Effects of Barley- and Oat-Based Diets on Some Gut Parameters and Microbiota Composition of the Small Intestine and Ceca of Broiler Chicken
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Experimental Design
2.2. Diets and Experimental Design
2.3. Ileal Chyme Viscosity
2.4. Cecal SCFA
2.5. DNA Extraction, 16S rRNA Gene Amplification, and Illumina MiSeq Sequencing
2.6. Bioinformatics and Statistical Analyses
3. Results
3.1. Ileal Chyme Viscosity and Cecal SCFA Content
3.2. Microbial Diversity
3.3. Jejunal and Cecal Microbial Abundances
4. Discussion
4.1. Ileal Viscosity and Cecal SCFA Concentration
4.2. Diversity of Gut Microbiota
4.3. Effects of Dietary Treatments on the Composition of Intestinal Microbiota
4.3.1. Jejunum Content Microbiota
4.3.2. Jejunum Mucosa Microbiota
4.3.3. Cecum Content Microbiota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Svihus, B. The Gizzard: Function, Influence of Diet Structure and Effects on Nutrient Availability. World’s Poult. Sci. J. 2011, 67, 207–224. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; Frikha, M.; de Coca-Sinova, A.; García, J.; Mateos, G.G. Oat Hulls and Sugar Beet Pulp in Diets for Broilers 1. Effects on Growth Performance and Nutrient Digestibility. Anim. Feed Sci. Technol. 2013, 182, 33–43. [Google Scholar] [CrossRef]
- Jeroch, H.; Dusel, G.; Kluge, H.; Nonn, H. The Effectiveness of Microbial Xylanase in Piglet Rations Based on Wheat, Wheat and Rye or Barley Respectively. Landbauforsch. Voelkenrode Sonderh. 1999, 193, 223–228. [Google Scholar]
- Smits, C.H.M.; Annison, G. Non-Starch Plant Polysaccharides in Broiler Nutrition—Towards a Physiologically Valid Approach to Their Determination. World’s Poult. Sci. J. 1996, 52, 203–221. [Google Scholar] [CrossRef]
- Bautil, A.; Verspreet, J.; Buyse, J.; Goos, P.; Bedford, M.; Courtin, C. Arabinoxylan-Oligosaccharides Kick-Start Arabinoxylan Digestion in the Aging Broiler. Poult. Sci. 2020, 99, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Zebeli, Q. Cereal β-Glucan Alters Nutrient Digestibility and Microbial Activity in the Intestinal Tract of Pigs, and Lower Manure Ammonia Emission: A Meta-Analysis. J. Anim. Sci. 2013, 91, 3188–3199. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Lee, I.; Feng, X.; Lee, H.Y.; Kim, J.; Ahn, D.U. Effect of Dietary Beta-Glucan on the Performance of Broilers and the Quality of Broiler Breast Meat. Asian-Australas. J. Anim. Sci. 2016, 29, 384–389. [Google Scholar] [CrossRef] [PubMed]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Friesen, O.; Guenter, W.; Marquardt, R.; Rotter, B. The Effect of Enzyme Supplementation on the Apparent Metabolizable Energy and Nutrient Digestibilities of Wheat, Barley, Oats, and Rye for the Young Broiler Chick. Poult. Sci. 1992, 71, 1710–1721. [Google Scholar] [CrossRef]
- Brenes, A.; Smith, M.; Guenter, W.; Marquardt, R.R. Effect of Enzyme Supplementation on the Performance and Digestive Tract Size of Broiler Chickens Fed Wheat- and Barley-Based Diets. Poult. Sci. 1993, 72, 1731–1739. [Google Scholar] [CrossRef]
- Lazaro, R.; Garcia, M.; Medel, P.; Mateos, G.G. Influence of Enzymes on Performance and Digestive Parameters of Broilers Fed Rye-Based Diets. Poult. Sci. 2003, 82, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Jozefiak, D.; Rutkowski, A.; Fratczak, M.; Boros, D. The Effect of Dietary Fibre Fractions from Different Cereals and Microbial Enzyme Supplementation on Performance, Ileal Viscosity and Short-Chain Fatty Acid Concentrations in the Caeca of Broiler Chickens. J. Anim. Feed Sci. 2004, 13, 487–496. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Carbohydrate and Lignin Contents of Plant Materials Used in Animal Feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; de Coca-Sinova, A.; González-Alvarado, J.M.; Mateos, G.G. Inclusion of Insoluble Fiber Sources in Mash or Pellet Diets for Young Broilers. 1. Effects on Growth Performance and Water Intake. Poult. Sci. 2016, 95, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hetland, H.; Svihus, B. Effect of Oat Hulls on Performance, Gut Capacity and Feed Passage Time in Broiler Chickens. Br. Poult. Sci. 2001, 42, 354–361. [Google Scholar] [CrossRef]
- Aerni, V.; El-Lethey, H.; Wechsler, B. Effect of Foraging Material and Food Form on Feather Pecking in Laying Hens. Br. Poult. Sci. 2000, 41, 16–21. [Google Scholar] [CrossRef]
- Carre, B.; Derouet, L.; Leclercq, B. The Digestibility of Cell-Wall Polysaccharides from Wheat (Bran or Whole Grain), Soybean-Meal, and White Lupin Meal in Cockerels, Muscovy Ducks, and Rats. Poult. Sci. 1990, 69, 623–633. [Google Scholar] [CrossRef]
- Denayrolles, M.; Arturo-Schaan, M.; Massias, B.; Bebin, K.; Elie, A.M.; Panheleux-Lebastard, M.; Urdaci, M.C. Effect of Diets with Different Fibrous Contents on Broiler Gut Microflora and Short-Chain Fatty Acid (SCFA) Production. In Proceedings of the World Poultry Science Association, 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 269–272. [Google Scholar]
- Dunkley, K.D.; Dunkley, C.S.; Njongmeta, N.L.; Callaway, T.R.; Hume, M.E.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Comparison of In Vitro Fermentation and Molecular Microbial Profiles of High-Fiber Feed Substrates Incubated with Chicken Cecal Inocula. Poult. Sci. 2007, 86, 801–810. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Eeckhaut, V.; Moore, R.J.; Choct, M.; Ducatelle, R. Beneficial Microbial Signals from Alternative Feed Ingredients: A Way to Improve Sustainability of Broiler Production? Microb. Biotechnol. 2017, 10, 1008–1011. [Google Scholar] [CrossRef]
- Donaldson, J.; Świątkiewicz, S.; Arczewka-Włosek, A.; Muszyński, S.; Szymańczyk, S.; Arciszewski, M.B.; Siembida, A.Z.; Kras, K.; Piedra, J.L.V.; Schwarz, T.; et al. Modern Hybrid Rye, as an Alternative Energy Source for Broiler Chickens, Improves the Absorption Surface of the Small Intestine Depending on the Intestinal Part and Xylanase Supplementation. Animals 2021, 11, 1349. [Google Scholar] [CrossRef]
- World’s Poultry Science Association Nutrition of the European Federation of Branches Subcommittee Energy of the Working Group. European Table of Energy Values for Poultry Feedstuffs, 3rd ed.; WPSA: Beekbergen, The Netherlands, 1989. [Google Scholar]
- Ross-Broiler Management Handbook. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 3 March 2018).
- Atteh, J.O.; Onagbesan, O.M.; Tona, K.; Decuypere, E.; Geuns, J.M.C.; Buyse, J. Evaluation of Supplementary Stevia (Stevia rebaudiana, Bertoni) Leaves and Stevioside in Broiler Diets: Effects on Feed Intake, Nutrient Metabolism, Blood Parameters and Growth Performance. J. Anim. Physiol. Anim. Nutr. 2008, 92, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 December 2022).
- Svihus, B.; Sacranie, A.; Denstadli, V.; Choct, M. Nutrient Utilization and Functionality of the Anterior Digestive Tract Caused by Intermittent Feeding and Inclusion of Whole Wheat in Diets for Broiler Chickens. Poult. Sci. 2010, 89, 2617–2625. [Google Scholar] [CrossRef]
- Choct, M. Enzymes for the Feed Industry: Past, Present and Future. World’s Poult. Sci. J. 2006, 62, 5–16. [Google Scholar] [CrossRef]
- Bedford, M.; Classen, H. Reduction of Intestinal Viscosity through Manipulation of Dietary Rye and Pentosanase Concentration Is Effected through Changes in the Carbohydrate Composition of the Intestinal Aqueous Phase and Results in Improved Growth Rate and Food Conversion Efficiency. J. Nutr. 1992, 122, 560–569. [Google Scholar] [CrossRef]
- Bao, Y.M.; Choct, M. Dietary NSP Nutrition and Intestinal Immune System for Broiler Chickens. World’s Poult. Sci. J. 2010, 66, 511–518. [Google Scholar] [CrossRef]
- Shakouri, M.D.; Iji, P.A.; Mikkelsen, L.L.; Cowieson, A.J. Intestinal Function and Gut Microflora of Broiler Chickens as Influenced by Cereal Grains and Microbial Enzyme Supplementation. J. Anim. Physiol. Anim. Nutr. 2009, 93, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Smulikowska, S. Viscosity Negatively Affects the Nutritional Value of Blue Lupin Seeds for Broilers. Animal 2018, 12, 1144–1153. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Zeng, D.; Luo, M.; Liu, L.; Li, G.; Pan, K.; Jing, B. Live Probiotic Lactobacillus Johnsonii BS15 Promotes Growth Performance and Lowers Fat Deposition by Improving Lipid Metabolism, Intestinal Development, and Gut Microflora in Broilers. Front. Microbiol. 2017, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
- Virden, W.S.; Kidd, M.T. Physiological Stress in Broilers: Ramifications on Nutrient Digestibility and Responses. J. Appl. Poult. Res. 2009, 18, 338–347. [Google Scholar] [CrossRef]
- Larbier, M.; Chagneau, A.M.; Geraert, P.A. Influence of Ambient Temperature on True Digestibility of Protein and Amino Acids of Rapeseed and Soybean Meals in Broilers. Poult. Sci. 1993, 72, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kaldhusdal, M.; Hofshagen, M. Barley Inclusion and Avoparcin Supplementation in Broiler Diets.: 2. Clinical, Pathological, and Bacteriological Findings in a Mild Form of Necrotic Enteritis. Poult. Sci. 1992, 71, 1145–1153. [Google Scholar] [CrossRef]
- Awad, W.A.; Molnár, A.; Aschenbach, J.R.; Ghareeb, K.; Khayal, B.; Hess, C.; Liebhart, D.; Dublecz, K.; Hess, M. Campylobacter Infection in Chickens Modulates the Intestinal Epithelial Barrier Function. Innate Immun. 2015, 21, 151–160. [Google Scholar] [CrossRef]
- Matos, M.; Dublecz, K.; Grafl, B.; Liebhart, D.; Hess, M. Pancreatitis Is an Important Feature of Broilers Suffering from Inclusion Body Hepatitis Leading to Dysmetabolic Conditions with Consequences for Zootechnical Performance. Avian Dis. 2017, 62, 57–64. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bedford, M.R.; Wu, S.-B.; Morgan, N.K. Soluble Non-Starch Polysaccharide Modulates Broiler Gastrointestinal Tract Environment. Poult. Sci. 2021, 100, 101183. [Google Scholar] [CrossRef]
- Molnár, A.; Hess, C.; Pál, L.; Wágner, L.; Awad, W.A.; Husvéth, F.; Hess, M.; Dublecz, K. Composition of Diet Modifies Colonization Dynamics of Campylobacter Jejuni in Broiler Chickens. J. Appl. Microbiol. 2015, 118, 245–254. [Google Scholar] [CrossRef]
- Choct, M.; Annison, G. Anti-Nutritive Effect of Wheat Pentosans in Broiler Chickens: Roles of Viscosity and Gut Microflora. Br. Poult. Sci. 1992, 33, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Alemka, A.; Corcionivoschi, N.; Bourke, B. Defense and Adaptation: The Complex Inter-Relationship between Campylobacter jejuni and Mucus. Front. Cell. Infect. Microbiol. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.B.; Kang, S.; Williams, B.A.; Grant, L.J.; McSweeney, C.S.; Gidley, M.J.; Mikkelsen, D. Addition of Arabinoxylan and Mixed Linkage Glucans in Porcine Diets Affects the Large Intestinal Bacterial Populations. Eur. J. Nutr. 2017, 56, 2193–2206. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Rossnagel, B.; Pieper, R.; Van Kessel, A.; Leterme, P. Barley and Oat Cultivars with Diverse Carbohydrate Composition Alter Ileal and Total Tract Nutrient Digestibility and Fermentation Metabolites in Weaned Piglets. Animal 2010, 4, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Jozefiak, D.; Rutkowski, A.; Kaczmarek, S.; Jensen, B.B.; Engberg, R.M.; Højberg, O. Effect of β-Glucanase and Xylanase Supplementation of Barley- and Rye-Based Diets on Caecal Microbiota of Broiler Chickens. Br. Poult. Sci. 2010, 51, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Jha, R.; Rossnagel, B.; Van Kessel, A.G.; Souffrant, W.B.; Leterme, P. Effect of Barley and Oat Cultivars with Different Carbohydrate Compositions on the Intestinal Bacterial Communities in Weaned Piglets. FEMS Microbiol. Ecol. 2008, 66, 556–566. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, G.; Bedford, M.R.; Bach Knudsen, K.E.; Courtin, C.M.; Classen, H.L. The Value of Fibre: Engaging the Second Brain for Animal Nutrition; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; ISBN 978-90-8686-342-6. [Google Scholar]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Feighner, S.D.; Dashkevicz, M.P. Effect of Dietary Carbohydrates on Bacterial Cholyltaurine Hydrolase in Poultry Intestinal Homogenates. Appl. Environ. Microbiol. 1988, 54, 337–342. [Google Scholar] [CrossRef]
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J.J.; Lee, M.D. Diversity and Succession of the Intestinal Bacterial Community of the Maturing Broiler Chicken. Appl. Environ. Microbiol. 2003, 69, 6816–6824. [Google Scholar] [CrossRef]
- Salanitro, J.P.; Blake, I.G.; Muirehead, P.A.; Maglio, M.; Goodman, J.R. Bacteria Isolated from the Duodenum, Ileum, and Cecum of Young Chicks. Appl. Environ. Microbiol. 1978, 35, 782–790. [Google Scholar] [CrossRef]
- Farkas, V.; Csitári, G.; Menyhárt, L.; Such, N.; Pál, L.; Husvéth, F.; Rawash, M.A.; Mezőlaki, Á.; Dublecz, K. Microbiota Composition of Mucosa and Interactions between the Microbes of the Different Gut Segments Could Be a Factor to Modulate the Growth Rate of Broiler Chickens. Animals 2022, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, M.; Rek-Ciepły, B.; Slot, A.; Lipińska, E.; Kubiński, T.; Barcz, I.; Afanasjew, B. The Effect of Rye, Probiotics and Nisine on Faecal Flora and Histology of the Small Intestine of Chicks. J. Anim. Feed Sci. 1993, 2, 73–81. [Google Scholar] [CrossRef]
- Smulikowska, S. Relationship between the Stage of Digestive Tract Development in Chicks and the Effect of Viscosity Reducing Enzymes on Fat Digestion. J. Anim. Feed Sci. 1998, 7, 125–134. [Google Scholar] [CrossRef]
- Choct, M.; Hughes, R.J.; Wang, J.; Bedford, M.R.; Morgan, A.J.; Annison, G. Increased Small Intestinal Fermentation Is Partly Responsible for the Anti-Nutritive Activity of Non-Starch Polysaccharides in Chickens. Br. Poult. Sci. 1996, 37, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Classen, H.L. Cereal Grain Starch and Exogenous Enzymes in Poultry Diets. Anim. Feed Sci. Technol. 1996, 62, 21–27. [Google Scholar] [CrossRef]
- Johnson, I.T.; Gee, J.M. Effect of Gel-Forming Gums on the Intestinal Unstirred Layer and Sugar Transport in Vitro. Gut 1981, 22, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Slominski, B.A.; Guenter, W. The Effect of Fat Type, Carbohydrase, and Lipase Addition on Growth Performance and Nutrient Utilization of Young Broilers Fed Wheat-Based Diets. Poult. Sci. 2004, 83, 1718–1727. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Salaheen, S.; Kim, S.-W.; Haley, B.J.; Van Kessel, J.A.S.; Biswas, D. Alternative Growth Promoters Modulate Broiler Gut Microbiome and Enhance Body Weight Gain. Front. Microbiol. 2017, 8, 2088. [Google Scholar] [CrossRef]
- Choct, M.; Annison, G. Anti-nutritive Activity of Wheat Pentosans in Broiler Diets. Br. Poult. Sci. 1990, 31, 811–821. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Z.; Tian, X.; Guo, Y.; Zhang, H. Yeast β-d-Glucans Induced Antimicrobial Peptide Expressions against Salmonella Infection in Broiler Chickens. Int. J. Biol. Macromol. 2016, 85, 573–584. [Google Scholar] [CrossRef] [PubMed]


| DM | c. Protein | c. Fat | c. Fiber | Ash | NFE | Ca | P | Starch | NDF | ADF | AMEn * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| winter barley (MvHV05-17) | 91.12 | 9.57 | 1.47 | 3.59 | 2.15 | 74.34 | 0.07 | 0.35 | 51.14 | 18.17 | 4.41 | 10.77 |
| 92.62 | 10.96 | 5.3 | 11.35 | 2.39 | 62.62 | 0.09 | 0.3 | 36.99 | 29.76 | 14.06 | 9.92 | |
| winter oat (MV HÓPEHELY) | 91.12 | 9.57 | 1.47 | 3.59 | 2.15 | 74.34 | 0.07 | 0.35 | 51.14 | 18.17 | 4.41 | 10.77 |
| 92.62 | 10.96 | 5.3 | 11.35 | 2.39 | 62.62 | 0.09 | 0.3 | 36.99 | 29.76 | 14.06 | 9.92 |
| Starter Diets | Grower Diets | Finisher Diets | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | O | C | B | O | C | B | O | |
| Maize | 430.0 | 28.0 | 219.0 | 400.0 | 92.0 | 277.0 | 459.0 | 152.0 | 336.0 |
| Wheat | 100.0 | 100.0 | |||||||
| Extracted soybean meal | 464.0 | 435.0 | 444.0 | 397.0 | 382.0 | 392.0 | 342.0 | 327.0 | 337.0 |
| Sunflower oil | 56.0 | 88.0 | 86.0 | 59.0 | 84.0 | 88.0 | 57.0 | 81.0 | 85.0 |
| Limestone | 18.0 | 18.0 | 17.0 | 15.0 | 15.0 | 14.0 | 14.0 | 15.0 | 14.0 |
| MCP | 16.0 | 15.0 | 17.0 | 15.0 | 13.0 | 15.0 | 14.0 | 12.0 | 14.0 |
| Barley | 400.0 | 400.0 | 400.0 | ||||||
| Oats | 200.0 | 200.0 | 200.0 | ||||||
| Lysine | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| DL-methionine | 4.0 | 4.0 | 4.0 | 3.0 | 3.0 | 3.0 | 3.0 | 2.0 | 3.0 |
| Threonine | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.5 | 0.5 | 0.5 |
| Valine | 0.5 | ||||||||
| Premix 1 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| NaCl | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| NaHCO3 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Phytase 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| NSP enzyme 3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Starter Diets | Grower Diets | Finisher Diets | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | O | C | B | O | C | B | O | |
| DM | 89.30 | 89.54 | 89.94 | 89.63 | 89.83 | 90.18 | 89.71 | 90.42 | 91.21 |
| crude protein | 22.87 | 23.24 | 21.88 | 21.43 | 22.38 | 21.36 | 19.91 | 19.96 | 19.63 |
| crude fat | 8.19 | 9.18 | 12.44 | 8.46 | 10.06 | 11.77 | 8.14 | 10.02 | 11.99 |
| crude fiber | 3.71 | 4.14 | 4.97 | 2.71 | 3.68 | 4.52 | 2.51 | 4.09 | 5.06 |
| soluble dietary fiber | 2.13 | 2.54 | 2.3 | 2.13 | 2.51 | 2.33 | 2.08 | 2.66 | 2.49 |
| ash | 6.49 | 6.67 | 6.57 | 5.71 | 6.15 | 6.12 | 5.70 | 5.82 | 5.74 |
| NFE | 48.04 | 45.31 | 44.08 | 51.32 | 47.56 | 46.41 | 53.45 | 50.53 | 48.79 |
| Ca | 0.98 | 1.07 | 1.10 | 0.86 | 0.95 | 0.97 | 0.85 | 0.89 | 0.90 |
| P | 0.71 | 0.75 | 0.72 | 0.69 | 0.67 | 0.72 | 0.70 | 0.60 | 0.66 |
| starch | 32.94 | 25.85 | 26.73 | 35.57 | 29.23 | 29.02 | 39.41 | 33.86 | 33.18 |
| NDF | 17.08 | 18.84 | 19.93 | 13.07 | 12.61 | 16.65 | 13.91 | 16.16 | 28.99 |
| ADF | 5.97 | 6.82 | 7.20 | 4.71 | 5.16 | 5.78 | 5.41 | 7.10 | 6.16 |
| AMEn (MJ/kg) * | 12.39 | 11.83 | 12.68 | 12.76 | 12.43 | 12.80 | 13.12 | 12.87 | 13.33 |
| CYS | 0.35 | 0.4 | 0.36 | 0.33 | 0.38 | 0.37 | 0.32 | 0.35 | 0.35 |
| MET | 0.71 | 0.71 | 0.65 | 0.61 | 0.59 | 0.58 | 0.5 | 0.43 | 0.46 |
| THR | 0.9 | 0.93 | 0.84 | 0.81 | 0.84 | 0.83 | 0.74 | 0.72 | 0.72 |
| VAL | 1.08 | 1.15 | 1.03 | 1.02 | 1.08 | 1.03 | 0.89 | 0.92 | 0.89 |
| ILE | 0.92 | 0.95 | 0.89 | 0.82 | 0.85 | 0.82 | 0.7 | 0.68 | 0.69 |
| PHE | 1.12 | 1.22 | 1.09 | 1.04 | 1.08 | 0.99 | 0.98 | 0.99 | 0.97 |
| HIS | 0.6 | 0.64 | 0.57 | 0.52 | 0.58 | 0.5 | 0.55 | 0.54 | 0.52 |
| LYS | 1.51 | 1.48 | 1.41 | 1.22 | 1.27 | 1.24 | 1.07 | 0.97 | 1.03 |
| ARG | 1.58 | 1.61 | 1.52 | 1.48 | 1.44 | 1.55 | 1.38 | 1.28 | 1.34 |
| Dietary Treatments | p—Value | |||||
|---|---|---|---|---|---|---|
| C Mean ± SD | B Mean ± SD | O Mean ± SD | ||||
| Ileum | Viscosity | mPa.s | 3.29 ± 0.151 b | 4.97 ± 0.250 a | 3.44 ± 0.155 b | <0.001 |
| Cecum | Acetate | SCFA µmol/g | 49.71 ± 7.63 a | 36.54 ± 15.95 b | 35.81 ± 17.17 ab | 0.041 |
| Propionate | 7.27 ± 2.36 a | 3.23 ± 1.95 b | 6.46 ± 3.85 ab | 0.007 | ||
| n-Butyrate | 0.49 ± 0.18 | 0.38 ± 0.23 | 0.50 ± 0.25 | 0.318 | ||
| Butyrate | 13.54 ± 3.99 | 9.81 ± 4.92 | 10.03 ± 5.37 | 0.191 | ||
| n-Valerate | 0.46 ± 0.25 | 0.34 ± 0.29 | 0.55 ± 0.31 | 0.201 | ||
| Valerate | 0.81 ± 0.16 ab | 0.49 ± 0.28 b | 0.79 ± 0.40 a | 0.026 | ||
| Total SCFA | 72.29 ± 11.07 a | 50.79 ± 22.14 b | 54.15 ± 26.17 ab | 0.037 | ||
| Chao1 Mean ± SD | Shannon Mean ± SD | Simpson Mean ± SD | ||
|---|---|---|---|---|
| JC | C | 129.94 ± 23.95 B | 2.30 ± 0.41 | 0.83 ± 0.05 |
| B | 184.85 ± 26.21 A | 2.68 ± 0.49 | 0.85 ± 0.10 | |
| O | 157.93 ± 43.21 B | 2.27 ± 0.30 | 0.81 ± 0.07 | |
| K–W Sig | 0.056 | 0.336 | 0.404 | |
| JM | C | 209.99 ± 96.05 | 2.73 ± 0.47 B | 0.87 ± 0.05 |
| B | 164.21 ± 89.54 | 3.44 ± 0.44 A | 0.92 ± 0.03 | |
| O | 144.92 ± 27.01 | 2.94 ± 0.36 B | 0.89 ± 0.04 | |
| K–W Sig | 0.357 | 0.093 | 0.177 | |
| CC | C | 501.04 ± 18.95 a | 4.64 ± 0.08 ab | 0.98 ± 0.004 |
| B | 406.75 ± 28.01 b | 4.40 ± 0.14 b | 0.97 ± 0.01 | |
| O | 496.48 ± 18.59 a | 4.72 ± 0.14 a | 0.98 ± 0.01 | |
| K–W Sig | 0.009 | 0.017 | 0.459 | |
| Actinobacteria | Actinobacteria | Bacteroidetes | Cyanobacteria | Deinococcus—Thermus | Firmicutes | Patescibacteria | Proteobacteria | Tenericutes | Verrucomicrobia | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| JC | C | 0.00 | 1.85 | 0.01 | 0.15 | 0.00 | 97.93 | 0.01 | 0.05 | 0.00 | 0.00 |
| B | 0.00 | 4.75 | 0.13 | 0.12 | 0.00 | 94.86 | 0.01 | 0.13 | 0.00 | 0.00 | |
| O | 0.00 | 3.19 | 0.01 | 0.08 | 0.00 | 96.59 | 0.02 | 0.11 | 0.00 | 0.00 | |
| Pooled SEM | 0.00 | 1.05 | 0.07 | 0.07 | 0.00 | 1.05 | 0.01 | 0.04 | 0.00 | 0.00 | |
| Asymp. Sig. | 1.000 | 0.179 | 0.426 | 0.779 | 1.000 | 0.208 | 0.580 | 0.230 | 0.368 | 0.368 | |
| JM | C | 0.01 | 0.44 | 1.03 | 0.05 | 0.00 | 97.69 a | 0.01 | 0.76 b | 0.00 b | 0.01 |
| B | 0.01 | 2.36 | 3.87 | 0.70 | 0.03 | 89.42 b | 0.00 | 3.47 a | 0.11 a | 0.02 | |
| O | 0.00 | 1.53 | 0.48 | 0.05 | 0.06 | 96.24 ab | 0.01 | 1.62 ab | 0.00 b | 0.00 | |
| Pooled SEM | 0.01 | 0.88 | 1.45 | 0.25 | 0.03 | 1.18 | 0.01 | 0.35 | 0.06 | 0.01 | |
| Asymp. Sig. | 0.409 | 0.185 | 0.281 | 0.426 | 0.161 | 0.004 | 0.581 | 0.006 | 0.032 | 0.291 | |
| CC | C | 0.00 | 0.29 ab | 9.28 | 0.10 | 0.00 | 89.59 | 0.00 | 0.20 | 0.07 ab | 0.46 |
| B | 0.00 | 6.73 a | 8.20 | 0.07 | 0.00 | 84.21 | 0.00 | 0.25 | 0.53 a | 0.01 | |
| O | 0.00 | 0.17 b | 11.32 | 0.24 | 0.00 | 87.64 | 0.00 | 0.40 | 0.07 b | 0.15 | |
| Pooled SEM | 0.00 | 0.95 | 1.43 | 0.05 | 0.00 | 1.29 | 0.00 | 0.08 | 0.06 | 0.23 | |
| Asymp. Sig. | 1.000 | 0.008 | 0.210 | 0.061 | 1.000 | 0.069 | 1.000 | 0.310 | 0.007 | 0.193 |
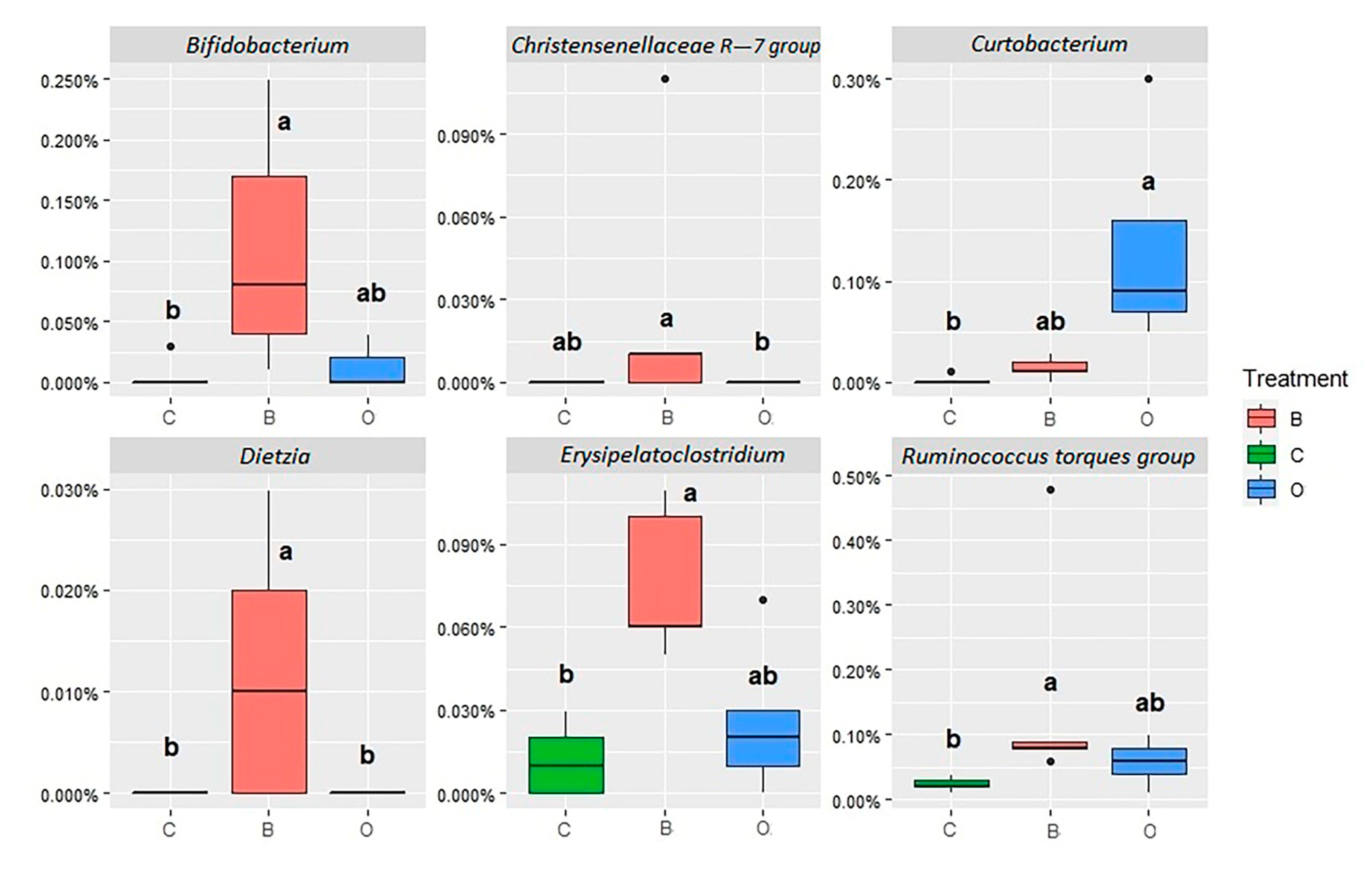
| Phylum Class | Family | Genus | C | B | O | Pooled SEM | Sig. |
|---|---|---|---|---|---|---|---|
| Actinobacteria Actinobacteria | Bifidobacteriaceae | Bifidobacterium | 0.21 ab | 6.63 a | 0.09 b | 0.950 | 0.005 |
| Bacteroidetes Bacteroidia | Bacteroidaceae | Bacteroides | 6.61 | 4.90 | 6.22 | 1.093 | 0.677 |
| Rikenellaceae | Alistipes | 2.67 | 3.30 | 5.10 | 0.992 | 0.326 | |
| Firmicutes Bacilli | Lactobacillaceae | Lactobacillus | 10.48 | 11.05 | 8.89 | 1.377 | 0.533 |
| Streptococcaceae | Streptococcus | 4.74 a | 2.54 ab | 0.67 b | 0.695 | 0.009 | |
| Firmicutes Clostridia | Christensenellaceae | Christensenellaceae R-7 gr. | 1.68 ab | 1.16 b | 2.31 a | 0.216 | 0.026 |
| Peptostreptococcaceae | Romboutsia | 4.96 | 4.10 | 3.45 | 0.921 | 0.275 | |
| Lachnospiraceae | CHKCI001 | 4.31 | 1.08 | 2.97 | 1.108 | 0.164 | |
| Ruminococcus torques gr. | 2.19 | 3.82 | 2.26 | 0.721 | 0.228 | ||
| Sellimonas | 1.10 b | 1.41 ab | 1.90 a | 0.164 | 0.032 | ||
| Eubacterium hallii gr. | 0.56 | 1.05 | 0.98 | 0.145 | 0.080 | ||
| Anaerostipes | 0.22 b | 1.30 a | 0.48 b | 0.215 | 0.010 | ||
| Eubacterium ventriosum gr. | 0.02 a | 0.00 b | 0.00 b | 0.004 | 0.031 | ||
| Marvinbryantia | 0.09 ab | 0.07 b | 0.19 a | 0.026 | 0.047 | ||
| Blautia | 2.91 | 1.42 | 2.52 | 0.486 | 0.059 | ||
| Lachnoclostridium | 0.37 | 0.70 | 0.50 | 0.082 | 0.071 | ||
| Lachnoclostridium 5 | 0.03 | 0.06 | 0.01 | 0.020 | 0.097 | ||
| Ruminococcaceae | Faecalibacterium | 7.81 | 9.34 | 12.36 | 1.393 | 0.125 | |
| Ruminococcaceae UCG-004 | 0.16 b | 0.19 ab | 0.28 a | 0.024 | 0.035 | ||
| Ruminococcaceae UCG-005 | 4.55 a | 1.15 b | 3.77 a | 0.663 | 0.009 | ||
| Ruminococcaceae UCG-008 | 1.00 ab | 0.00 b | 1.48 a | 0.353 | 0.008 | ||
| Ruminococcaceae UCG-014 | 3.70 ab | 1.87 b | 4.39 a | 0.525 | 0.034 | ||
| Ruminococcaceae NK4A214 gr. | 0.32 b | 0.23 b | 0.75 a | 0.064 | 0.006 | ||
| Subdoligranulum | 2.76 | 3.93 | 2.62 | 0.520 | 0.196 | ||
| Butyricicoccus | 2.00 | 1.82 | 1.48 | 0.454 | 0.887 | ||
| Ruminococcus 2 | 0.58 a | 0.02 b | 0.50 a | 0.109 | 0.008 | ||
| Ruminiclostridium 5 | 1.09 | 1.73 | 1.05 | 0.231 | 0.176 | ||
| Eubacterium coprostanoligenes gr. | 0.78 | 0.69 | 1.01 | 0.147 | 0.405 | ||
| Anaerotruncus | 0.03 ab | 0.004 b | 0.06 a | 0.009 | 0.016 | ||
| Anaerofilum | 0.08 ab | 0.02 b | 0.09 a | 0.023 | 0.025 | ||
| GCA-900066225 | 0.14 a | 0.065 ab | 0.058 b | 0.023 | 0.038 | ||
| Family XIII | Family XIII UCG-001 | 0.002 | 0.02 | 0.00 | 0.006 | 0.088 | |
| Clostridiales vadin-BB60 group | uncultured Clostridia bacterium | 0.04 | 0.20 | 0.14 | 0.062 | 0.080 | |
| Firmicutes Erysipelotrichia | Erysipelotrichaceae | Turicibacter | 3.00 | 1.69 | 1.10 | 0.527 | 0.112 |
| Erysipelatoclostridium | 2.57 b | 7.35 a | 3.94 ab | 0.798 | 0.016 | ||
| Not Assigned | 18.67 | 17.22 | 17.84 | 0.900 | 0.357 | ||
| Other genera | 7.56 | 7.86 | 8.56 | 1.758 | |||
| 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawash, M.A.; Farkas, V.; Such, N.; Mezőlaki, Á.; Menyhárt, L.; Pál, L.; Csitári, G.; Dublecz, K. Effects of Barley- and Oat-Based Diets on Some Gut Parameters and Microbiota Composition of the Small Intestine and Ceca of Broiler Chicken. Agriculture 2023, 13, 169. https://doi.org/10.3390/agriculture13010169
Rawash MA, Farkas V, Such N, Mezőlaki Á, Menyhárt L, Pál L, Csitári G, Dublecz K. Effects of Barley- and Oat-Based Diets on Some Gut Parameters and Microbiota Composition of the Small Intestine and Ceca of Broiler Chicken. Agriculture. 2023; 13(1):169. https://doi.org/10.3390/agriculture13010169
Chicago/Turabian StyleRawash, Mohamed Ali, Valéria Farkas, Nikoletta Such, Ákos Mezőlaki, László Menyhárt, László Pál, Gábor Csitári, and Károly Dublecz. 2023. "Effects of Barley- and Oat-Based Diets on Some Gut Parameters and Microbiota Composition of the Small Intestine and Ceca of Broiler Chicken" Agriculture 13, no. 1: 169. https://doi.org/10.3390/agriculture13010169
APA StyleRawash, M. A., Farkas, V., Such, N., Mezőlaki, Á., Menyhárt, L., Pál, L., Csitári, G., & Dublecz, K. (2023). Effects of Barley- and Oat-Based Diets on Some Gut Parameters and Microbiota Composition of the Small Intestine and Ceca of Broiler Chicken. Agriculture, 13(1), 169. https://doi.org/10.3390/agriculture13010169








