hurry, V.; Linder, S.; Näsholm, T.; et al. Quantification of effects of season and nitrogen supply on tree below- ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 2010, 187, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.S.; Hattenschwiler, S.; Lu, X.T.; Kardol, P.; Zhang, Y.; Wei, C.Z.; Han, X.G. Carbon limitation overrides acidification in mediating soil microbial activity to nitrogen enrichment in a temperate grassland. Glob. Chang. Biol. 2021, 27, 5976–5988. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.Q.; Dou, Y.X.; Cheng, H.; Liu, L.X.; An, S.S. Linkage between soil ectoenzyme stoichiometry ratios and microbial diversity following the conversion of cropland into grassland. Agric. Ecosyst. Environ. 2021, 314, 107418. [Google Scholar] [CrossRef]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S. The pH optimum of soil exoenzymes adapt to long term changes in soil pH. Soil Biol. Biochem. 2019, 138, 107601–107610. [Google Scholar] [CrossRef]
- Shen, D.; Ye, C.; Hu, Z.; Chen, X.; Guo, H.; Li, J.; Du, G.; Adl, S.; Liu, M. Increased chemical stability but decreased physical protection of soil organic carbon in response to nutrient amendment in a Tibetan alpine meadow. Soil Biol. Biochem. 2018, 126, 11–21. [Google Scholar] [CrossRef]
- Wang, R.Z.; Dorodnikov, M.; Yang, S.; Zhang, Y.Y.; Filley, T.R.; Turco, R.F.; Jiang, Y. Responses of enzymatic activities within soil aggregates to 9-year nitrogen and water addition in a semi-arid grassland. Soil Biol. Biochem. 2015, 81, 159–167. [Google Scholar] [CrossRef]
- Jing, H.; Li, J.J.; Yan, B.S.; Wei, F.R.; Wang, G.L.; Liu, G.B. The effects of nitrogen addition on soil organic carbon decomposition and microbial C-degradation functional genes abundance in a Pinus tabulaeformis forest. For. Ecol. Manag. 2021, 489, 119098. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Tang, M.; Ding, Z.J.; Jiang, L.; Li, P.; Ma, S.H.; Tian, D.; Xu, L.C.; Zhu, J.X.; et al. Nitrogen deposition has minor effect on soil extracellular enzyme activities in six Chinese forests. Sci. Total Environ. 2017, 607–608, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.W.; Du, H.J.; Gao, Y.L.; Qiu, W.F. Analysis on Metabolic Functions of Stored Rice Microbial Communities by BIOLOG ECO Microplates. Front. Microbiol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Bhattacharyya, P. Variation of functional diversity of soil microbial community in sub-humid tropical rice-rice cropping system under long-term organic and inorganic fertilization. Ecol. Indic. 2017, 73, 536–543. [Google Scholar] [CrossRef]
- He, Y.T.; Qi, Y.C.; Dong, Y.S.; Xiao, S.S.; Peng, Q.; Liu, X.C. Effects of nitrogen fertilization on soil microbial biomass and community functional diversity in temperate grassland in inner mongolia, china. Clean Soil Air Water 2013, 41, 1216–1221. [Google Scholar] [CrossRef]
- Yuan, Y.H. Effects of nitrogen deposition on soil microbial biomass, community functional diversity and enzyme activities in fir plantation subtropical China. Adv. Mater. Res. 2013, 610–613, 323–330. [Google Scholar]
- Shen, W.S.; Lin, X.G.; Shi, W.M.; Min, J. Higher rates of nitrogen fertilization decrease soil enzyme activities, microbial functional diversity and nitrification capacity in a chinese polytunnel greenhouse vegetable land. Plant Soil 2010, 337, 137–150. [Google Scholar] [CrossRef]
- Preece, C.; Farré-Armengol, G.; Peuelas, J. Drought is a stronger driver of soil respiration and microbial communities than nitrogen or phosphorus addition in two mediterranean tree species. Sci. Total Environ. 2020, 735, 139554. [Google Scholar] [CrossRef]
- Cui, J.; Wang, J.J.; Xu, J.; Xu, C.H.; Xu, X.N. Changes in soil bacterial communities in an evergreen broad-leaved forest in east china following 4 years of nitrogen addition. J. Soils Sediments 2017, 17, 2156–2164. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, T.X.; Zhang, X.Z. Nutrient budget and soil nutrient status in greenhouse system. Agric. Sci. China 2010, 9, 871–879. [Google Scholar] [CrossRef]
- Song, H.; Guo, J.H.; Ren, T.; Chen, Q.; Li, B.G.; Wang, J.G. Increase of soil pH in a solar greenhouse vegetable production system. Soil Sci. Soc. Am. J. 2012, 76, 2074–2082. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Beare, M.H. Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci. Soc. Am. J. 1993, 57, 1007–1012. [Google Scholar] [CrossRef]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for Chemical Analysis of Soils; New Zealand Soil Bureau Scientific Report 10A; Department of Scientific and Industrial Research: Dunedin, New Zealand, 1987.
- Nobile, C.M.; Bravin, M.N.; Becquer, T.; Paillat, J.M. Phosphorus sorption and availability in an andosol after a decade of organic or mineral fertilizer applications: Importance of pH and organic carbon modifications in soil as compared to phosphorus accumulation. Chemosphere 2020, 239, 124709–124719. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphrous. In Methods of Soil Analysis; ASA and SSSA: Madison, WI, USA, 1982. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Velasco, G.V.; Probanza, A.; Mañero, F.J.G. Effect of fire and retardant on soil microbial activity and functional diversity in a Mediterranean pasture. Geoderma 2009, 153, 186–193. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, Y.; Wang, Y.; Wan, L.; Wang, J.; Butterbach-Bahl, K.; Lin, S. Conventional flooding irrigation and over fertilization drives soil pH decrease not only in the top- but also in subsoil layers in solar greenhouse vegetable production systems. Geoderma 2020, 363, 114156. [Google Scholar] [CrossRef]
- Hao, T.X.; Zhu, Q.C.; Zeng, M.F.; Shen, J.B.; Shi, X.J.; Liu, X.J.; Zhang, F.S.; Vries, W.D. Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.P.; Chen, H.Y.H.; Searle, E.B.; Sardans, J.; Ciais, P.; Peñuelas, J.; Huang, Z.Q. Whole soil acidification and base cation reduction across subtropical China. Geoderma 2020, 361, 114107. [Google Scholar] [CrossRef]
- Wu, C.X.; Wang, J.J.; Zhang, S.Z.; Zhang, Z.M. Adsorption and desorption of methiopyrsulfuron in soils. Pedosphere 2011, 21, 380–388. [Google Scholar] [CrossRef]
- Long, M.; Wu, H.H.; Smith, M.D.; La Pierre, K.G.; Lv, X.T.; Zhang, H.Y. Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 2016, 408, 475–484. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Li, T.; Cheng, H.; An, S.S. Soil extracellular enzyme stoichiometry reflects the shift from P- to N-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Schleuss, P.M.; Widdig, M.; Heintz-Buschart, A.; Guhr, A.; Martin, S.; Kirkman, K.; Spohn, M. Stoichiometric controls of soil carbon and nitrogen cycling after long-term nitrogen and phosphorus addition in a mesic grassland in South Africa. Soil Biol. Biochem. 2019, 135, 294–303. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Chang. Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Liu, N.N.; Bai, W.M.; Li, L.H.; Chen, J.Q.; Reich, P.B.; Yu, Q.; Guo, D.; Smith, M.D.; Knapp, A.K.; et al. A novel soil manganese mechanism drives plant species loss with increased nitrogen deposition in a temperate steppe. Ecology 2016, 97, 65–74. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of longterm nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Gao, M.; Dong, Y.; Zhang, Z.; Song, Z. Effect of dibutyl phthalate on microbial function diversity and enzyme activity in wheat rhizosphere and non-rhizosphere soils. Environ. Pollut. 2020, 265, 114800. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Hui, D.F.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Talbot, J.M.; Martin, F.; Kohler, A.; Henrissat, B.; Peay, K.G. Functional guild classification predicts the enzymatic role offungi in litter and soil biogeochemistry. Soil Biol. Biochem. 2015, 88, 441–456. [Google Scholar] [CrossRef]
- Luo, R.; Luo, J.; Fan, J.; Liu, D.; He, J.S.; Perveen, N.; Ding, W. Responses of soil microbial communities and functions associated with organic carbon mineralization to nitrogen addition in a Tibetan grassland. Pedosphere 2020, 30, 214–225. [Google Scholar] [CrossRef]
- Zhang, N.L.; Wan, S.Q.; Li, L.H.; Bi, J.; Zhao, M.M.; Ma, K.P. Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 2008, 311, 19–28. [Google Scholar] [CrossRef]
- Wan, W.J.; Tan, J.D.; Wang, Y.; Qin, Y.; He, H.M.; Wu, H.Q.; Zuo, W.L.; He, D.L. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Meng, M.J.; Zhai, L.; Zhang, B.; Jia, Z.H.; Zhang, J.C. The use of Biolog Eco microplates to compare the effects of sulfuric and nitric acid rain on the metabolic functions of soil microbial communities in a subtropical plantation within the Yangtze River Delta region. Catena 2021, 198, 105039. [Google Scholar] [CrossRef]
- Xi, N.X.; Bloor, J.M.G. Interactive effects of precipitation and nitrogen spatial pattern on carbon use and functional diversity in soil microbial communities. Appl. Soil Ecol. 2016, 100, 207–210. [Google Scholar] [CrossRef]
- Yang, T.; Lupwayi, N.; Marc, S.A.; Siddique, K.H.M.; Bainard, L.D. Anthropogenic drivers of soil microbial communities and impacts on soil biological functions in agroecosystems. Glob. Ecol. Conserv. 2021, 27, e01521. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xie, F.X.; Zhang, F.F.; Zhou, K.; Sun, H.B.; Zhao, Y.J.; Yang, Q. Analysis of bacterial community functional diversity in late-stage shrimp (Litopenaeus vannamei) ponds using Biolog EcoPlates and PICRUSt2. Aquaculture 2022, 546, 737288. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Z.L.; Barberán, A.; Yang, Y.; Hu, S.J.; Guo, H. Nitrogen-induced acidification plays a vital role driving ecosystem functions: Insights from a 6-year nitrogen enrichment experiment in a Tibetan alpine meadow. Soil Biol. Biochem. 2021, 153, 108107. [Google Scholar] [CrossRef]
- Arshad, A.; Dalcin Martins, P.; Frank, J.; Jetten, M.S.M.; Op den Camp, H.J.M.; Welte, C.U. Mimicking microbial interactions under nitrate-reducing conditions in an anoxic bioreactor: Enrichment of novel Nitrospirae bacteria distantly related to Thermodesulfovibrio. Environ. Microbiol. 2017, 19, 4965–4977. [Google Scholar] [CrossRef]
- Xing, W.; Lu, X.M.; Ying, J.Y.; Lan, Z.C.; Chen, D.M.; Bai, Y.F. Disentangling the effects of nitrogen availability and soil acidification on microbial taxa and soil carbon dynamics in natural grasslands. Soil Biol. Biochem. 2022, 164, 108495. [Google Scholar] [CrossRef]
- Hu, C.; Sadras, V.O.; Lu, G.; Zhang, R.; Yang, X.; Zhang, S. Root pruning enhances wheat yield, harvest index and water-use efficiency in semiarid area. Field Crop. Res. 2019, 230, 62–71. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.W.; Yao, Y.L.; Zhu, F.X.; Wang, W.P.; Chang, X.J. The influence of different concentrations of bio-organic fertilizer on cucumber Fusarium wilt and soil microflora alterations. PLoS ONE 2017, 12, e0171490. [Google Scholar] [CrossRef] [Green Version]
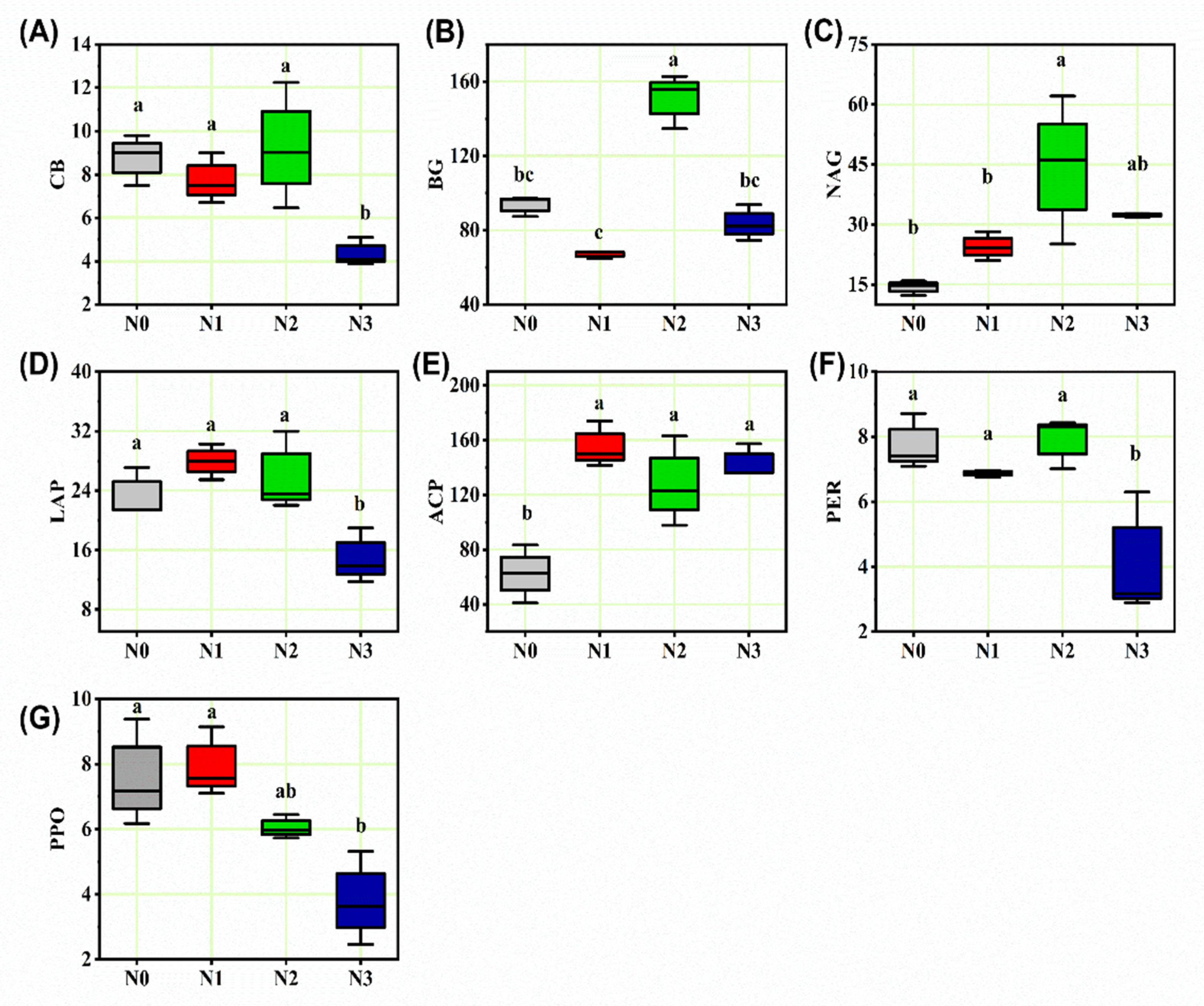
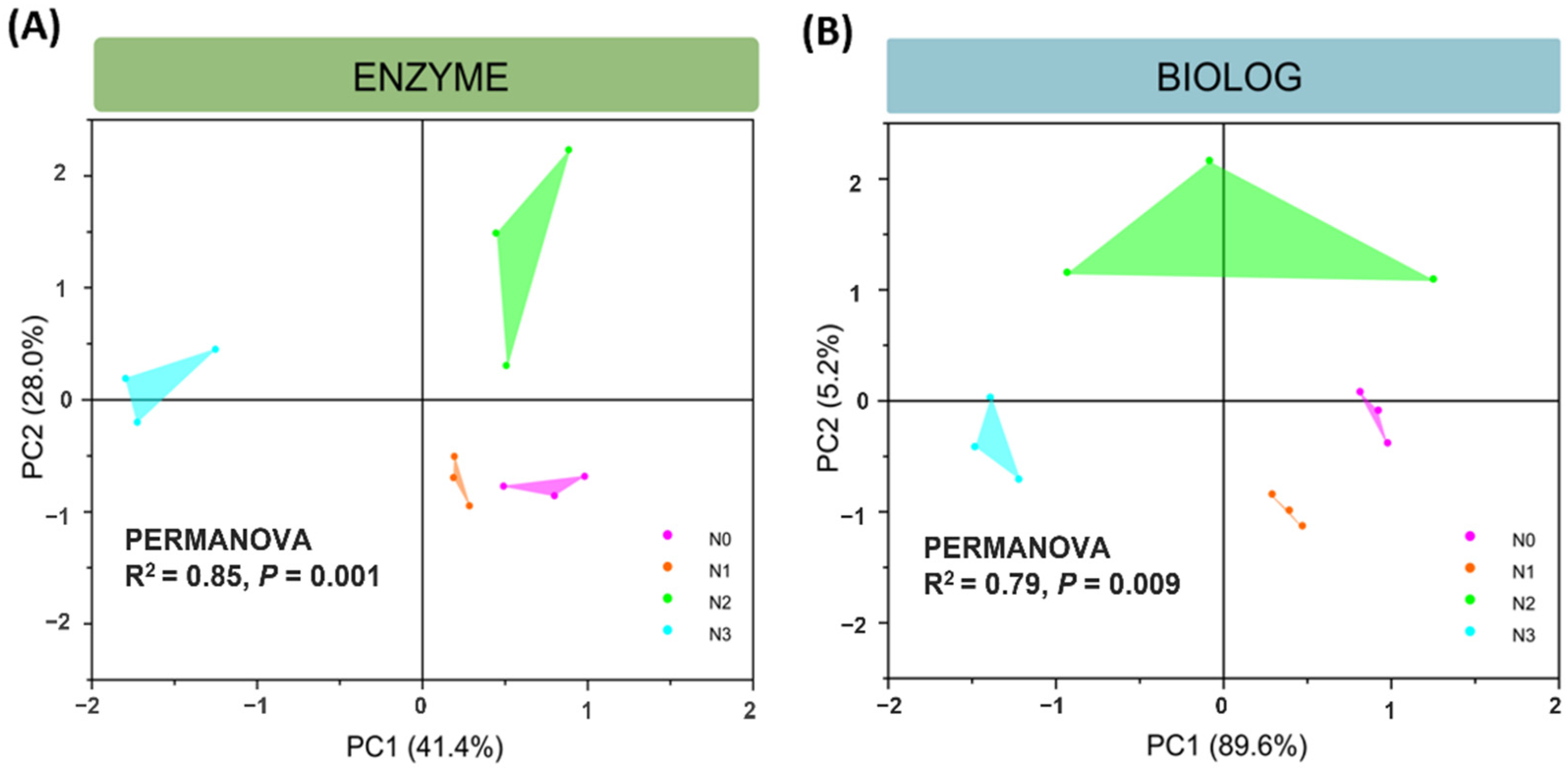

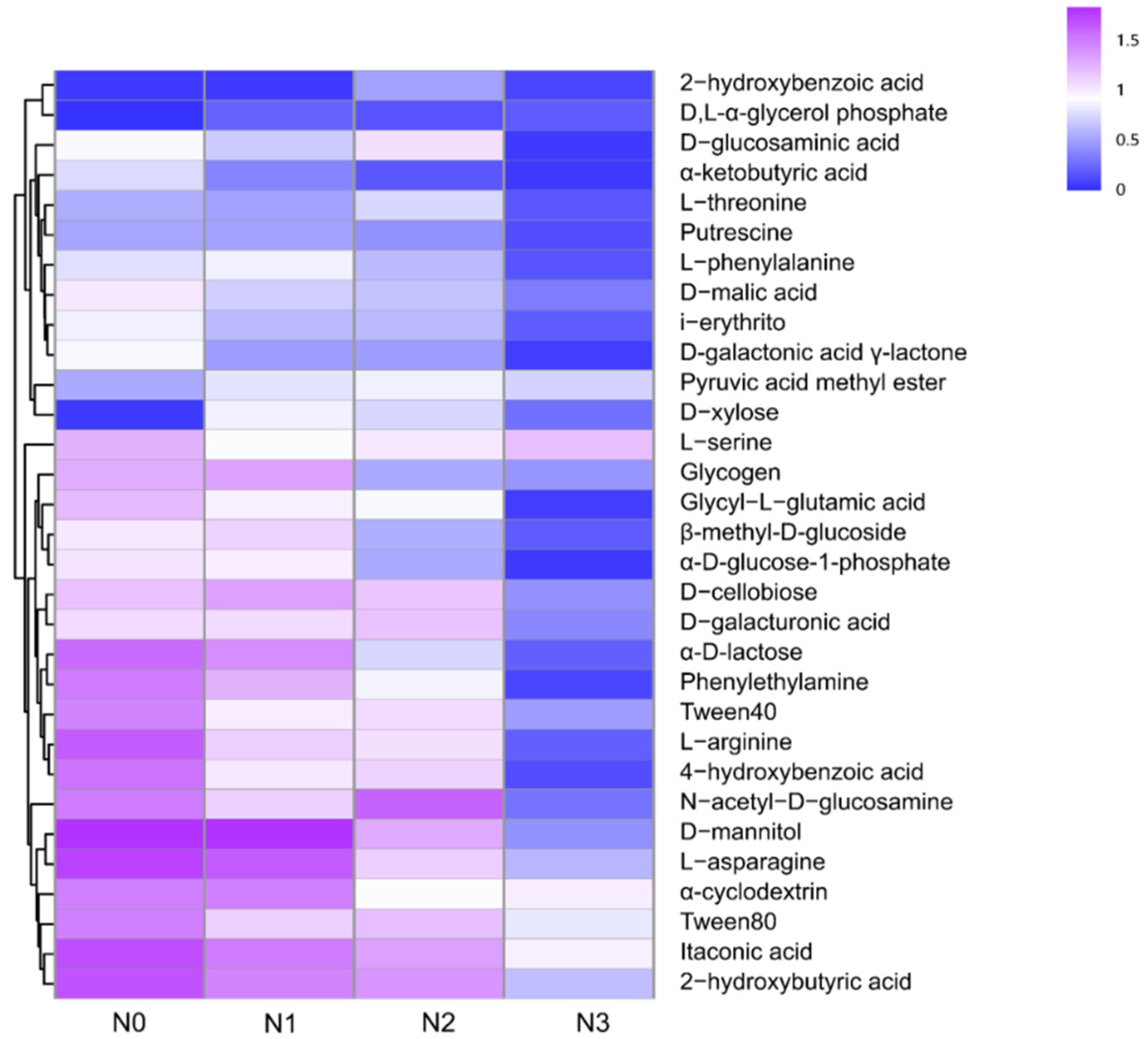



| Enzyme | Abbreviation | EC Number | Substrate |
|---|---|---|---|
| β-1,4-glucosidase | BG | 3.2.1.21 | 4-MUB-β-D-glucoside |
| Cellobiohydrolase | CB | 3.2.1.91 | 4-MUB-β-D-cellobioside |
| Leucine aminopeptidase | LAP | 3.4.11.1 | Leucine-7-amino-4-methylcoumarin |
| β-1,4-N-acetyl-glucosaminidase | NAG | 3.1.6.1 | 4-MUB-N-acetyl-B-D-glucosaminide |
| Acid phosphatase | ACP | 3.1.3.2 | 4-MUB-phosphate |
| Peroxidase | PER | 1.11.1.7 | L-DOPA |
| Polyphenol oxidase | PPO | 1.10.3.2 | L-DOPA |
| Environmental Attributes | N0 | N1 | N2 | N3 |
|---|---|---|---|---|
| pH | 7.01 ± 0.11 a | 6.38 ± 0.34 b | 5.83 ± 0.09 c | 5.49 ± 0.11 c |
| EC (ms cm−1) | 0.33 ± 0.11 c | 0.57 ± 0.12 bc | 0.7 ± 0.3 b | 1.45 ± 0.11 a |
| NH4+-N (mg kg−1) | 1.98 ± 0.57 c | 5.51 ± 2.06 c | 34.72 ± 4.07 b | 62.25 ± 1.83 a |
| NO3−N (mg kg−1) | 2.57 ± 0.61 b | 29.67 ± 13.06 b | 177.94 ± 66.87 a | 199.57 ± 16.09 a |
| TN (g kg−1) | 1.37 ± 0.1 c | 1.7 ± 0.03 b | 2.01 ± 0.22 a | 2.03 ± 0.18 a |
| AP (mg kg−1) | 236.18 ± 14.54 b | 225.48 ± 7.17 b | 304.21 ± 8.19 a | 240.38 ± 0.62 b |
| AK (mg kg−1) | 721.05 ± 90.91 b | 839 ± 109.99 ab | 1031.69 ± 182.27 a | 1001.66 ± 83.9 a |
| TP (g kg−1) | 1.09 ± 0.12 b | 1.24 ± 0.02 b | 1.45 ± 0.05 a | 1.46 ± 0.17 a |
| TK (g kg−1) | 25.67 ± 1.7 a | 23.53 ± 0.52 a | 22.93 ± 1.36 a | 24.96 ± 3.5 a |
| SOC (g kg−1) | 24.21 ± 1.4 b | 28.56 ± 2.6 a | 29.39 ± 1.8 a | 26.8 ± 2.4 ab |
| MBC (mg kg−1) | 396.8 ± 26.03 a | 402.06 ± 7.07 a | 241.52 ± 13.4 b | 121.08 ± 10.59 c |
| Soil C/N | 17.7 ± 2.01 a | 16.79 ± 1.47 ab | 14.74 ± 2.16 ab | 13.24 ± 1.61 b |
| Yield (kg plant−1) | 0.66 ± 0.06 b | 1.16 ± 0.38 a | 0.76 ± 0.04 ab | 0.39 ± 0.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, S.; Bian, T.; Song, Q.; Wu, G.; Awais, M.; Liu, Y.; Fu, H.; Sun, Z. Effects of Nitrogen Addition on Soil Microbial Functional Diversity and Extracellular Enzyme Activities in Greenhouse Cucumber Cultivation. Agriculture 2022, 12, 1366. https://doi.org/10.3390/agriculture12091366
Wang Z, Wang S, Bian T, Song Q, Wu G, Awais M, Liu Y, Fu H, Sun Z. Effects of Nitrogen Addition on Soil Microbial Functional Diversity and Extracellular Enzyme Activities in Greenhouse Cucumber Cultivation. Agriculture. 2022; 12(9):1366. https://doi.org/10.3390/agriculture12091366
Chicago/Turabian StyleWang, Zhen, Shuang Wang, Ting Bian, Qiaobo Song, Guorui Wu, Muhammad Awais, Yufeng Liu, Hongdan Fu, and Zhouping Sun. 2022. "Effects of Nitrogen Addition on Soil Microbial Functional Diversity and Extracellular Enzyme Activities in Greenhouse Cucumber Cultivation" Agriculture 12, no. 9: 1366. https://doi.org/10.3390/agriculture12091366






