Do Metals Increase or Decrease Nitrous Oxide Emissions and Maize Yields from Upland Soils?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Field Management
2.3. Measurement of N2O Emission
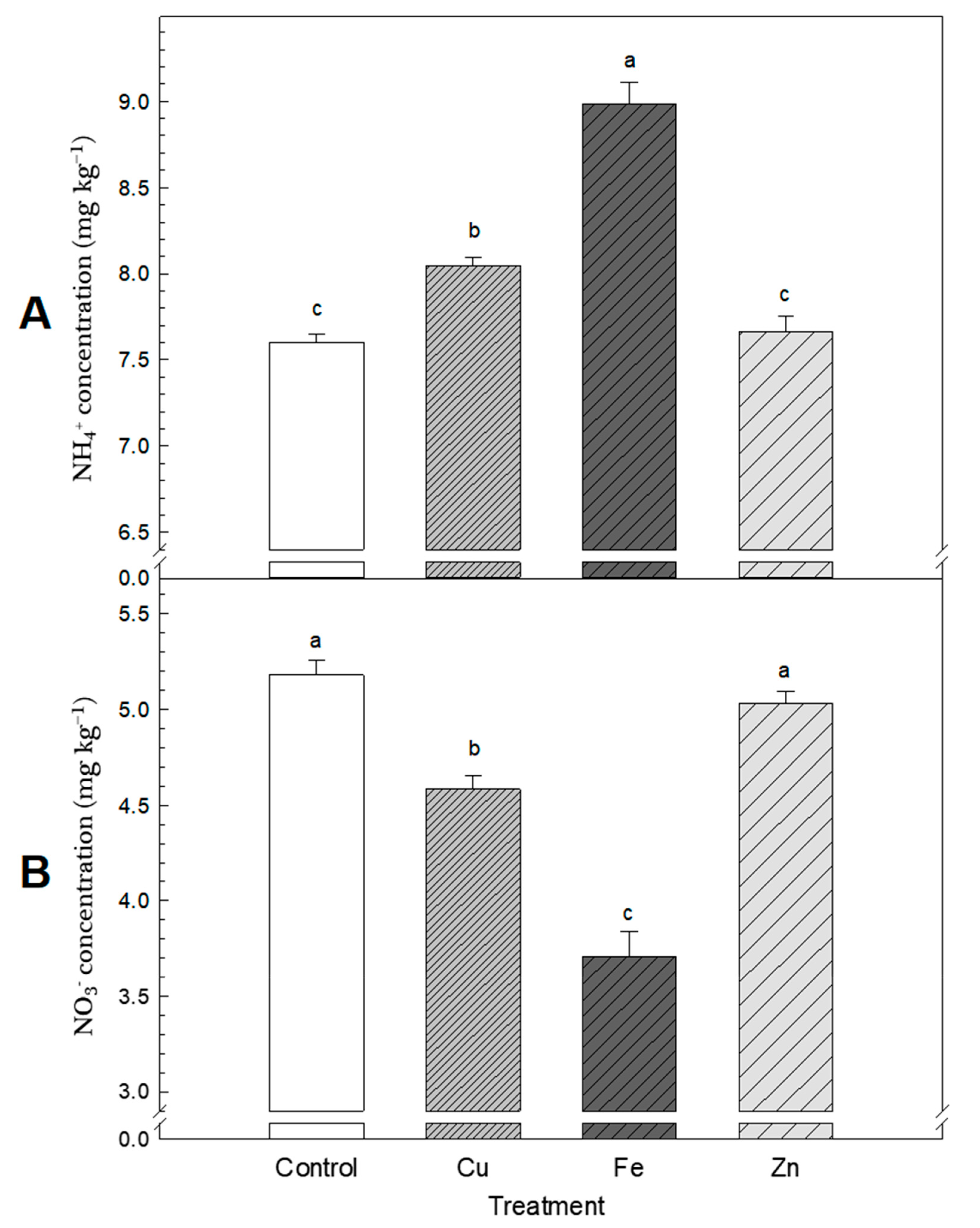
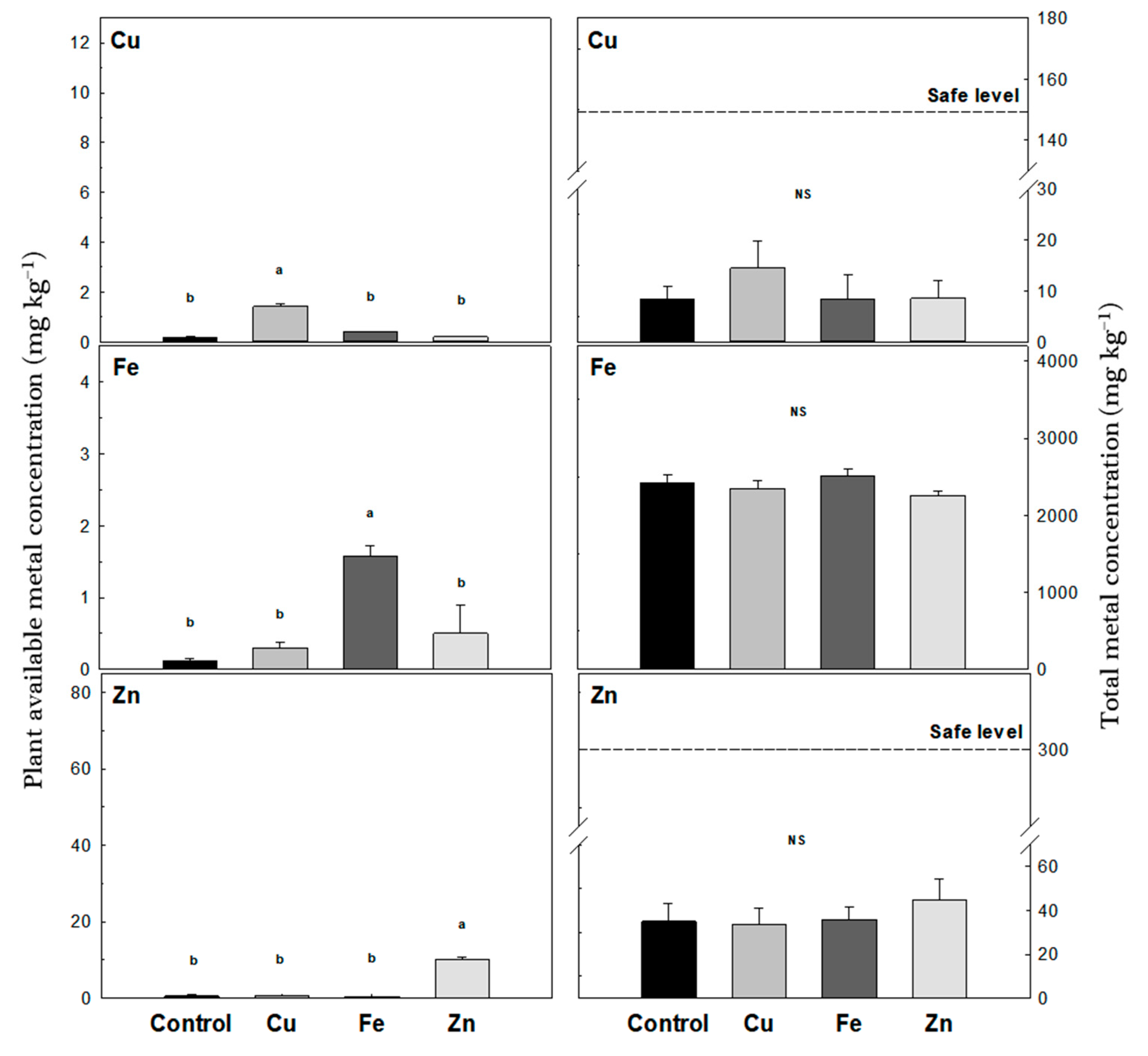
2.4. Physical and Chemical Analyses
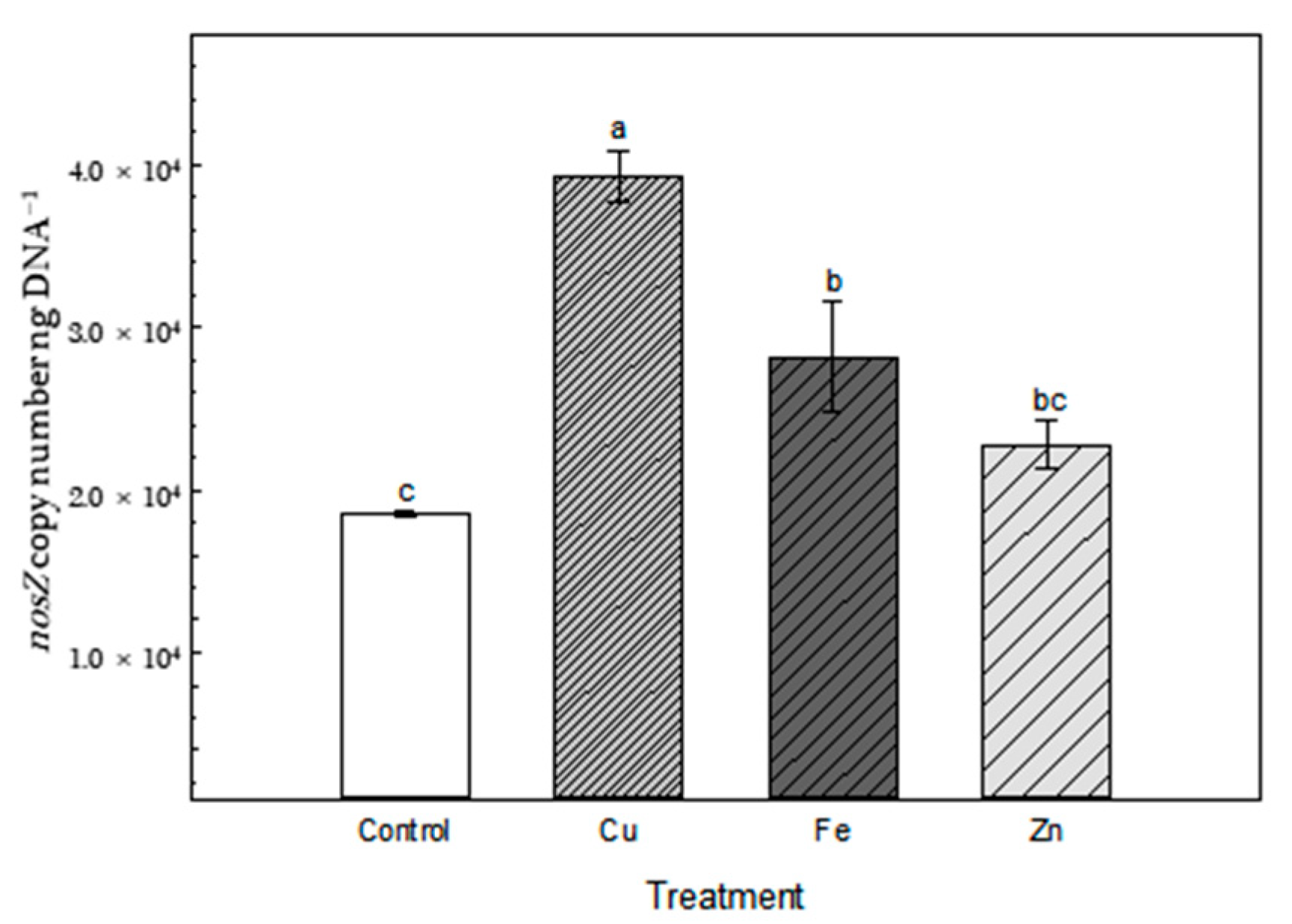
2.5. Total DNA Extraction and Real-Time PCR
2.6. Statistical Analysis
3. Results and Discussion
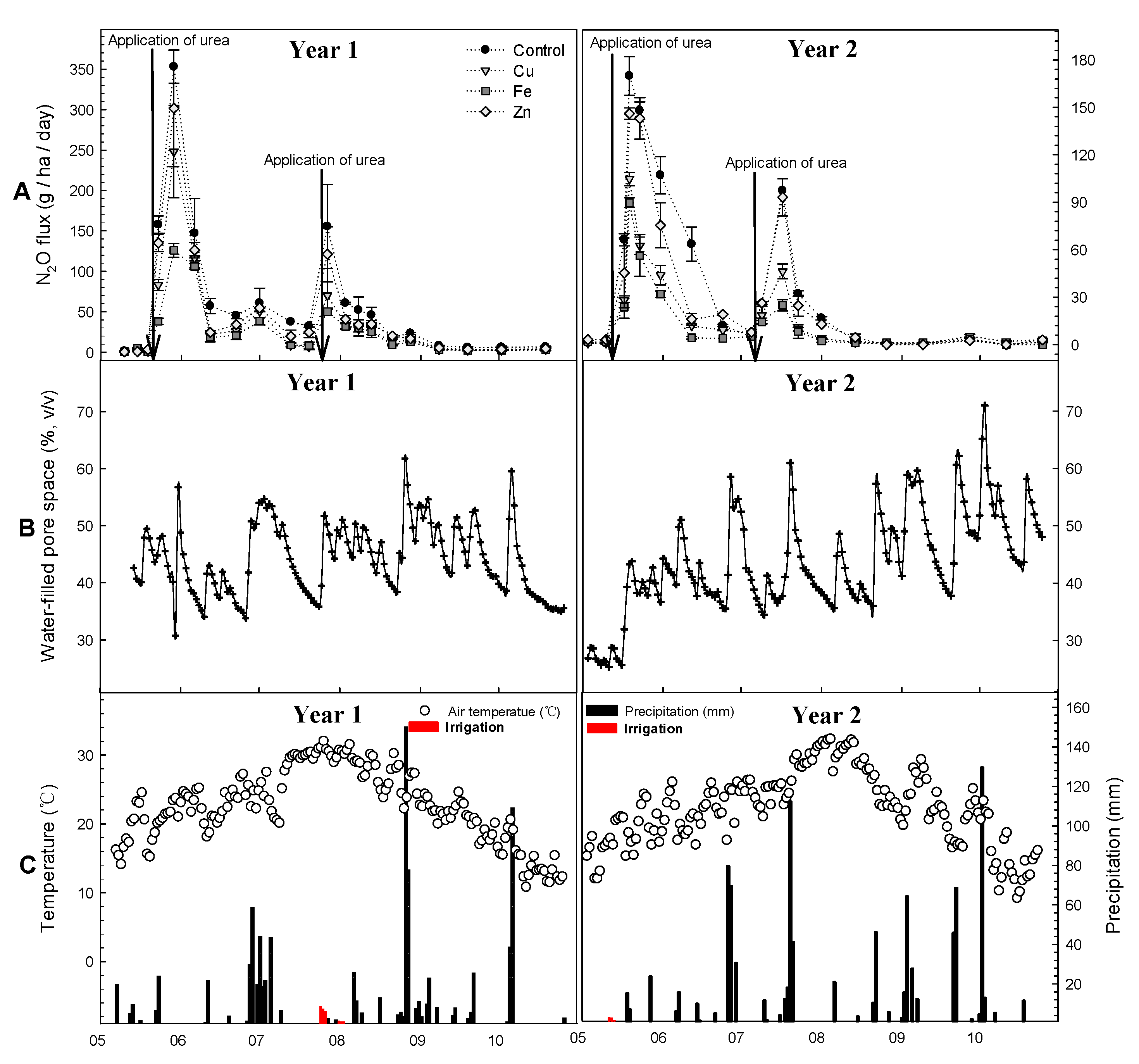
3.1. N2O Flux
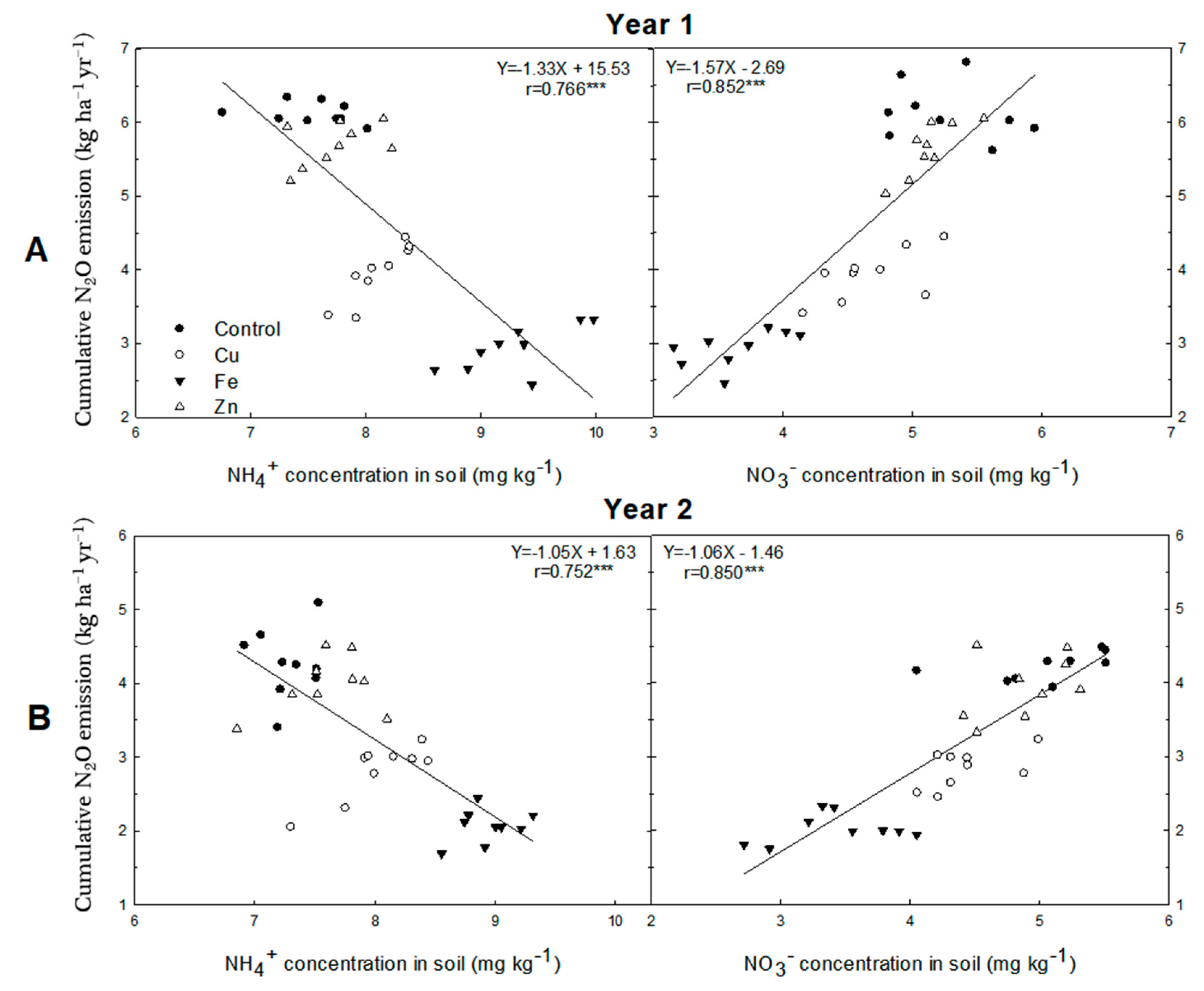
3.2. Cumulative N2O Emission
3.3. Yield-Scaled N2O Emission
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Calvo, E.; Guendehou, S.; Limmeechokchai, B.; Pipatti, R.; Rojas, Y.; Sturgiss, R.; Tanabe, K.; Wirth, T. Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019; Volume 5, p. 194.
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. Ser. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.R.; Cassman, N.A.; Kielak, A.M.; Pijl, A.; Carmo, J.B.; Lourenço, K.S.; Kuramae, E.E. Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci. Rep. 2016, 6, 30349. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, S. Relation of available micronutrients in soils and plants. J. Indian Soc. Soil Sci. 1996, 44, 800–802. [Google Scholar]
- Glass, J.B.; Orphan, V.J. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Igarashi, N.; Moriyama, H.; Fujiwara, T.; Fukumori, Y.; Tanaka, N. The 2.8 Å structure of hydroxylamine oxidoreductase from a nitrifying chemoautotrophic bacterium, Nitrosomonas europaea. Nat. Struct. Biol. 1997, 4, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Cantera, J.J.L.; Stein, L.Y. Molecular diversity of nitrite reductase genes (nirK) in nitrifying bacteria. Environ. Microbiol. 2007, 9, 765–776. [Google Scholar] [CrossRef]
- Cantera, J.J.L.; Stein, L.Y. Role of nitrite reductase in the ammonia-oxidizing pathway of Nitrosomonas europaea. Arch. Microbiol. 2007, 188, 349–354. [Google Scholar] [CrossRef]
- Casciotti, K.L.; Ward, B.B. Dissimilatory nitrite reductase genes from autotrophic ammoniaoxidizing bacteria. Appl. Environ. Microbiol. 2001, 67, 2213–2221. [Google Scholar] [CrossRef]
- Beaumont, H.J.E.; Van Schooten, B.; Lens, S.I.; Westerhoff, H.V.; Van Spanning, R.J.M. Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J. Bacteriol. 2004, 186, 4417–4421. [Google Scholar] [CrossRef]
- Casciotti, K.L.; Ward, B.B. Phylogenetic analysis of nitric oxide reductase gene homologues from aerobic ammonia oxidizing bacteria. FEMS Microbiol. Ecol. 2005, 52, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, A.K.; Hooper, A.B.; Hendrich, M.P. NO reductase activity of the tetraheme cytochrome c554 of Nitrosomonas europaea. J. Am. Chem. Soc. 2006, 128, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y. Surveying N2O producing pathways in bacteria. Methods Enzymol. 2011, 486, 131–152. [Google Scholar] [PubMed]
- Brown, K.; Tegoni, M.; Prudêncio, M.; Pereira, A.; Besson, S.; Moura, J.; Cambillau, C. A novel type of catalytic copper cluster in nitrous oxide reductase. Nat. Struct. Biol. 2000, 7, 191–195. [Google Scholar] [CrossRef]
- Rosenzweig, A.C. Nitrous oxide reductase from CuA to CuZ. Nat. Struct. Biol. 2000, 7, 169–171. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Chen, H.; Li, X.; Peng, Y.; Wang, S. Minimizing nitrous oxide in biological nutrient removal from municipal wastewater by controlling copper ion concentrations. Appl. Microbiol. Biotechnol. 2013, 97, 1325–1334. [Google Scholar] [CrossRef]
- Sharma, N.; Flynn, E.D.; Catalano, J.G.; Giammar, D.E. Copper availability governs nitrous oxide accumulation in wetland soils and stream sediments. Geochim. Cosmochim. Acta 2022, 327, 96–115. [Google Scholar] [CrossRef]
- Arezoo, T.T.; Tim, C.; Søren, O.P.; Lars, E. Nitrous oxide dynamics in agricultural peat soil in response to availability of nitrate, nitrite, and iron sulfides. Geomicrobiol. J. 2020, 37, 76–85. [Google Scholar]
- Deng, S.; Peng, S.; Ngo, H.H.; Jin-An Oh, S.; Hu, Z.; Yao, H.; Li, D. Characterization of nitrous oxide and nitrite accumulation during iron (Fe (0))- and ferrous iron (Fe(II))-driven autotrophic denitrification: Mechanisms, environmental impact factors and molecular microbial characterization. Chem. Eng. J. 2022, 438, 135627. [Google Scholar] [CrossRef]
- Montoya, M.; Guardia, G.; Recio, J.; Castellano-Hinojosa, A.; Ginés, C.; Bedmar, E.; Álvarez, J.M.; Vallejo, A. Zinc-nitrogen co-fertilization influences N2O emissions and microbial communities in an irrigated maize field. Geoderma 2021, 383, 114735. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, Y.; Yao, H.; Ge, C. Effect of zinc oxide nanoparticles on nitrous oxide emissions in agricultural soil. Agriculture 2021, 11, 730. [Google Scholar] [CrossRef]
- Braun, T. Biopolitics and the molecularization of life. Cult. Geogr. 2007, 13, 6–28. [Google Scholar] [CrossRef]
- Frank, A.; Howe, G.T.; Sperisen, C.; Brang, P.; St. Clair, J.B.; Schmatz, D.R.; Heiri, C. Risk of genetic maladaption due to climate change in three major European species. Glob. Chang. Biol. 2017, 23, 5358–5371. [Google Scholar] [CrossRef] [PubMed]
- Epstain, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates, an Imprint of Oxford University Press: Oxford, UK, 2005; p. 52. [Google Scholar]
- Guo, X.Y.; Zuo, Y.B.; Wang, B.R.; Li, J.M.; Ma, Y.B. Toxicity and accumulation of copper and nickel in maize plants cropped on calcareous and acidic field soils. Plant Soil 2010, 333, 365–373. [Google Scholar] [CrossRef]
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Takkar, P.N.; Mann, M.S. Toxic levels of soil and plant zinc for maize and wheat. Plant Soil 1978, 49, 667–669. [Google Scholar] [CrossRef]
- Conen, F.; Smith, K.A. A re-examination of closed flux chamber methods for the measurement of trace gas emissions from soils to the atmosphere. Eur. J. Soil Sci. 1998, 49, 701–707. [Google Scholar] [CrossRef]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Wolf, B. Determination of nitrate, nitrite, and Ammonium Nitrogen rapid photometric determination in soil and plant extracts. J. Ind. Eng. Chem. 1944, 16, 446–447. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.C.; Hooper, A.B.; Klotz, M.G.; Woebken, D.; Lam, P.; Kuypers, M.M.M.; Jetten, M.S.M. Environmental detection of octahaem cytochromechydroxylamine/hydrazine oxidoreductase genes of aerobic and anaerobic ammonium-oxidizing bacteria. Environ. Microbiol. 2008, 10, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- López-Gutiérrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef] [PubMed]
- Braker, G.; Tiedje, J.M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosz gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006, 72, 5181–5189. [Google Scholar] [CrossRef]
- Klappenbach, J.A.; Saxman, P.R.; Cole, J.R.; Schmidt, T.M. rrndb: The ribosomal RNA Operon copy number database. Nucleic Acids Res. 2000, 29, 181–184. [Google Scholar] [CrossRef]
- Shen, J.; Treu, R.; Wang, J.; Nicholson, F.; Bhogal, A.; Thorman, R. Modeling nitrous oxide emissions from digestate and slurry applied to three agricultural soils in the United Kingdom: Fluxes and emission factors. Environ. Pollut. 2018, 243, 1952–1965. [Google Scholar] [CrossRef]
- Westphal, M.; Tenuta, M.; Entz, M.H. Nitrous oxide emissions with organic crop production depends on fall soil moisture. Agric. Ecosyst. Environ. 2018, 254, 41–49. [Google Scholar] [CrossRef]
- Gregorutti, V.C.; Caviglia, O.P. Nitrous oxide emission after the addition of organic residues on soil surface. Agric. Ecosyst. Environ. 2017, 246, 234–242. [Google Scholar] [CrossRef]
- Kuang, W.; Gao, X.; Gui, D.; Tenuta, M.; Flaten, D.N.; Yin, M.; Zeng, F. Effects of fertilizer and irrigation management on nitrous oxide emission from cotton fields in an extremely arid region of northwestern China. Field Crop. Res. 2018, 229, 17–26. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Q.; Noll, L.; Hu, Y.; Wanek, W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2019, 135, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L. Chemical Equilibria in Soils; The Blackburn Press: Caldwell, NJ, USA, 1979; p. 32. [Google Scholar]
- Dalsgaard, T.; Bak, F. Nitrate reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil:sulfide inhibition, kinetics, and regulation. Appl. Environ. Microbiol. 1994, 60, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Sahrawat, K.L. Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur. J. Soil Sci. 2001, 52, 667–673. [Google Scholar] [CrossRef]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204–218. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Anna, G.; Karolina, G.; Jarosław, G.; Magdalena, F.; Jerzy, K. Microbial community diversity and the interaction of soil under maize growth in different cultivation techniques. Plant Soil Environ. 2017, 63, 264–270. [Google Scholar] [CrossRef]
- Huang, T.; Yang, H.; Huang, C.; Ju, X. Effect of fertilizer N rates and straw management on yield-scaled nitrous oxide emissions in a maize-wheat double cropping system. Field Crops Res. 2017, 204, 1–11. [Google Scholar] [CrossRef]
- Ni, K.; Ding, W.; Zaman, M. Nitrous oxide emissions from a rainfed-cultivated black soil in Northeast China: Effect of fertilization and maize crop. Biol. Fertil. Soils 2012, 48, 973–979. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Del Grosso, S.J.; Alluvione, F. nitrogen source effects on nitrous oxide emissions from irrigated no-till corn. J. Environ. Qual. 2010, 39, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Loick, N.; Dixon, E.; Matthews, G.P.; Müller, C.; Ciganda, V.S.; López-Aizpún, M.; Repullo, M.A.; Cardenas, L.M. Application of a triple 15N tracing technique to elucidate N transformations in a UK grassland soil. Geoderma 2021, 385, 114844. [Google Scholar] [CrossRef]
- Shahid, M.; Shukla, A.K.; Bhattacharyya, P.; Tripathi, R.; Mohanty, S.; Kumar, A.; Lal, B.; Gautam, P.; Raja, R.; Panda, B.B.; et al. Micronutrients (Fe, Mn, Zn and Cu) balance under long-term application of fertilizer and manure in a tropical rice-rice system. J. Soils Sediments 2016, 16, 737–747. [Google Scholar] [CrossRef]
- Hall, S.J.; Matson, P.A.; Roth, P.M. NOx Emissions from soil: Implications for air quality modeling in agricultural regions. Annu. Rev. Energy Environ. 1996, 21, 311–346. [Google Scholar] [CrossRef]
- Ruangcharus, C.; Kim, S.U.; Yoo, G.Y.; Choi, E.J.; Kumar, S.; Kang, N.G.; Hong, C.O. Nitrous oxide emission and sweet potato yield in upland soil: Effects of different type and application rate of composted animal manures. Environ. Pollut. 2021, 279, 116892. [Google Scholar] [CrossRef]

| Parameter | Mean ± Standard Deviation |
|---|---|
| pH (1:5, H2O) | 6.72 ± 0.38 |
| Organic matter (g kg−1) | 26.6 ± 1.33 |
| Total nitrogen (g kg−1) | 1.09 ± 0.02 |
| Inorganic nitrogen | |
| NH4+ (mg kg−1) | 3.38 ± 0.23 |
| NO3− (mg kg−1) | 1.07 ± 0.29 |
| Available P2O5 (mg kg−1) | 113 ± 2.33 |
| Exchangeable cation (cmolc kg−1) | |
| K | 0.73 ± 0.05 |
| Ca | 4.20 ± 0.30 |
| Mg | 1.17 ± 0.07 |
| Bulk density (g cm−3) | 1.27 ± 0.12 |
| Particle size distribution (%) | |
| Sand | 43.4 ± 2.27 |
| Silt | 44.5 ± 3.15 |
| Clay | 12.1 ± 1.01 |
| Soil texture | Sandy clay loam |
| Primer | Sequence (5′→3′) | Size (bp) | Reference |
|---|---|---|---|
| 16s rRNA-1097F | CGGCAACGAGCGCAACCC | 146 | [32] |
| 16s rRNA-1242R | CCATTGTAGCACGTGTGTAGCC | ||
| amoA-1F | GGGGTTTCTACTGGTGGT | 491 | [33] |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | ||
| hao-1F | TGCGTGGAAGTGCTCAC | 992 | [34] |
| hao-3R | AGAGTAAGGAGTCTCGGGCAAA | ||
| narG-2F | TA(CT) GT(GC) GGG CAG GA(AG) AAA CTG | 110 | [35] |
| narG-2R | CGTAGAAGAAGCTGGTGCTGTT | ||
| nirS-1F | CCTAYTGGCCGCCRCART | 890 | [36] |
| nirS-6R | CGTTGAACTTRCCGGT | ||
| cnorB-2F | GACAAGNNNTACTGGTGGT | 389 | [37] |
| cnorB-6R | GAANCCCCANACNCCNGC | ||
| nosZ-1F | WCSYTGTTCMTCGACAGCCAG | 700 | [38] |
| nosZ-2R | CAKRTGCAKSGCRTGGCAGAA |
| Parameter | Source of Variation | ||
|---|---|---|---|
| Metal (M) | Year (Y) | M × Y | |
| df | 3 | 1 | 3 |
| Cumulative N2O emissions | <0.001 | <0.01 | <0.001 |
| NH4+ | <0.001 | NS | NS |
| NO3− | <0.001 | NS | NS |
| 16S rRNA | <0.001 | - | - |
| amoA | <0.01 | - | - |
| hao | NS § | - | - |
| narG | NS | - | - |
| nirS | NS | - | - |
| cnorB | NS | - | - |
| nosZ | <0.001 | - | - |
| Maize grain yield | NS | NS | NS |
| Yield-scaled N2O emission | <0.01 | <0.05 | <0.01 |
| Metal | Cumulative N2O Emission (kg ha−1 yr−1) | ||
|---|---|---|---|
| Year 1 | Year 2 | Year Mean §§ | |
| Control | 6.13 a | 4.25 a | 5.19 a |
| Cu | 3.93 b | 2.81 b | 3.37 b |
| Fe | 2.97 c | 1.99 c | 2.48 b |
| Zn | 5.69 a | 3.96 a | 4.82 a |
| Metal mean § | 4.68 A | 3.25 B | |
| Metal | Maize Grain Yield (Mg ha−1) | ||
| Year 1 | Year 2 | Year Mean§§ | |
| Control | 5.77 a | 5.83 a | 5.80 a |
| Cu | 5.85 a | 5.85 a | 5.85 a |
| Fe | 5.06 a | 5.06 a | 5.06 a |
| Zn | 5.87 a | 5.87 a | 5.87 a |
| Metal mean § | 5.64 A | 5.65 A | |
| Metal | Yield-Scaled N2O Emission (kg Mg−1) | ||
| Year 1 | Year 2 | Year Mean§§ | |
| Control | 1.22 a | 1.03 a | 1.12 a |
| Cu | 0.68 c | 0.41 b | 0.55 b |
| Fe | 0.57 c | 0.31 b | 0.44 b |
| Zn | 0.95 b | 0.61 b | 0.78 ab |
| Metal mean § | 0.86 A | 0.59 B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.L.; Lee, H.H.; Kim, S.U.; Kang, N.; Hong, C.O. Do Metals Increase or Decrease Nitrous Oxide Emissions and Maize Yields from Upland Soils? Agriculture 2022, 12, 1458. https://doi.org/10.3390/agriculture12091458
Park YL, Lee HH, Kim SU, Kang N, Hong CO. Do Metals Increase or Decrease Nitrous Oxide Emissions and Maize Yields from Upland Soils? Agriculture. 2022; 12(9):1458. https://doi.org/10.3390/agriculture12091458
Chicago/Turabian StylePark, Ye Lim, Hyun Ho Lee, Sung Un Kim, Namgoo Kang, and Chang Oh Hong. 2022. "Do Metals Increase or Decrease Nitrous Oxide Emissions and Maize Yields from Upland Soils?" Agriculture 12, no. 9: 1458. https://doi.org/10.3390/agriculture12091458
APA StylePark, Y. L., Lee, H. H., Kim, S. U., Kang, N., & Hong, C. O. (2022). Do Metals Increase or Decrease Nitrous Oxide Emissions and Maize Yields from Upland Soils? Agriculture, 12(9), 1458. https://doi.org/10.3390/agriculture12091458







