Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Pretreatments
2.2. Incubation for A. platensis after Pretreatments
2.3. Determination of Total Protein by Bradford Method
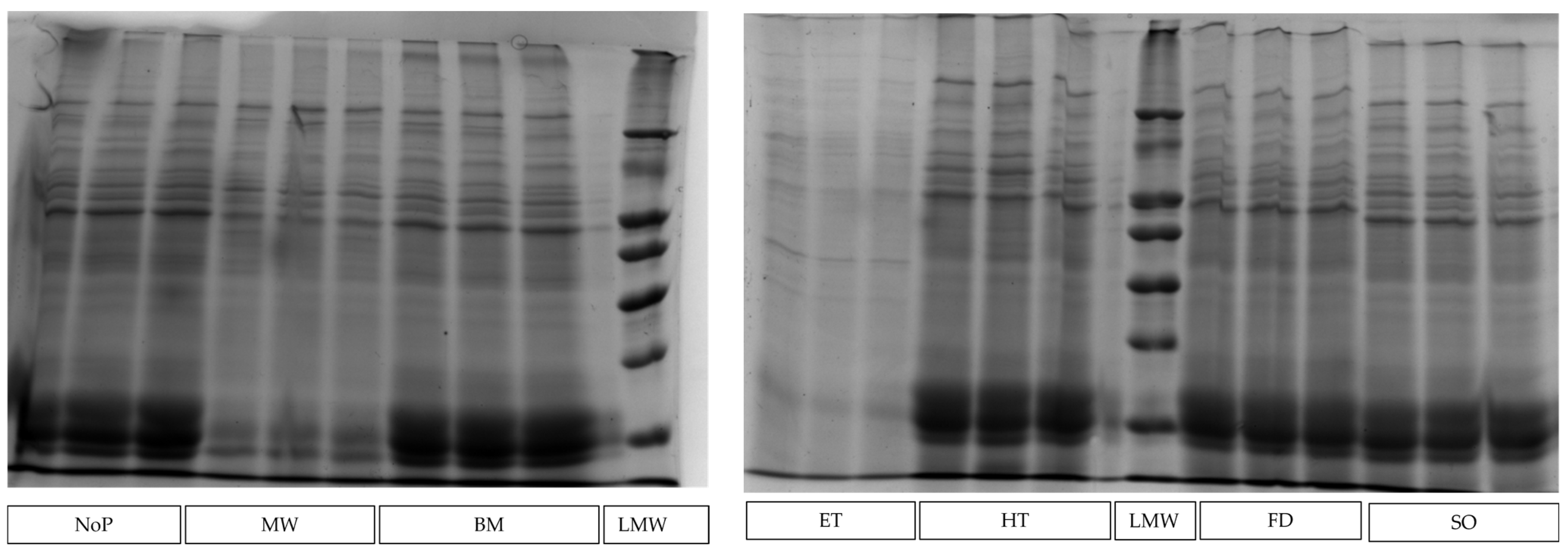
2.4. Electrophoretic Analysis of Proteins by SDS-PAGE
2.5. Determination of Total Peptides by O-Phthaldialdehyde (OPA) Assay
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Safi, C.; Charton, M.; Ursu, A.V.; Laroche, C.; Zebib, B.; Pontalier, P.-Y.; Vaca-Garcia, C. Release of hydro-soluble microalgal proteins using mechanical and chemical treatments. Algal Res. 2014, 3, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.; Skomedal, H.; Skjånes, K.; Mazur-Marzec, H.; Toruńska-Sitarz, A.; Catala, M.; Isleten Hosoglu, M.; García-Vaquero, M. Microalgal proteins for feed, food and health. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 347–368. [Google Scholar]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Digestive Constraints of Arthrospira platensis in Poultry and Swine Feeding. Foods 2022, 11, 2984. [Google Scholar] [CrossRef] [PubMed]
- Palmegiano, G.B.; Agradi, E.; Forneris, G.; Gai, F.; Gasco, L.; Rigamonti, E.; Sicuro, B.; Zoccarato, I. Spirulina as a nutrient source in diets for growing sturgeon (Acipenser baeri). Aquac. Res. 2005, 36, 188–195. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 396-399, 14–19. [Google Scholar] [CrossRef]
- Martins, C.F.; Pestana Assunção, J.; Ribeiro Santos, D.M.; Madeira, M.S.M.d.S.; Alfaia, C.M.R.P.M.; Lopes, P.A.A.B.; Coelho, D.F.M.; Cardoso Lemos, J.P.; de Almeida, A.M.; Mestre Prates, J.A.; et al. Effect of dietary inclusion of Spirulina on production performance, nutrient digestibility and meat quality traits in post-weaning piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 247–259. [Google Scholar] [CrossRef]
- Pestana, J.M.; Puerta, B.; Santos, H.; Madeira, M.S.; Alfaia, C.M.; Lopes, P.A.; Pinto, R.M.A.; Lemos, J.P.C.; Fontes, C.M.G.A.; Lordelo, M.M.; et al. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020, 99, 2519–2532. [Google Scholar] [CrossRef]
- Beynen, A.C. Microalgae in petfood. Creat. Companion 2019, 40, 42. [Google Scholar]
- Satyaraj, E.; Reynolds, A.; Engler, R.; Labuda, J.; Sun, P. Supplementation of Diets With Spirulina Influences Immune and Gut Function in Dogs. Front. Nutr. 2021, 8, 667072. [Google Scholar] [CrossRef]
- Böcker, L.; Hostettler, T.; Diener, M.; Eder, S.; Demuth, T.; Adamcik, J.; Reineke, K.; Leeb, E.; Nyström, L.; Mathys, A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food Chem. 2020, 316, 126374. [Google Scholar] [CrossRef]
- Buecker, S.; Grossmann, L.; Loeffler, M.; Leeb, E.; Weiss, J. Thermal and acidic denaturation of phycocyanin from Arthrospira platensis: Effects of complexation with λ-carrageenan on blue color stability. Food Chem. 2022, 380, 132157. [Google Scholar] [CrossRef]
- Agboola, J.O.; Teuling, E.; Wierenga, P.A.; Gruppen, H.; Schrama, J.W. Cell wall disruption: An effective strategy to improve the nutritive quality of microalgae in African catfish (Clarias gariepinus). Aquac. Nutr. 2019, 25, 783–797. [Google Scholar] [CrossRef]
- Middelberg, A.P.J. Process-scale disruption of microorganisms. Biotechnol. Adv. 1995, 13, 491–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Sousa, C.V.d. Microalgas: Do Tratamento de Efluentes Para a Biorrefinaria. Master’s Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2014. [Google Scholar]
- Ahmed, J.; Kumar, V. Effect of high-pressure treatment on oscillatory rheology, particle size distribution and microstructure of microalgae Chlorella vulgaris and Arthrospira platensis. Algal Res. 2022, 62, 102617. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Rodriguez, C.; Durrant, A. Sustainable Approaches to Microalgal Pre-Treatment Techniques for Biodiesel Production: A Review. Sustainability 2022, 14, 9953. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Zhou, W.; Yuan, W.; Wang, D. Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Res. 2020, 46, 101794. [Google Scholar] [CrossRef]
- Annamalai, S.N.; Das, P.; Thaher, M.I.A.; Abdul Quadir, M.; Khan, S.; Mahata, C.; Al Jabri, H. Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications. Sustainability 2021, 13, 13211. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, H.; Chen, S.; Wen, S.; Wu, X.; Zhang, D.; Yuan, Q.; Cong, W. Microalgal cell disruption via extrusion for the production of intracellular valuables. Energy 2018, 142, 339–345. [Google Scholar] [CrossRef]
- Al Hattab, M.; Ghaly, A. Microalgae Oil Extraction Pre-treatment Methods: Critical Review and Comparative Analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Meade, S.J.; Reid, E.A.; Gerrard, J.A. The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 2005, 88, 904–922. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Lopes, P.A.; Coelho, D.; Prates, J.A.M. Testimony on a successful lab protocol to disrupt Chlorella vulgaris microalga cell wall. PLoS ONE 2022, 17, e0268565. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Acién, F.G.; Alarcón, F.J. Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Sedighi, M.; Jalili, H.; Darvish, M.; Sadeghi, S.; Ranaei-Siadat, S.-O. Enzymatic hydrolysis of microalgae proteins using serine proteases: A study to characterize kinetic parameters. Food Chem. 2019, 284, 334–339. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility−Digestibility Relationship of Legume Proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: A comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Krzemiñska, I.; Tys, J. Physical methods of microalgal biomass pretreatment. Int. Agrophys 2014, 28, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Viner, K.J.; Champagne, P.; Jessop, P.G. Comparison of cell disruption techniques prior to lipid extraction from Scenedesmus sp. slurries for biodiesel production using liquid CO2. Green Chem. 2018, 20, 4330–4338. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L.; Show, P.L. Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique. Chem. Eng. J. 2019, 367, 1–8. [Google Scholar] [CrossRef]
- Unterlander, N.; Champagne, P.; Plaxton, W.C. Lyophilization pretreatment facilitates extraction of soluble proteins and active enzymes from the oil-accumulating microalga Chlorella vulgaris. Algal Res. 2017, 25, 439–444. [Google Scholar] [CrossRef]
- Abbassi, A.; Ali, M.; Watson, I.A. Temperature dependency of cell wall destruction of microalgae with liquid nitrogen pretreatment and hydraulic pressing. Algal Res. 2014, 5, 190–194. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Garrido-Fernández, A.; Mijlkovic, A.; Krona, A.; Martínez-Abad, A.; Coll-Marqués, J.M.; López-Rubio, A.; Lopez-Sanchez, P. Composition and rheological properties of microalgae suspensions: Impact of ultrasound processing. Algal Res. 2020, 49, 101960. [Google Scholar] [CrossRef]

| Nutritional Composition | |
| Energy (MJ/kg) | 13.9 |
| Crude protein (% dry matter) | 62.6 |
| Ash (% dry matter) | 14.9 |
| Crude carbohydrates (% dry matter) | 6.06 |
| Crude fibre (% dry matter) | 9.78 |
| Crude fat (% dry matter) | 6.70 |
| Pigment composition | |
| Phycocyanin (% dry matter) | 11.2 |
| Item | Pretreatments 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| NoP | BM | ET | FD | HT | MW | SO | ||
| Total protein (mg/mL) | ||||||||
| Bradford method | 1.42 ± 0.112 ab | 1.30 ± 0.061 b | 0.07 ± 0.060 c | 1.20 ± 0.105 b | 1.29 ± 0.125 b | 0.41 ± 0.054 c | 1.72 ± 0.418 a | <0.001 |
| SDS-PAGE gel | 9.9 ± 1.58 a | 10.1 ± 0.20 a | 6.50 ± 1.035 b | 9.6 ± 0.99 a | 10.5 ± 1.61 a | 7.0 ± 0.32 b | 10.7 ± 0.54 a | <0.001 |
| Proteins (mg/mL) in SDS-PAGE gel | ||||||||
| Proteins 18–26 kDa | 2.41 ± 0.066 ab | 2.35 ± 0.107 ab | 1.44 ± 0.176 c | 2.25 ± 0.030 b | 2.74 ± 0.315 a | 1.73 ± 0.256 c | 2.65 ± 0.244 a | <0.001 |
| Proteins 40–48 kDa | 1.71 ± 0.094 bc | 2.02 ± 0.205 ab | 1.19 ± 0.188 d | 1.94 ± 0.113 ab | 2.10± 0.273 a | 1.41 ± 0.057 cd | 2.00 ± 0.056 ab | <0.001 |
| Other proteins | 5.74 ± 1.553 a | 5.76 ± 0.152 a | 3.87 ± 0.755 bc | 5.39 ± 0.909 abc | 5.61 ± 1.027 ab | 3.81 ± 0.327 c | 6.09 ± 0.588 a | 0.001 |
| Proteins (% total protein) in SDS-PAGE gel | ||||||||
| Proteins 18–26 kDa | 22.3 ± 5.22 ab | 23.2 ± 1.30 ab | 18.7 ± 2.07 b | 23.7 ± 2.49 ab | 26.4 ± 1.17 a | 24.9 ± 3.58 a | 24.7 ± 2.72 a | 0.011 |
| Proteins 40–48 kDa | 17.7 ± 3.15 | 19.9 ± 1.85 | 18.3 ± 0.95 | 20.3 ± 1.25 | 20.2 ± 0.56 | 20.3 ± 0.36 | 18.6 ± 0.52 | 0.047 |
| Other proteins | 60.0 ± 7.46 ab | 56.8 ± 0.79 ab | 63.0 ± 1.48 a | 56.0 ± 3.64 ab | 53.5 ± 1.70 b | 54.9 ± 3.89 b | 56.6 ± 3.13 ab | 0.008 |
| Proteins (PTRAT/PCON) in SDS-PAGE gel | ||||||||
| Total protein | nd | 1.05 ± 0.196 abc | 0.68 ± 0.207 c | 1.00 ± 0.206 abc | 1.07 ± 0.206 ab | 0.72 ± 0.135 b c | 1.12 ± 0.223 a | 0.005 |
| Proteins 18–26 kDa | nd | 1.18 ± 0.110 a | 0.69 ± 0.105 b | 1.13 ± 0.046 a | 1.23 ± 0.190 a | 0.82 ± 0.038 b | 1.17 ± 0.054 a | <0.001 |
| Proteins 40–48 kDa | nd | 0.98 ± 0.028 ab | 0.60 ± 0.076 c | 0.93 ± 0.021 b | 1.13 ± 0.108 a | 0.72 ± 0.102 c | 1.10 ± 0.127 ab | <0.001 |
| Other proteins | nd | 1.08 ± 0.358 | 0.74 ± 0.320 | 1.01 ± 0.335 | 1.03 ± 0.336 | 0.71 ± 0.202 | 1.14 ± 0.426 | 0.237 |
| Total peptides (µg/mL) | ||||||||
| o-phthaldialdehyde assay | 42.7 ± 2.20 a | 50.4 ± 9.20 a | 24.6 ± 4.81 b | 44.4 ± 9.84 a | 38.5 ± 4.39 a | 39.0 ± 5.02 a | 43.4 ± 3.69 a | <0.001 |
| Item | Pretreatments 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| NoP | BM | ET | FD | HT | MW | SO | ||
| Total protein (mg/mL) | ||||||||
| Bradford method | 0.83 ± 0.094 bc | 1.13 ± 0.312 b | 0.03 ± 0.039 d | 0.71 ± 0.091 c | 0.67 ± 0.101 c | 1.13 ± 0.076 b | 2.36 ± 0.246 a | <0.001 |
| SDS-PAGE gel | 11.5 ± 0.59 | 12.9 ± 0.79 | 13.8 ± 5.07 | 11.5 ± 1.85 | 11.3 ± 0.31 | 13.6 ± 0.64 | 10.2 ± 1.39 | 0.115 |
| Protein quantification (mg/mL) by SDS-PAGE gel | ||||||||
| Proteins 18–26 kDa | 2.23 ± 0.026 | 2.40 ± 0.057 | 2.14 ± 0.237 | 2.29 ± 0.132 | 2.16 ± 0.067 | 2.38 ± 0.080 | 2.40 ± 0.337 | 0.075 |
| Proteins 40–48 kDa | 1.95 ± 0.032 | 2.14 ± 0.088 | 1.97 ± 0.255 | 1.98 ± 0.264 | 1.96 ± 0.045 | 2.20 ± 0.090 | 2.19 ±0.440 | 0.279 |
| Other proteins | 7.36 ± 0.591 | 8.33 ± 0.673 | 9.66 ± 5.041 | 7.20 ± 1.456 | 7.16 ± 0.214 | 8.99 ± 0.698 | 5.60 ± 2.054 | 0.110 |
| Protein proportion (% total) by SDS-PAGE gel | ||||||||
| Proteins 18–26 kDa | 19.3 ± 0.87 ab | 18.7 ± 0.80 ab | 16.7 ± 4.01 b | 20.3 ± 2.51 ab | 19.1 ± 0.15 ab | 17.6 ± 1.14 b | 24.2 ± 6.39 a | 0.020 |
| Proteins 40–48 kDa | 16.9 ± 0.63 | 16.6 ± 0.29 | 15.9 ± 0.59 | 17.4 ± 1.20 | 17.4 ± 5.55 | 16.3 ± 1.06 | 22.2 ± 7.31 | 0.129 |
| Other proteins | 63.7 ± 1.91 | 64.7 ± 1.37 | 67.3 ± 9.53 | 62.4 ± 3.09 | 63.5 ± 0.22 | 66.2 ± 2.30 | 53.6 ± 13.69 | 0.056 |
| Protein proportion (PTRAT/PCON) by SDS-PAGE gel | ||||||||
| Total protein | nd | 1.11 ± 0.037 | 1.21 ± 0.505 | 0.99 ± 0.126 | 0.98 ± 0.071 | 1.18 ± 0.083 | 0.88 ± 0.093 | 0.169 |
| Proteins 18–26 kDa | nd | 1.08 ± 0.014 | 0.96 ± 0.117 | 1.03 ± 0.049 | 0.97 ± 0.040 | 1.07 ± 0.037 | 1.08 ± 0.162 | 0.132 |
| Proteins 40–48 kDa | nd | 1.10 ± 0.051 | 1.01 ± 0.140 | 1.02 ± 0.144 | 1.00 ± 0.021 | 1.13 ± 0.052 | 1.12 ± 0.214 | 0.385 |
| Other proteins | nd | 1.13 ± 0.044 | 1.36 ± 0.812 | 0.97 ± 0.150 | 0.98 ± 0.102 | 1.23 ± 0.134 | 0.75 ± 0.236 | 0.145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga. Agriculture 2023, 13, 221. https://doi.org/10.3390/agriculture13010221
Spínola MP, Costa MM, Prates JAM. Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga. Agriculture. 2023; 13(1):221. https://doi.org/10.3390/agriculture13010221
Chicago/Turabian StyleSpínola, Maria P., Mónica M. Costa, and José A. M. Prates. 2023. "Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga" Agriculture 13, no. 1: 221. https://doi.org/10.3390/agriculture13010221
APA StyleSpínola, M. P., Costa, M. M., & Prates, J. A. M. (2023). Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga. Agriculture, 13(1), 221. https://doi.org/10.3390/agriculture13010221







