Comprehensive Analysis of lncRNA and mRNA Reveals the Effect of ZBED6 on Spleen Growth in Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Slaughtering Experiment
2.2. Histological Analysis
2.3. Total RNA Extraction and RNA Sequencing
2.4. RNA-Seq Analysis
2.5. lncRNAs Analysis
2.6. lncRNA Target Gene Prediction
2.7. Clustering and Correlation and GO Enrichment Analysis
2.8. Association Analysis of lncRNA and mRNA
2.9. qRT-PCR
2.10. Statistical Analysis
3. Results
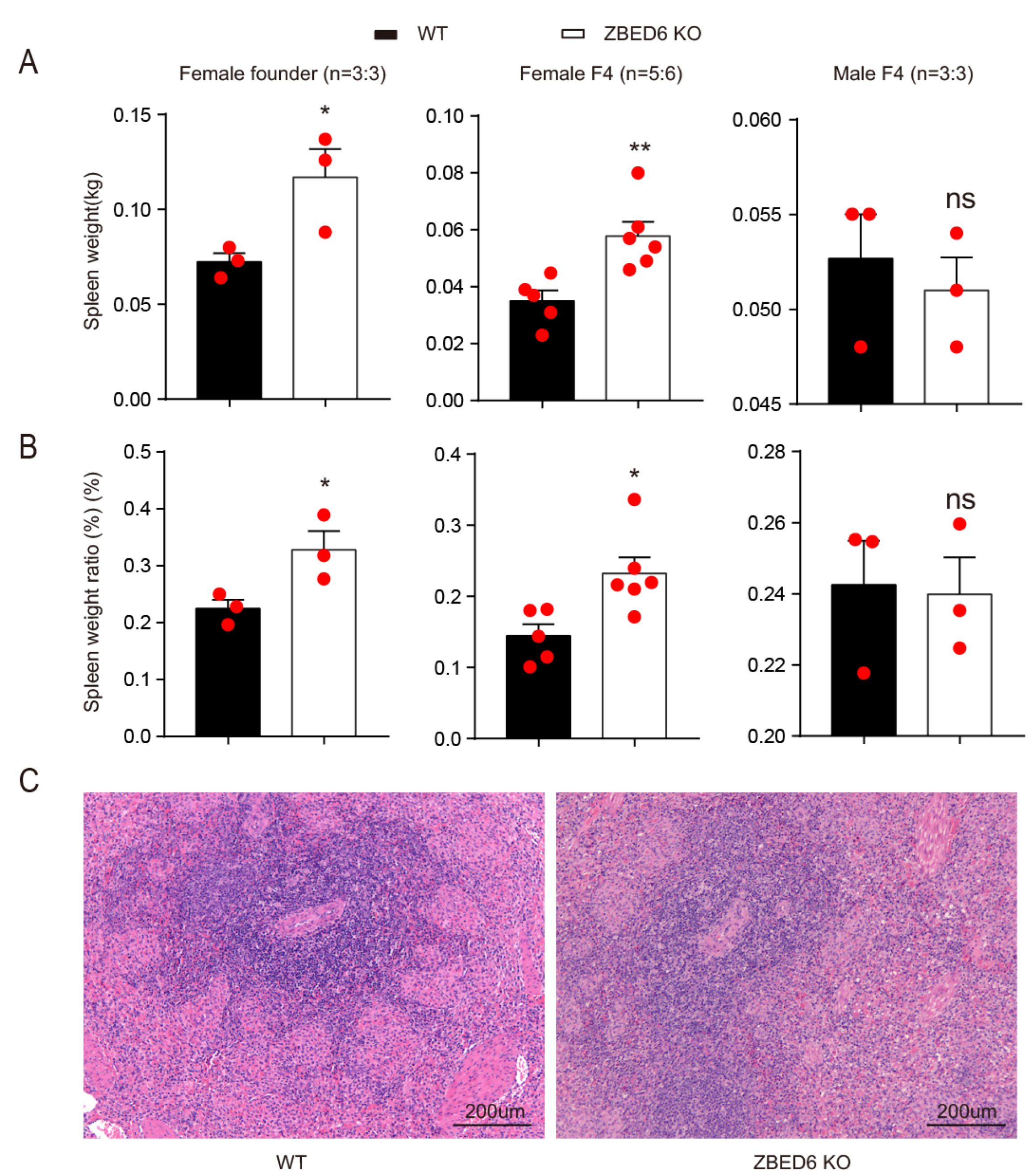
3.1. ZBED6 Knockout Promotes Pig Spleen Growth
3.2. Whole-Transcriptome Sequencing Results
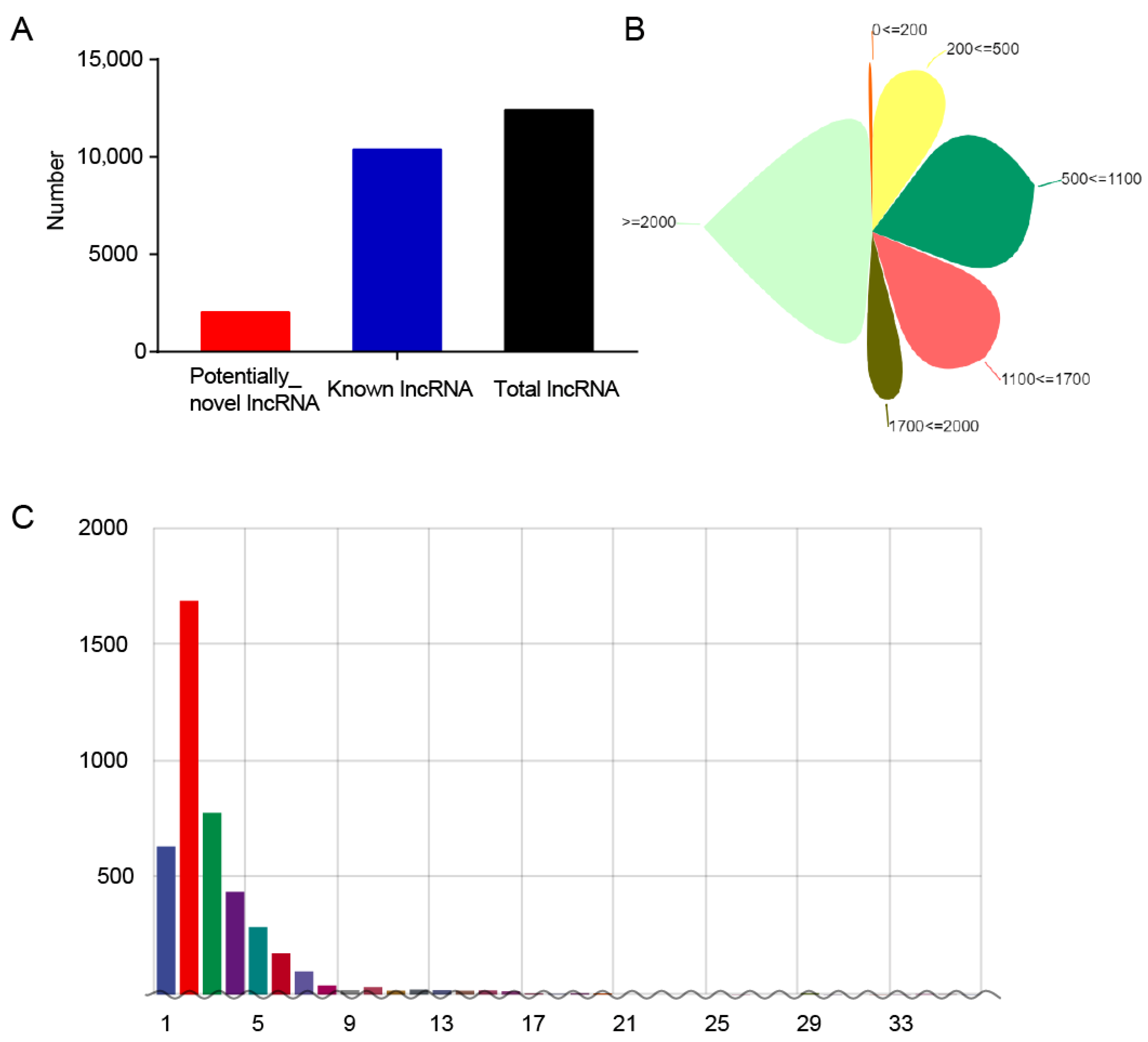
3.3. Identification and Characteristics of lncRNAs
3.4. Analysis of DEGs and DE-lncRNAs
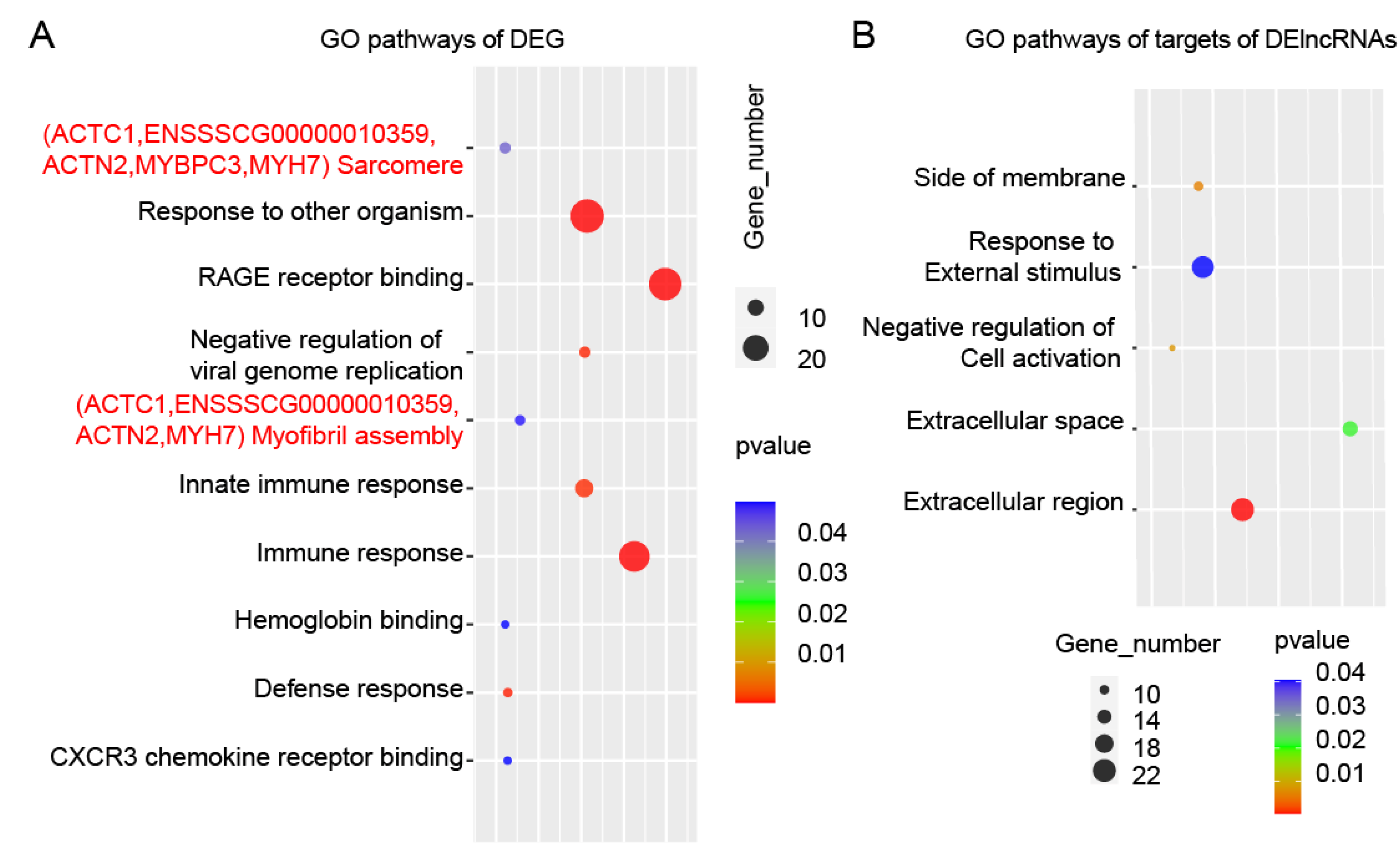
3.5. Prediction of DE-lncRNA Target Genes
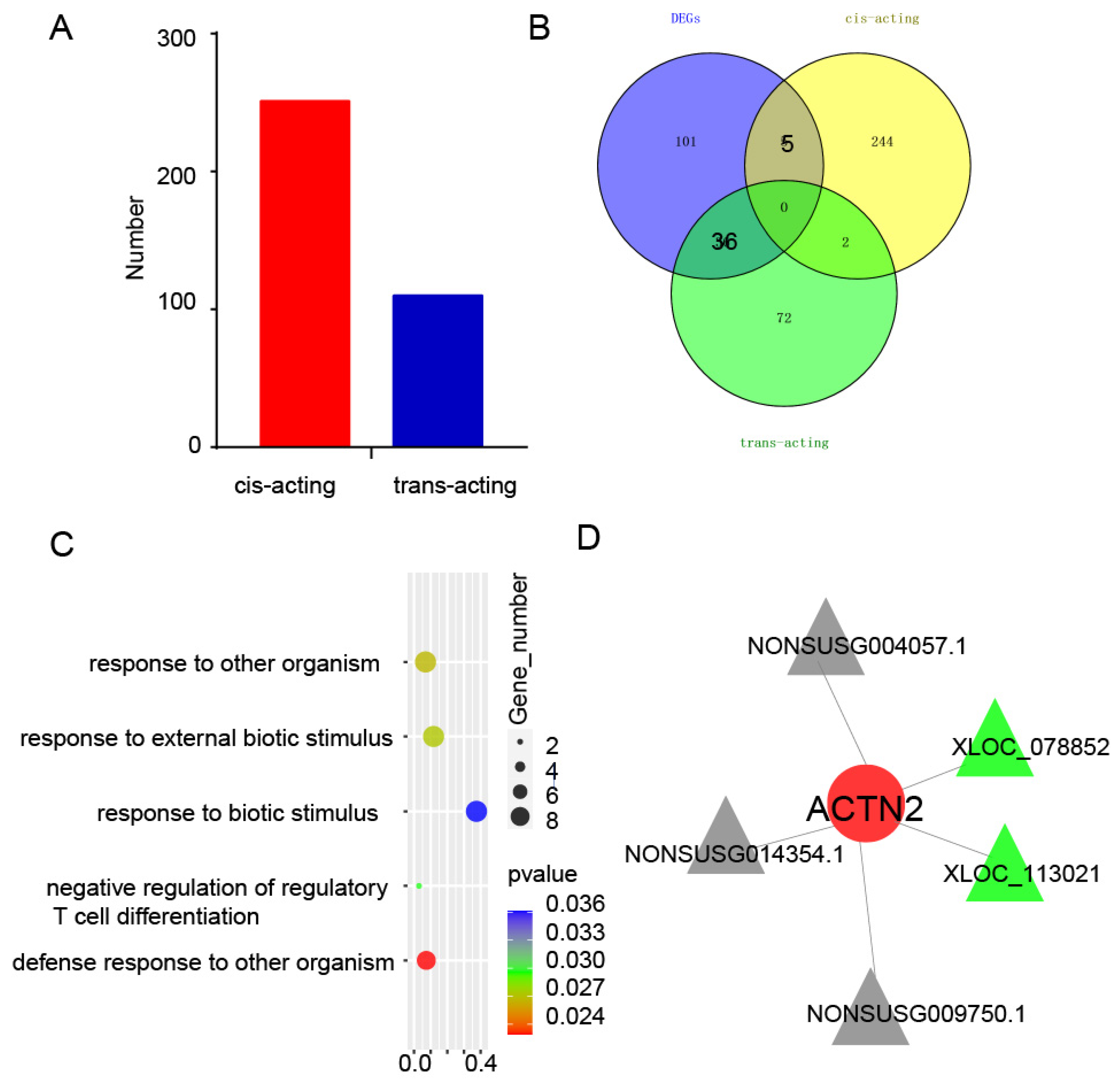
3.6. Association Analysis of lncRNA and mRNA
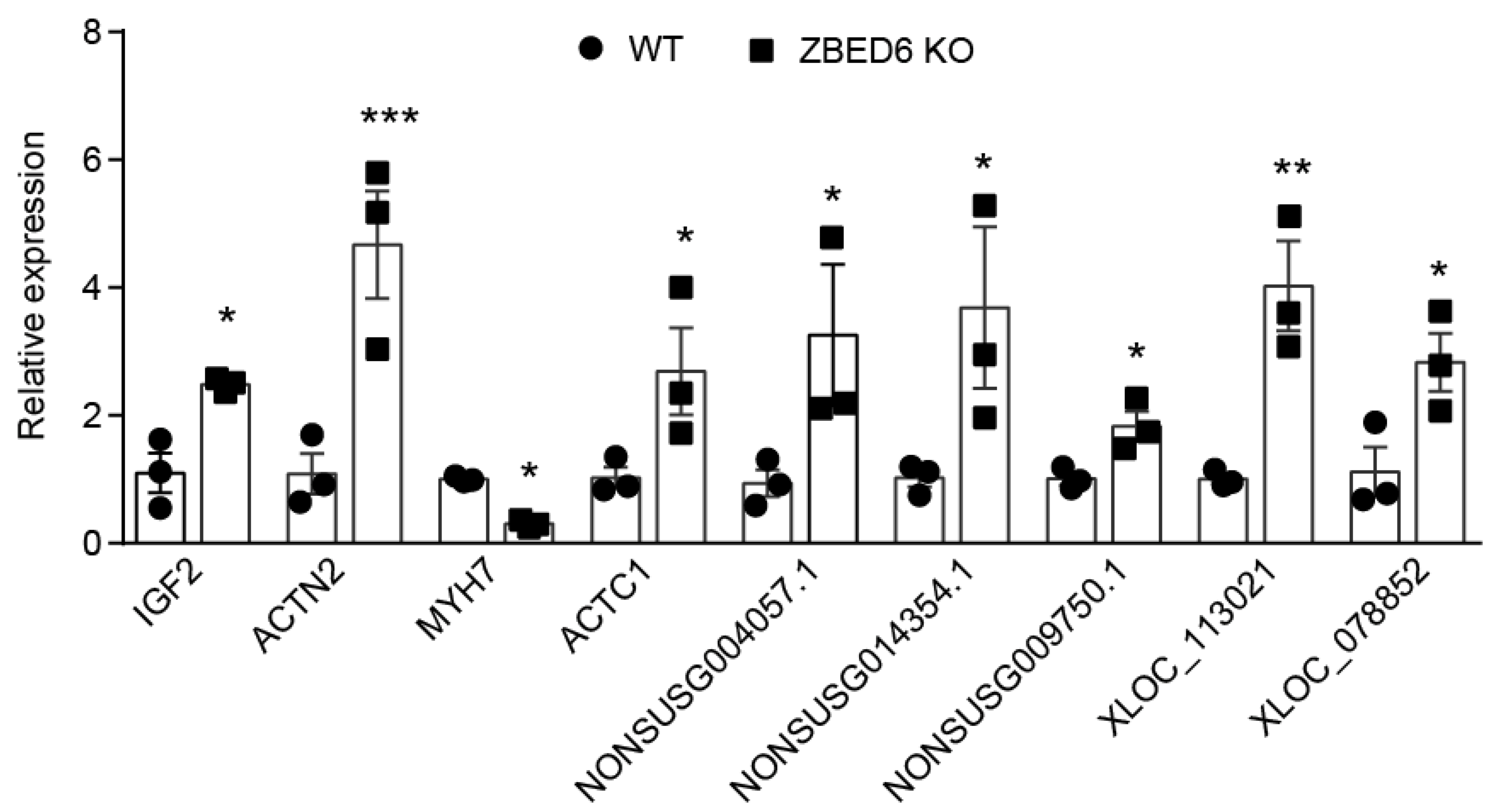
3.7. RT-qPCR Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The Reference Human Genome Annotation for the ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin Signature Reveals over a Thousand Highly Conserved Large Non-Coding RNAs in Mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique Features of Long Non-Coding RNA Biogenesis and Function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, W.F. Is Junk DNA Bunk? A Critique of ENCODE. Proc. Natl. Acad. Sci. USA 2013, 110, 5294–5300. [Google Scholar] [CrossRef]
- Zhao, W.; Mu, Y.; Ma, L.; Wang, C.; Tang, Z.; Yang, S.; Zhou, R.; Hu, X.; Li, M.H.; Li, K. Systematic Identification and Characterization of Long Intergenic Non-Coding RNAs in Fetal Porcine Skeletal Muscle Development. Sci. Rep. 2015, 5, 8957. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, G.; Niu, G.; Zhang, Y.; Zhou, R.; Wang, Y.; Mu, Y.; Tang, Z.; Li, K. Comparative Analysis of DNA Methylome and Transcriptome of Skeletal Muscle in Lean-, Obese-, and Mini-Type Pigs. Sci. Rep. 2017, 7, 39883. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.-F.; Ma, Q.; Mo, D.-L.; Sun, J.-J.; Ren, Q.-L.; Zhang, J.-Q.; Lu, Q.-X.; Xing, B.-S. Identification and Characterization of CircRNAs Related to Meat Quality during Embryonic Development of the Longissimus Dorsi Muscle in Two Pig Breeds. Front. Genet. 2022, 13, 1019687. [Google Scholar] [CrossRef]
- Curtis, D. Weighted Burden Analysis in 200,000 Exome-Sequenced Subjects Characterises Rare Variant Effects on BMI. Int. J. Obes. 2022, 46, 782–792. [Google Scholar] [CrossRef]
- Tripathi, V.; Shen, Z.; Chakraborty, A.; Giri, S.; Freier, S.M.; Wu, X.; Zhang, Y.; Gorospe, M.; Prasanth, S.G.; Lal, A.; et al. Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB. PLoS Genet. 2013, 9, e1003368. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Ye, S.; Liu, Y.; Chen, Q.; Guan, W.; Pu, Y.; Jiang, L.; He, X.; Ma, Y.; et al. Integrated Analysis of Lncrna and Mrna Reveals Novel Insights into Wool Bending in Zhongwei Goat. Animals 2021, 11, 3326. [Google Scholar] [CrossRef]
- Joo, M.S.; Shin, S.-B.; Kim, E.J.; Koo, J.H.; Yim, H.; Kim, S.G. Nrf2-LncRNA Controls Cell Fate by Modulating P53-Dependent Nrf2 Activation as an MiRNA Sponge for Plk2 and P21(Cip1). FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 7953–7969. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Min, L.; Qiu, X.; Wu, X.; Liu, C.; Ma, J.; Zhang, D.; Zhu, L. Biological Function of Long Non-Coding RNA (LncRNA) Xist. Front. Cell Dev. Biol. 2021, 9, 645647. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, K.; Shan, B.; Wei, S.; Li, D.; Han, H.; Wei, W.; Chen, J.; Liu, H.; Zhang, L. A Genome-Wide Landscape of MRNAs, LncRNAs, and CircRNAs during Subcutaneous Adipogenesis in Pigs. J. Anim. Sci. Biotechnol. 2018, 9, 76. [Google Scholar] [CrossRef]
- Markljung, E.; Jiang, L.; Jaffe, J.D.; Mikkelsen, T.S.; Wallerman, O.; Larhammar, M.; Zhang, X.; Wang, L.; Saenz-Vash, V.; Gnirke, A.; et al. ZBED6, a Novel Transcription Factor Derived from a Domesticated DNA Transposon Regulates IGF2 Expression and Muscle Growth. PLoS Biol. 2009, 7, e1000256. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.T.; Carlborg, O.; Törnsten, A.; Giuffra, E.; Amarger, V.; Chardon, P.; Andersson-Eklund, L.; Andersson, K.; Hansson, I.; Lundström, K.; et al. A Paternally Expressed QTL Affecting Skeletal and Cardiac Muscle Mass in Pigs Maps to the IGF2 Locus. Nat. Genet. 1999, 21, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Nezer, C.; Moreau, L.; Brouwers, B.; Coppieters, W.; Detilleux, J.; Hanset, R.; Karim, L.; Kvasz, A.; Leroy, P.; Georges, M. An Imprinted QTL with Major Effect on Muscle Mass and Fat Deposition Maps to the IGF2 Locus in Pigs. Nat. Genet. 1999, 21, 155–156. [Google Scholar] [CrossRef]
- Van Laere, A.-S.; Nguyen, M.; Braunschweig, M.; Nezer, C.; Collette, C.; Moreau, L.; Archibald, A.L.; Haley, C.S.; Buys, N.; Tally, M.; et al. A Regulatory Mutation in IGF2 Causes a Major QTL Effect on Muscle Growth in the Pig. Nature 2003, 425, 832–836. [Google Scholar] [CrossRef]
- Jiang, L.; Wallerman, O.; Younis, S.; Rubin, C.-J.; Gilbert, E.R.; Sundström, E.; Ghazal, A.; Zhang, X.; Wang, L.; Mikkelsen, T.S.; et al. ZBED6 Modulates the Transcription of Myogenic Genes in Mouse Myoblast Cells. PLoS One 2014, 9, e94187. [Google Scholar] [CrossRef]
- Younis, S.; Schönke, M.; Massart, J.; Hjortebjerg, R.; Sundström, E.; Gustafson, U.; Björnholm, M.; Krook, A.; Frystyk, J.; Zierath, J.R.; et al. The ZBED6-IGF2 Axis Has a Major Effect on Growth of Skeletal Muscle and Internal Organs in Placental Mammals. Proc. Natl. Acad. Sci. USA 2018, 115, E2048–E2057. [Google Scholar] [CrossRef]
- Zou, H.; Yu, D.; Yao, S.; Ding, F.; Li, J.; Li, L.; Li, X.; Zhao, S.; Pang, Y.; Hao, H.; et al. Efficient Editing of the ZBED6-Binding Site in Intron 3 of IGF2 in a Bovine Model Using the CRISPR/Cas9 System. Genes 2022, 13, 1132. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Jiang, X.; Wang, W.; Chen, Y.Q. Free Fatty Acid Receptor 4 Deletion Attenuates Colitis by Modulating Treg Cells via ZBED6-IL33 Pathway. EBioMedicine 2022, 80, 104060. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pan, D.; Xie, B.; Wang, S.; Xing, X.; Liu, X.; Ma, Y.; Andersson, L.; Wu, J.; Jiang, L. Porcine ZBED6 Regulates Growth of Skeletal Muscle and Internal Organs via Multiple Targets. PLoS Genet. 2021, 17, e90972. [Google Scholar] [CrossRef]
- Wang, D.; Pu, Y.; Li, Y.; Pan, D.; Wang, S.; Tian, W.; Ma, Y.; Jiang, L. Comprehensive Analysis of LncRNAs Involved in Skeletal Muscle Development in ZBED6-Knockout Bama Pigs. BMC Genomics 2021, 22, 593. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, W.; Pan, D.; Liu, L.; Xu, C.; Ma, Y.; Wang, D.; Jiang, L. A Comprehensive Analysis of the Myocardial Transcriptome in ZBED6-Knockout Bama Xiang Pigs. Genes 2022, 13, 1382. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wang, M.; Li, R.; Zeng, J.; Mo, D.; Cong, P.; Liu, X.; Chen, Y.; He, Z. Disruption of the ZBED6 Binding Site in Intron 3 of IGF2 by CRISPR/Cas9 Leads to Enhanced Muscle Development in Liang Guang Small Spotted Pigs. Transgenic Res. 2019, 28, 141–150. [Google Scholar] [CrossRef]
- Xiang, G.; Ren, J.; Hai, T.; Fu, R.; Yu, D.; Wang, J.; Li, W.; Wang, H.; Zhou, Q. Editing Porcine IGF2 Regulatory Element Improved Meat Production in Chinese Bama Pigs. Cell. Mol. Life Sci. 2018, 75, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Zhan, Z.-Y.; Sun, Y.-J.; Wang, J.; Li, M.-X.; Lan, X.-Y.; Lei, C.-Z.; Zhang, C.-L.; Chen, H. Comparative Analysis of the IGF2 and ZBED6 Gene Variants and Haplotypes Reveals Significant Effect of Growth Traits in Cattle. Genome 2013, 56, 327–334. [Google Scholar] [CrossRef]
- Akhtar Ali, M.; Younis, S.; Wallerman, O.; Gupta, R.; Andersson, L.; Sjöblom, T. Transcriptional Modulator ZBED6 Affects Cell Cycle and Growth of Human Colorectal Cancer Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 7743–7748. [Google Scholar] [CrossRef]
- Pauli, A.; Valen, E.; Lin, M.F.; Garber, M.; Vastenhouw, N.L.; Levin, J.Z.; Fan, L.; Sandelin, A.; Rinn, J.L.; Regev, A.; et al. Systematic Identification of Long Noncoding RNAs Expressed during Zebrafish Embryogenesis. Genome Res. 2012, 22, 577–591. [Google Scholar] [CrossRef]
- Ulitsky, I.; Shkumatava, A.; Jan, C.H.; Sive, H.; Bartel, D.P. Conserved Function of LincRNAs in Vertebrate Embryonic Development despite Rapid Sequence Evolution. Cell 2011, 147, 1537–1550. [Google Scholar] [CrossRef]
- Lee, J.T.; Bartolomei, M.S. X-Inactivation, Imprinting, and Long Noncoding RNAs in Health and Disease. Cell 2013, 152, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Wapinski, O.; Chang, H.Y. Long Noncoding RNAs and Human Disease. Trends Cell Biol. 2011, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yang, M.; Li, Y.; Rouzi, M.; Zhao, Q.; Pu, Y.; He, X.; Mwacharo, J.M.; Yang, N.; Ma, Y.; et al. Genome-Wide Discovery of LincRNAs with Spatiotemporal Expression Patterns in the Skin of Goat during the Cashmere Growth Cycle. BMC Genomics 2018, 19, 495. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Liang, B.; Zhao, Y.; Han, J.; Pu, Y.; Wang, C.; Ma, Y.; Jiang, L. Transcriptome Analysis Reveals Candidate Genes Regulating the Skin and Hair Diversity of Xinji Fine-wool Sheep and Tan Sheep. Agric. 2022, 12, 15. [Google Scholar] [CrossRef]
- Ling, Y.; Zheng, Q.; Sui, M.; Zhu, L.; Xu, L.; Zhang, Y.; Liu, Y.; Fang, F.; Chu, M.; Ma, Y.; et al. Comprehensive Analysis of LncRNA Reveals the Temporal-Specific Module of Goat Skeletal Muscle Development. Int. J. Mol. Sci. 2019, 20, 3950. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.N.; Zhou, X.-B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.-S.; Zhang, H.; et al. The Imprinted H19 LncRNA Antagonizes Let-7 MicroRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Ahmed, W.; Liu, Z.-F. Long Non-Coding RNAs: Novel Players in Regulation of Immune Response Upon Herpesvirus Infection. Front. Immunol. 2018, 9, 761. [Google Scholar] [CrossRef]
- Gloss, B.S.; Dinger, M.E. The Specificity of Long Noncoding RNA Expression. Biochim. Biophys. Acta 2016, 1859, 16–22. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, Y.; Du, W.; Fairchild, L.; Melnick, A.; Elemento, O. Transcriptome Sequencing Reveals Thousands of Novel Long Non-Coding RNAs in B Cell Lymphoma. Genome Med. 2015, 7, 110. [Google Scholar] [CrossRef]
- Lv, J.; Huang, Z.; Liu, H.; Liu, H.; Cui, W.; Li, B.; He, H.; Guo, J.; Liu, Q.; Zhang, Y.; et al. Identification and Characterization of Long Intergenic Non-Coding RNAs Related to Mouse Liver Development. Mol. Genet. Genomics 2014, 289, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhang, Y.; Zhang, T.; Han, J.; Ma, Y.; Liu, X. Identification of Novel LncRNAs Differentially Expressed in Placentas of Chinese Ningqiang Pony and Yili Horse Breeds. Animals 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Gotoda, Y.; Hatakeyama, A.; Nakagawa, T.; Miyatake, Y.; Kuroda, M.; Masumoto, S.; Tsutsumi, R.; Nakaya, Y.; Sakaue, H. Differential Regulation of Actn2 and Actn3 Expression during Unfolded Protein Response in C2C12 Myotubes. J. Muscle Res. Cell Motil. 2020, 41, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Masita Silviana, N.; Andarini, S.; Lyrawati, D.; Hidayat, M. Masticatory Functional Load Increases the MRNA Expression Levels of ACTN2 and ACTN3 and the Protein Expression of α-Actinin-2 in Rat Masseter Muscle. Turkish J. Pharm. Sci. 2021, 18, 28–33. [Google Scholar] [CrossRef]
- Lu, S.H.-A.; Lee, K.-Z.; Hsu, P.W.-C.; Su, L.-Y.; Yeh, Y.-C.; Pan, C.-Y.; Tsai, S.-Y. Alternative Splicing Mediated by RNA-Binding Protein RBM24 Facilitates Cardiac Myofibrillogenesis in a Differentiation Stage-Specific Manner. Circ. Res. 2022, 130, 112–129. [Google Scholar] [CrossRef]
- Tiso, N.; Majetti, M.; Stanchi, F.; Rampazzo, A.; Zimbello, R.; Nava, A.; Danieli, G.A. Fine Mapping and Genomic Structure of ACTN2, the Human Gene Coding for the Sarcomeric Isoform of Alpha-Actinin-2, Expressed in Skeletal and Cardiac Muscle. Biochem. Biophys. Res. Commun. 1999, 265, 256–259. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, S.; Tian, W.; Ma, Y.; Jiang, L. Comprehensive Analysis of lncRNA and mRNA Reveals the Effect of ZBED6 on Spleen Growth in Pigs. Agriculture 2023, 13, 108. https://doi.org/10.3390/agriculture13010108
Wang D, Wang S, Tian W, Ma Y, Jiang L. Comprehensive Analysis of lncRNA and mRNA Reveals the Effect of ZBED6 on Spleen Growth in Pigs. Agriculture. 2023; 13(1):108. https://doi.org/10.3390/agriculture13010108
Chicago/Turabian StyleWang, Dandan, Shengnan Wang, Wenjie Tian, Yuehui Ma, and Lin Jiang. 2023. "Comprehensive Analysis of lncRNA and mRNA Reveals the Effect of ZBED6 on Spleen Growth in Pigs" Agriculture 13, no. 1: 108. https://doi.org/10.3390/agriculture13010108
APA StyleWang, D., Wang, S., Tian, W., Ma, Y., & Jiang, L. (2023). Comprehensive Analysis of lncRNA and mRNA Reveals the Effect of ZBED6 on Spleen Growth in Pigs. Agriculture, 13(1), 108. https://doi.org/10.3390/agriculture13010108






