Abstract
Melatonin acts as a seed germination activator, plant growth regulator, leaf senescence retardant, and, in general, has a multifunctional role as a ‘defence molecule’; furthermore, by interacting with other molecules, such as phytohormones and gaseous molecules, it greatly enhances plant adaptation to different environments. However, there are not enough studies about the use of melatonin on horticultural crops, and even fewer studies have outlined the differences related to this phytohormone use between protected environment and in open field. The two latter systems have different growing conditions that could lead to diversified application doses. As the choice of melatonin dose depends on all crop system components, the present research aimed to assess the effects of three melatonin concentrations (1 ppm, 5 ppm and 10 ppm) plus an untreated control, on yield, quality, and antioxidants of four strawberry cultivars (i.e., Kabarla, Fortuna, Sweet Ann, Festival) grown either in greenhouse or in open field. Research was conducted to assess the yield parameters were better affected by greenhouse than open field, and mean fruit weight was the highest in cultivar Sweet Ann. In open field 10 ppm showed the highest values of fruit number and yield, but in greenhouse did not differ from 5 ppm which led to the highest fruit number. At all melatonin doses, cultivar Kabarla demonstrated the highest yield, compared to the other cultivars, with the maximum value of about 46 t·ha−1. Plant dry weight was 90% higher under greenhouse than in open field conditions, and 52% or 132% higher with cultivar Kabarla in comparison with Fortuna and Sweet Ann, respectively. The melatonin dose of 10 p.p.m showed 56% higher plant dry weight in comparison to the untreated control. Fortuna showed higher values of fruit dry matter, soluble solids, and glucose than Sweet Ann. The fruit dry matter was 7% lower at 10 ppm melatonin than in the untreated control. Fructose was higher under 1 ppm melatonin with 245 mg·g−1 d.w. compared to the untreated control with 220 mg·g−1 d.w. in Festival, whereas in Fortuna was the highest in the control fruits, the latter also showing the highest titratable acidity in Fortuna and Sweet Ann. The highest phenolics content was recorded under 10 ppm melatonin in open field, and with 5 ppm in greenhouse; the phenolics content was the highest under 1 ppm melatonin dose in Kabarla and 5 ppm in Fortuna. Under the protected environment 5 and 10 p.p.m. melatonin elicited the highest accumulation of ascorbic acid; 10 ppm were more effective in Fortuna and Sweet Ann, and 5 ppm in Festival. The most enhanced antioxidant activity was recorded under 5 p.p.m. melatonin dose in Fortuna and Festival. The present study confirms that the dose of melatonin to apply to strawberry crop closely relates both to cultivar and crop system.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) is a species belonging to the Rosaceae family and is a temperate crop, widely cultivated from autumn to spring [1]. Strawberry is grown in many world regions as it can adapt to different soil and climate conditions [2], and indeed USA, Turkey, Egypt, Mexico, Spain and Italy are important producers of this fruit [3].
Many people include strawberry fruits in their daily diets because of their high nutritional and phytochemical properties. Soluble solids content (SSC) and titratable acidity (TA), as well as their ratio, are among the major attributes of strawberry fruit quality [4,5], and have a remarkable impact on the consumers’ preferences [6,7], with a higher demand for the varieties showing higher values of soluble solids [8]. The beneficial effect of strawberry fruits on human health is associated to their anti-cancer properties due to the high content of ellagic acid [9], vitamin C [10], phenolics and anthocyanins resulting in high antioxidant capacity [11], which allows for prevent the cell damage caused by oxidation, by inhibiting or reducing the formation and activity of free radicals [12].
The varieties of this species are classified into two groups according to photoperiodism [13]: short-day cultivars, commonly preferred in lower altitude regions or coastal areas in Turkey; day-neutral varieties, usually chosen in high-altitude regions or highlands.
Melatonin (N-acetyl-5-methoxytryptamine) was first discovered in 1958 in the bovine pineal gland [14], and in the following decades, it was found in several other higher plants [15]. However, its effects on mammals and animals (birds, fishes, etc,) have been better studied [16,17] than on plants [18]. Melatonin has several important physiological roles in mammals, including circadian rhythms, sleep, body temperature, food intake, sexual behaviour and the immune system [19]. Moreover, this substance may act as a plant growth regulator which may stimulate root and shoot growth, activate seed germination and delay leaf senescence. Moreover, melatonin role in plants’ response against abiotic stress conditions is related to its antioxidant properties [20,21]. Zhang et al. [20] reported that melatonin application reduced chlorophyll degradation, increased the rate of photosynthesis in seedlings, thus reversing the effect of water stress, and restored the chloroplast structure of cucumber leaves under drought stress. Melatonin applications were also reported to increase the ascorbic acid content in plants [22]. In a study conducted on soybeans, it was found that melatonin application increased plant growth, increased crop yield and provided tolerance to abiotic stress conditions [18]. The antioxidant properties of melatonin were demonstrated in some plant species, including apple, rice and grape [22,23,24]. As reported in the review by Agathokleous et al. [25], many studies have been conducted on higher plants in which different doses of melatonin, used as a biostimulant, were applied, even under non-stressful conditions.
The available literature reports suggest that melatonin has significant roles in plant growth and development, but it is not clear yet if the application dose for each species differs based on cultivar and cropping system simultaneously; in fact, we hypothesise that the application dose could depend on the interaction between cultivar and cropping system. The present study was conducted to compare the effects of different melatonin doses application on fruit yield and quality of four strawberry cultivars grown both in greenhouse and open field in the Isparta region in Turkey.
2. Materials and Methods
2.1. Experimental Design and Growing Conditions
Research was carried out during the 2015-16 and 2016-17 growing seasons, at the Agricultural Research and Application Centre of Süleyman Demirel University, Isparta region, with the aim to assess the effects of two crop systems (greenhouse, open field) in factorial combination with four cultivars (i.e., Kabarla, Fortuna, Sweet Ann, Festival) and three melatonin doses (i.e., 1 ppm, 5 ppm and 10 ppm) plus an untreated control, on fruit yield, quality, and antioxidants of strawberry. A split split plot design with three replicates was used for the treatment distribution both in greenhouse and open field, and the experimental unit covered a 15 m2 (5 × 3 m) surface area.
The four strawberry cultivars used in the present experiment are among the most spread in the research agricultural Region and were provided by the private company Yaltır AŞ (Adana city, Turkey).
Melatonin application started on March 4th and 6th in greenhouse, and on June 25th and 27th in open field, when new shoots had sprung and the plants were well established in the soil, in 2015 and 2016, respectively, and was repeated after a week. One litre of Melatonin solution was sprayed on the leaves at a volume of 1 L per 40 plants.
Strawberry was transplanted on June 10th and 12th, in 2015 and 2016 respectively, on 100 cm wide raised beds, mulched with 50 µm black polythene, in double rows with 30 cm × 30 cm spacing between and along the rows, with 5.3 plants·m−2 density.
Each year 280 kg·ha−1 N, 130 kg·ha−1 P2O5 and 460 kg·ha−1 K2O were supplied: half dose was given two days prior to transplanting as ammonium sulphate, single superphosphate and potassium sulphate, while the remaining 50% was applied on dressing through fertigation at two-week intervals, using potassium nitrate and monoammonium phosphate. Drip irrigation was activated when the AWC decreased to 80%. In early January, weeds and plant withered leaves were removed. Thiram, Abamectin and Imidacloprid were used for plant pest and disease control treatments.
Harvests of marketable fruit were performed every five days on average, and: began on May 27th in greenhouse, and on June 6th and 7th in open field, in 2016 and 2017 respectively; ended on June 21st in greenhouse and July 1st and 2nd in open field, in 2016 and 2017 respectively.
2.2. Yield and Quality Analysis
At each harvest, the weight and number of marketable fruits (regularly shaped and sized) were determined on 32 plants of the two central double rows in all the plots, and the mean fruit weight on 50-fruit samples. Total yield was determined as the sum of all harvest productions.
A 30-fruit sample was randomly collected in each plot in the middle of the harvest period and immediately transferred to the laboratories for quality analyses. In addition, a nine-plant sample was randomly collected in each plot at the end of crop cycle and transferred to the laboratories for quality analyses.
Both plant and fruit dry matter were assessed in an oven at 70 °C until constant weight.
The soluble solids content (SSC) was measured with a refractometer and expressed in °Brix; glucose, fructose and sucrose were determined by HPLC, and titratable acidity (TA) was determined according to the standard NaOH titration method, as g 100 g−1 citric acid [26].
The spectrophotometric method was used for determining the ascorbic acid content, and 2,6-Dichlorophenolindephenol (2,6-D) dyestuff was used for the reduction by ascorbic acid. In this respect, standard curves were obtained through solutions prepared with oxalic acid, ascorbic acid, and 2,6-D dyestuff. Ten ml of fruit juice was squeezed and diluted 10 times with oxalic acid, then a 1 mL aliquot was mixed with 9 mL of distilled water, and another 1 mL aliquot with 9 mL of 2,6-D dye. The solutions obtained were read by the spectrophotometer at 518 nm wavelength. By replacing Abs values in the standard curve, the corresponding amounts of ascorbic acid were determined [26].
The total phenolic content (TPC) was determined by using Folin-Ciocalteu reagent according to the method described by Singleton and Rossi [27]. The fruit juice was mixed with Folin-Ciocalteu reagent and distilled water at a ratio of 1:1:18, left to rest for 8 min, and then 7% sodium carbonate was added into the mixture. The latter was incubated in a dark room for 2 h and the absorbance (at 750 nm) of the bluish solution was measured using a spectrophotometer. The results were expressed as gallic acid equivalent, calculated as µg GAE per g of fresh weight.
Free radical scavenging activity percentage (DPPH), i.e., antioxidant activity was measured according to the method of Boskou et al. [28], by determining the scavenging rate of the methanolic extracts in the DPPH radical samples. One hundred µL of methanolic extract were diluted at a 1/10 ratio, mixed with 3.9 mL of 6 × 105 M DPPH solution, and left to rest for 30 min. The results were measured using a double-beam UV-Vis spectrophotometer at 515 nm, in 3 parallel runs. The percentage of DPPH scavenging activity was calculated according to the equation of “%DPPH = [(Ac − As)/Ac] × 100”, where Ac represents the absorbance of the negative control, and As the sample absorbance.
2.3. Data Statistical Analysis
The experimental data were statistically processed by three-way analysis of variance (ANOVA), using the software SPSS version 22.0, and the mean separations were performed through Tukey’s honesty test at p ≤ 0.05.
3. Results
No yield, quality and phytochemical parameters were significantly affected by the year of research and, therefore, the average values of the two years of the experiment have been reported, both in Tables and Figures.
3.1. Yield Parameters
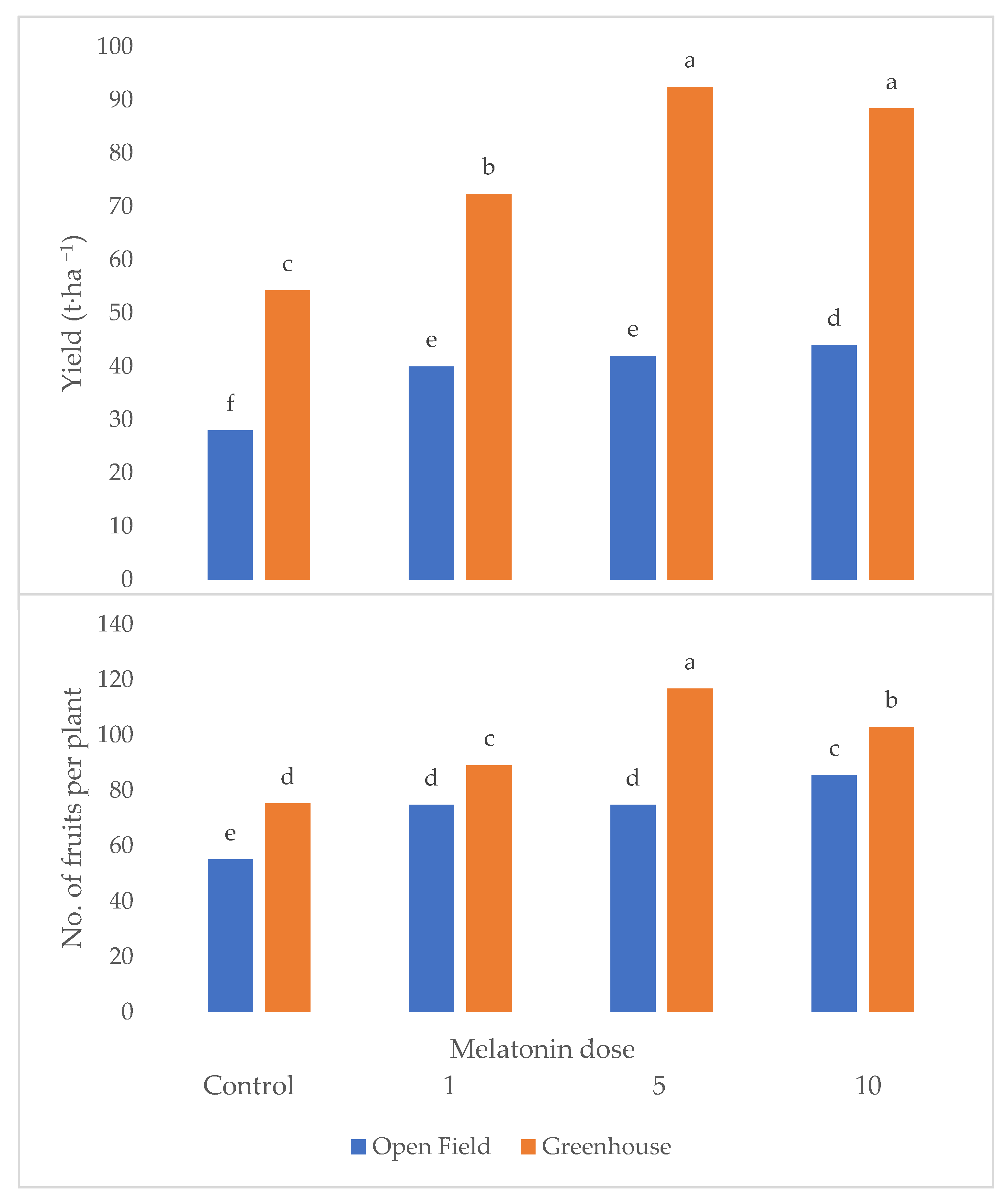
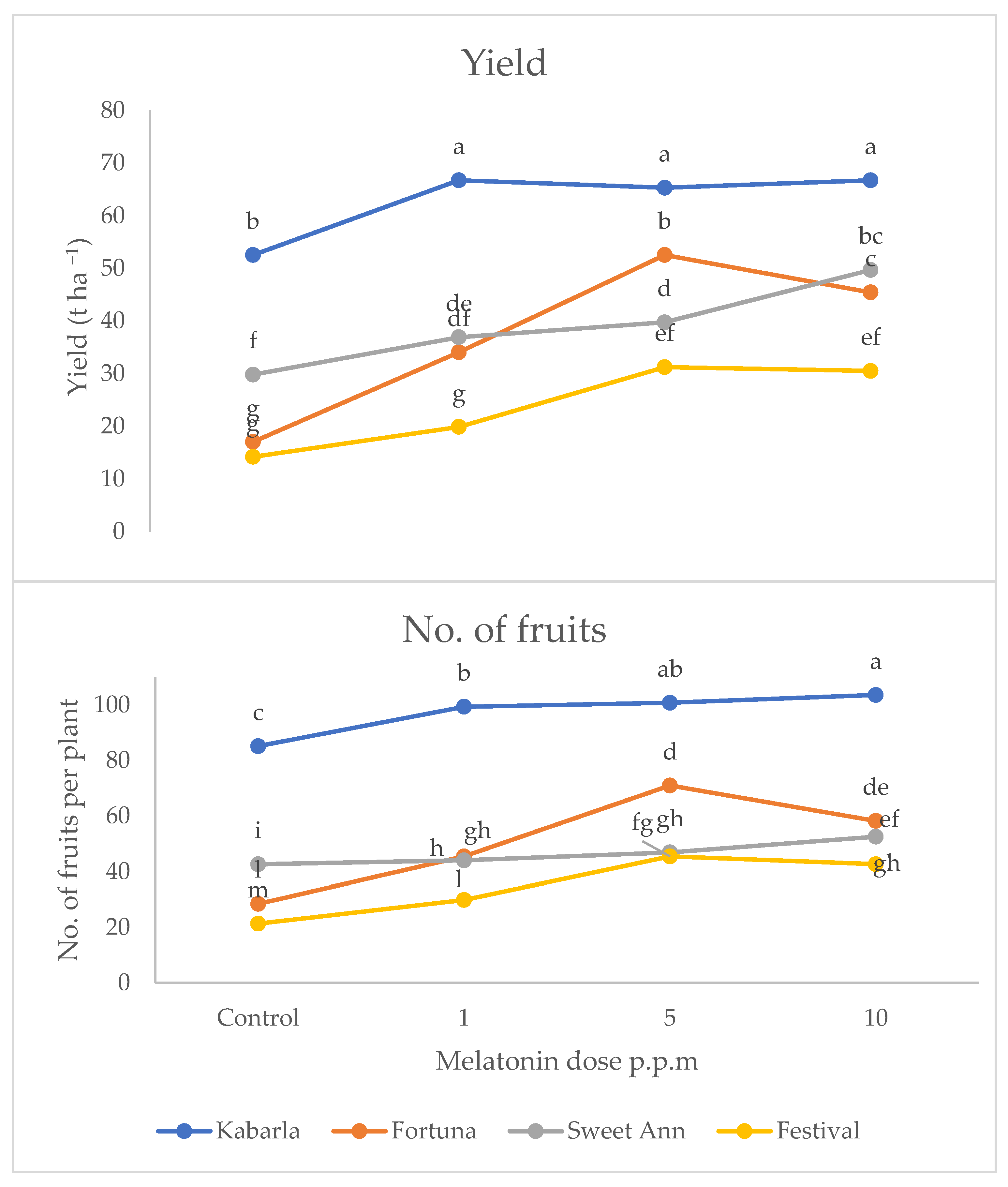
Yield and fruit number were significantly affected by the interaction between cultivar and melatonin dose (Figure 1 and Figure 2). In fact, both variables attained the highest values under the melatonin application compared to the control, with cultivar Kabarla showing the best yield performances with 47 t ha−1 and about 70 fruits per plant. Fortuna was best affected by the 5 p.p.m. concentration, reaching about 36 t ha−1, and about 50 fruits per plant, instead, Sweet Ann by the 10 p.p.m. dose, and Festival both by 5 and 10 ppm melatonin levels. All the cultivars showed a higher yield under the protected environment compared to open field, whereas the production consequences of the melatonin treatment were controversial.
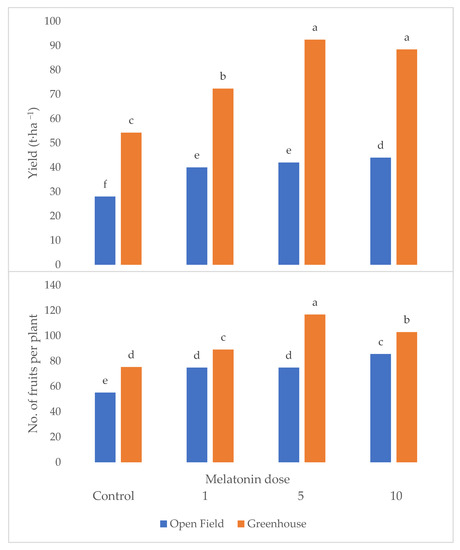
Figure 1.
Interaction between crop system and melatonin dose on yield and fruit number per plant of strawberry grown in Isparta region (Turkey); different letters are statistically different according to Tukey’s HSD test at p ≤ 0.05.
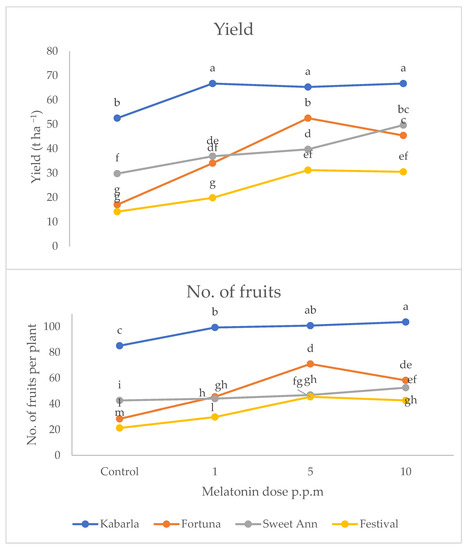
Figure 2.
Interaction between cultivar and melatonin dose on yield and fruit number per plant of strawberry grown in Isparta (Turkey), the values followed by different letters are statistically different according to Tukey’s HSD test at p ≤ 0.05.
As can be observed in Table 1, the mean fruit weight was significantly higher in greenhouse than in open field, in cultivar Sweet Ann compared to the other three varieties but was not affected by the melatonin dose. Plant dry weight attained the highest values under greenhouse, with cultivar Kabarla and upon the 10 p.p.m. melatonin dose supply. From the significant interaction between crop system and melatonin dose, it arose that 10 ppm was the most effective concentration either on yield (Figure 1) or fruit number (Figure 2) in open field, with average values 57% higher in comparison to control, whereas in greenhouse it did not significantly differ in terms of yield from 5 ppm which elicited the highest fruit number (+55% compared to control).

Table 1.
Effects of crop system, cultivar and melatonin concentration on yield and growth parameters of strawberry grown in Turkey.
3.2. Quality and Phytochemical Parameters
Regarding quality parameters (Table 2), the crop system did not significantly affect any of the variables examined. Between the cultivars, Fortuna showed higher values of dry matter, soluble solids and glucose than Sweet Ann (from 5 to 7 % more, respectively), whereas no differences arose regarding sucrose content. Dry matter negatively reacted to 10 ppm melatonin application compared to the untreated control by 6% less, whereas soluble solids, glucose and sucrose were not significantly affected.

Table 2.
Effects of crop system, cultivar and melatonin dose on quality attributes of strawberry fruits grown in Turkey.
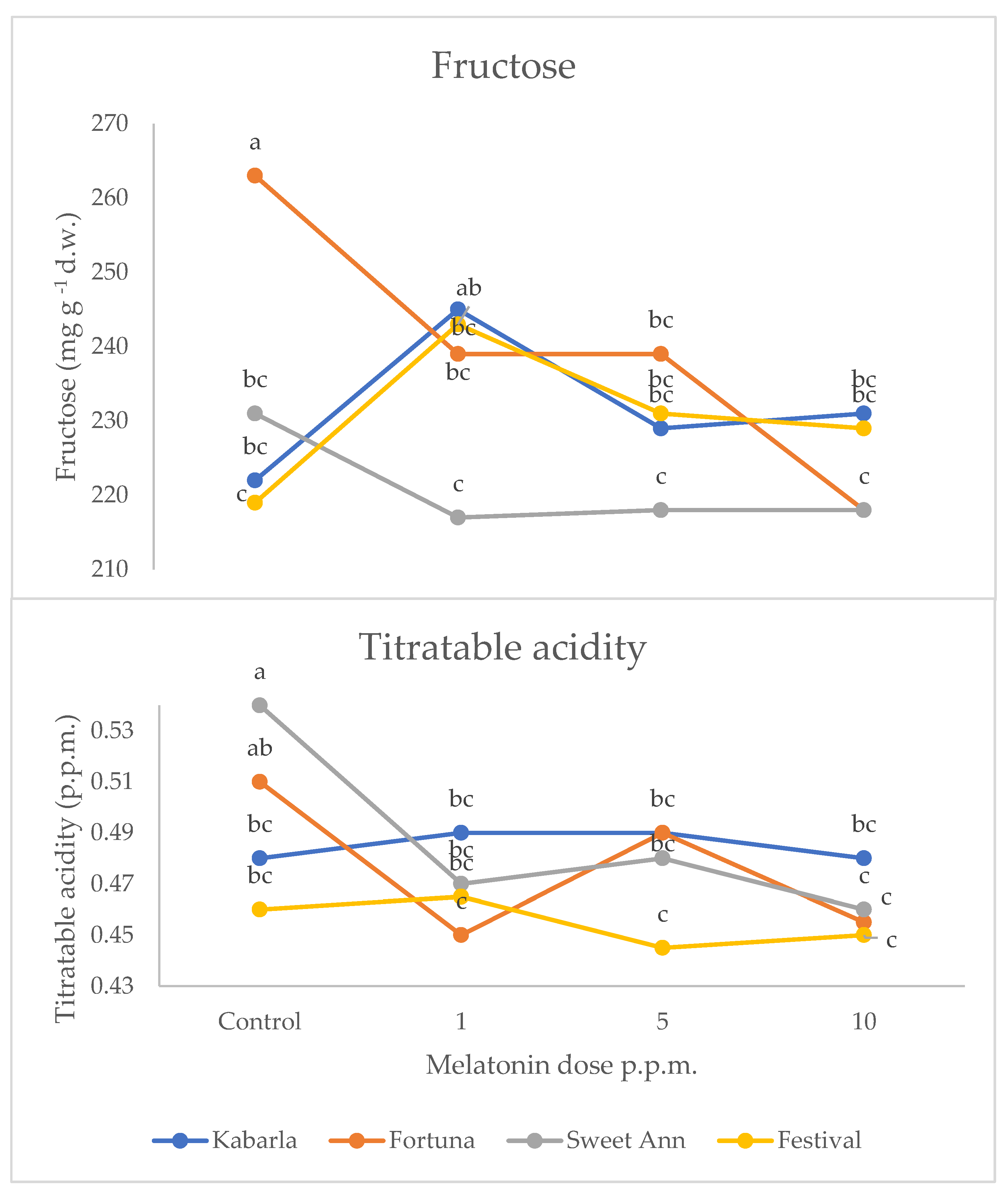
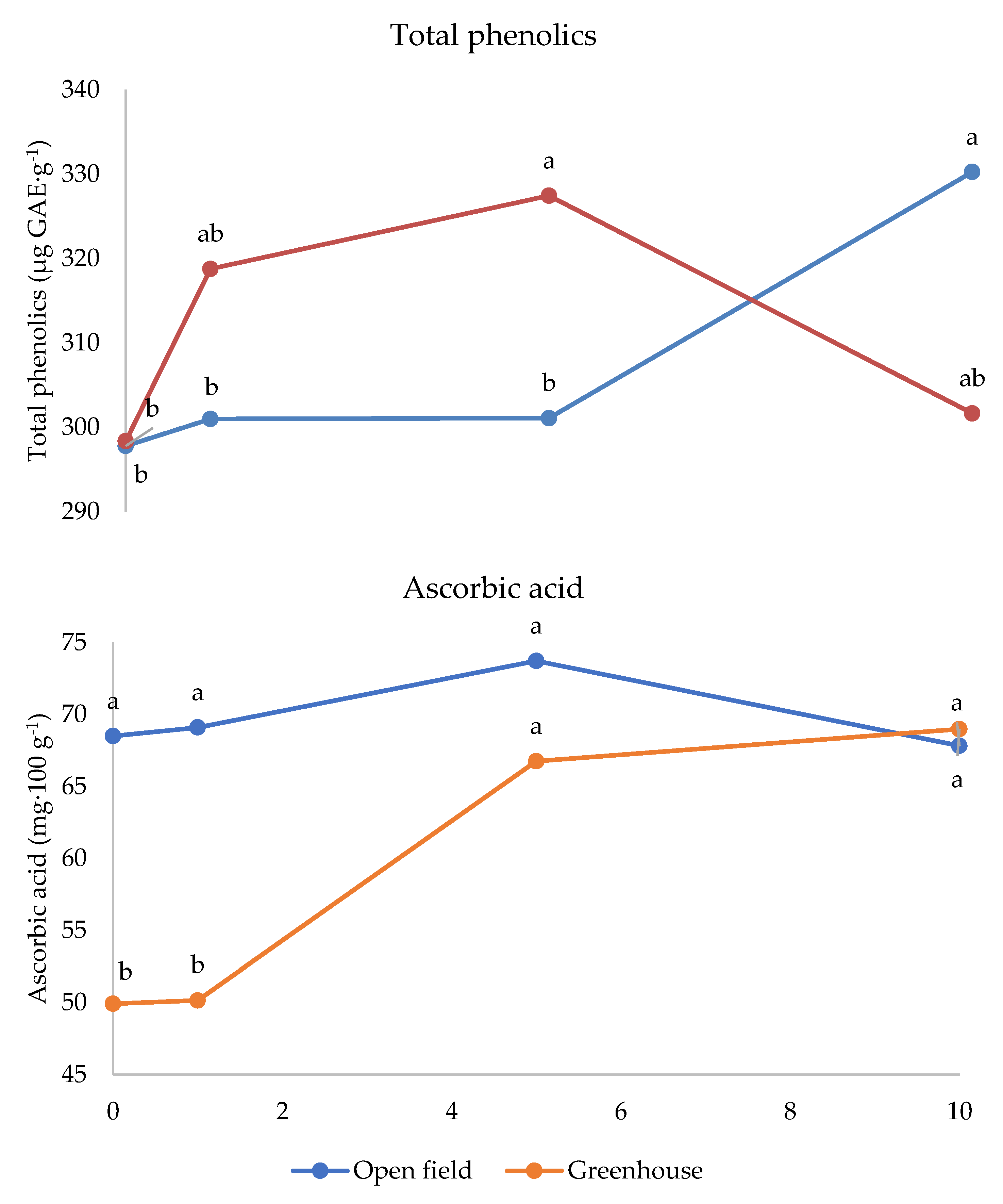
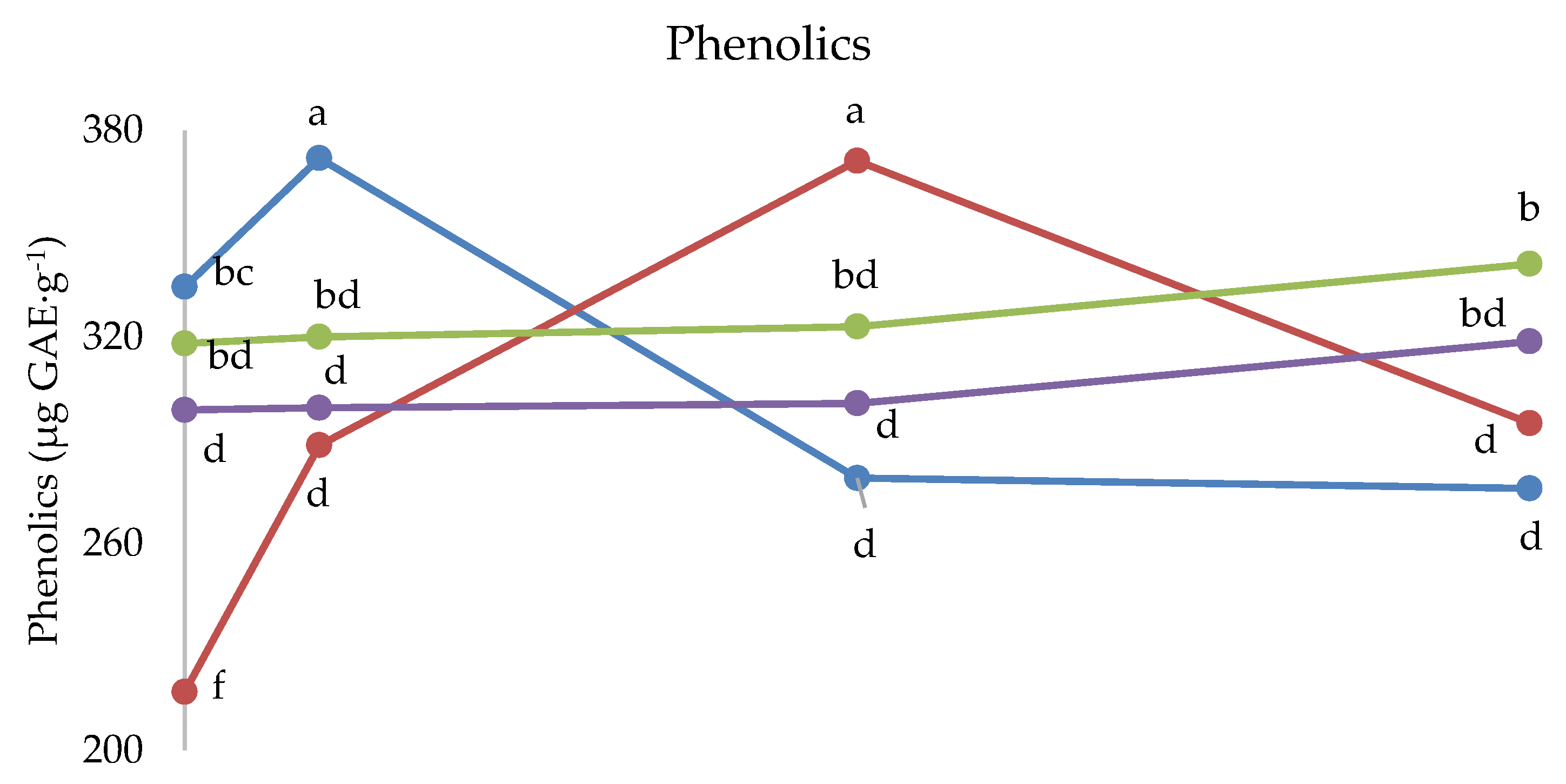
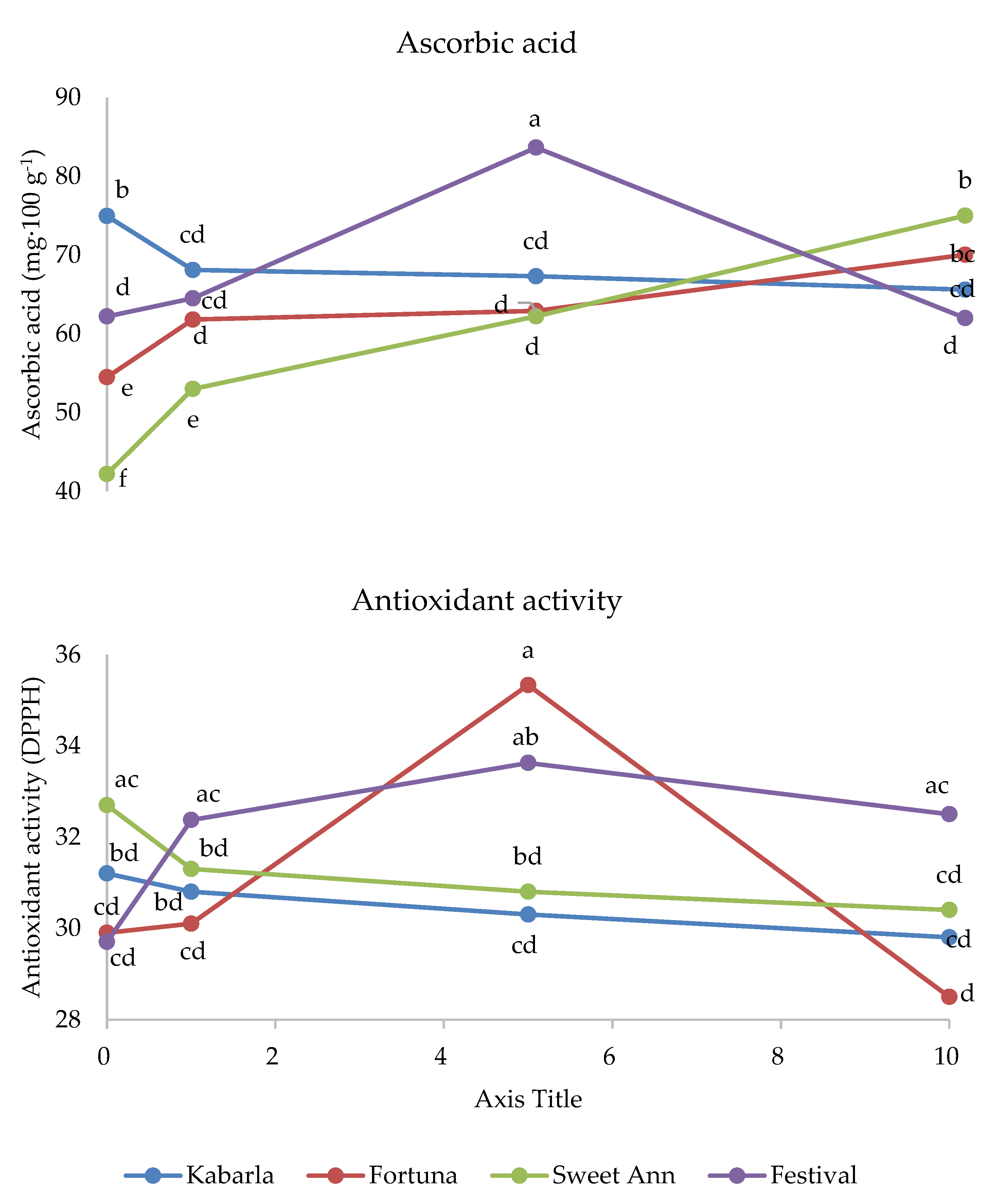
The interaction between cultivar and melatonin dose was significant on fructose and titratable acidity (Figure 3). Fructose was higher under 1 ppm application compared to the untreated control in Festival (+11%), whereas in cultivar Fortuna it was the highest in the control (+10%). The latter cultivar also showed the highest value compared to the control fruits of the other cultivars, whereas the 1 ppm application in Festival was more effective than the same treatment in Sweet Ann. The untreated control fruits showed the highest values of titratable acidity in Fortuna and Sweet Ann, compared to melatonin treatments. Sweet Ann fruits had higher titratable acidity than Kabarla and Festival ones only in the control, whereas no significant differences arose between cultivars corresponding to each melatonin dose. The antioxidant activity was not significantly affected by the crop system (Table 3). The interaction between the crop system and melatonin dose was significant for both phenolics and ascorbic acid (Figure 4). Indeed, the 10 ppm melatonin application resulted in the highest phenolics in open field, whereas 5 ppm was the most effective in greenhouse and even higher compared to the same treatment applied in open field conditions. Ascorbic acid was not significantly affected by melatonin application in open field, whereas under protected environment 5 and 10 p.p.m. elicited the highest accumulation of this antioxidant, 30% more. Moreover, the untreated control and 1 p.p.m. melatonin-supplied fruits showed higher ascorbic acid values in open field than in greenhouse.
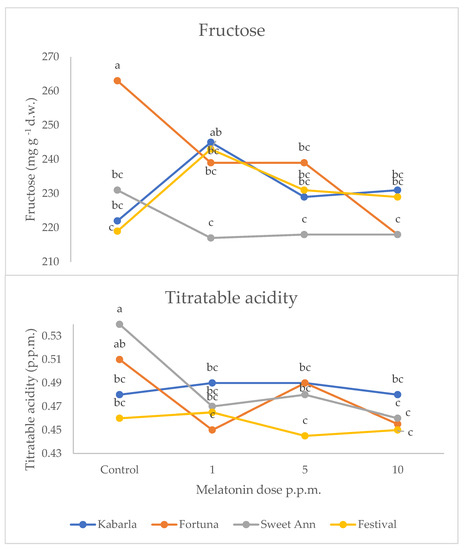
Figure 3.
Interaction between Cultivar and Melatonin dose on fructose content and titratable acidity of strawberry fruits grown in Isparta region (Turkey), the values followed by different letters are statistically different according to Tukey’s HSD test at p ≤ 0.05.

Table 3.
Effects of crop system, cultivar and melatonin dose on antioxidant compounds and activity of strawberry fruits grown in Turkey.
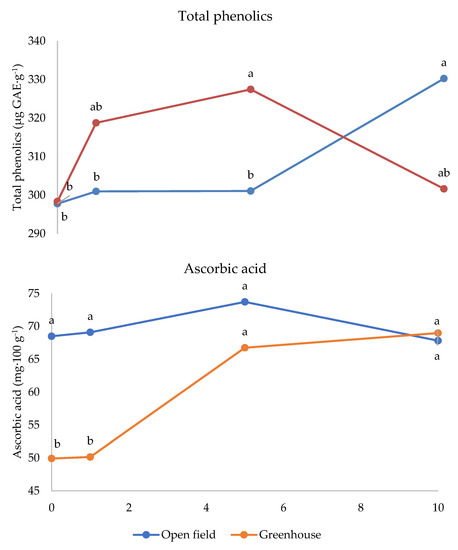
Figure 4.
Interaction between Crop system and Melatonin dose on total phenolics and ascorbic acid content of strawberry fruits grown in Isparta region (Turkey), the values followed by different letters are statistically different according to Tukey’s HSD test at p ≤ 0.05.
The interaction between cultivar and melatonin dose was significant on phenolics, ascorbic acid and antioxidant activity (Figure 5). Indeed, the phenolics content was the highest under 1 ppm melatonin dose in Kabarla and 5 ppm in Fortuna, both about 380 µg GAE·g−1, whereas the other two cultivars were not significantly affected by melatonin application. Regarding the comparison between cultivars, the highest phenolics levels were recorded in Kabarla, both in the fruits of the control, not significantly different from Sweet Ann, and in those grown under the 1 ppm melatonin treatment; in Fortuna fruits supplied with the 5 p.p.m. melatonin dose; in Sweet Ann fruits under 10 ppm melatonin application, not significantly different from Festival.
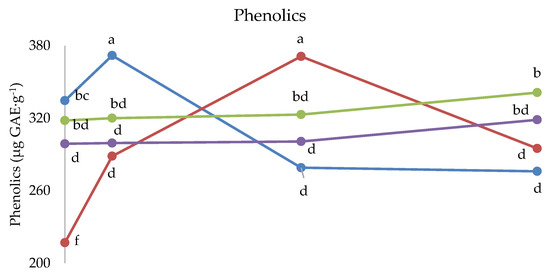
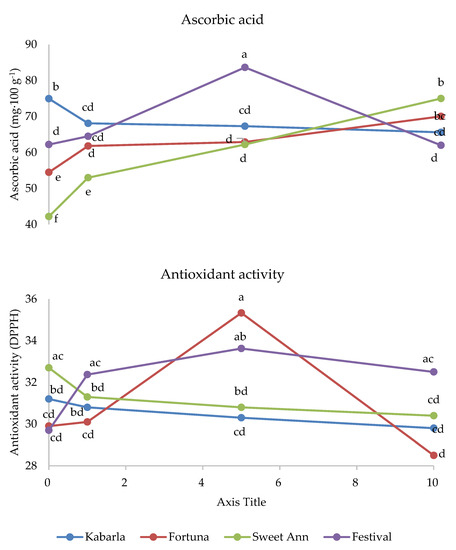
Figure 5.
Interaction between Cultivar and Melatonin dose on phenolics and ascorbic acid content, and on antioxidant activity of strawberry fruits grown in Isparta region (Turkey), the values followed by different letters are statistically different according to Tukey’s HSD test at p ≤ 0.05.
The ascorbic acid concentration was the highest under 10 ppm melatonin in Fortuna and Sweet Ann fruits, and 5 ppm in Festival, whereas this antioxidant was adversely affected by melatonin application in Kabarla. Moreover, Kabarla showed the highest value of ascorbic acid in control fruits, Sweet Ann the lowest under 1 ppm melatonin treatment and the highest with 10 ppm, and Festival the highest content corresponding to 5 ppm supply.
The most enhanced antioxidant activity was recorded under 5 p.p.m. melatonin dose in Fortuna and Festival, though in the latter cultivar not significantly different from 1 and 10 p.p.m., whereas the other two cultivars were not affected by this experimental factor. As for the comparison between cultivars, no significant differences in antioxidant activity arose in the fruits of untreated control and in those grown under 1 ppm melatonin dose; Fortuna showed the highest antioxidant activity under 5 p.p.m. melatonin application, not significantly different from Festival; the latter cultivar had a higher level of this variable than Fortuna at 10 p.p.m. melatonin supply.
4. Discussion
In our research, melatonin application affected cultivar performance and provided more benefits to strawberry crops grown in protected environment compared to the open field ones, presumably due to the more favourable environmental conditions.
Melatonin, sharing the same biosynthetic prospector as the Indole-3-acetic acid, is a type of indoleamine (IAA), i.e., an auxinic hormone found in monocots such as wheat, canary grass, oat, and barley as well as dicots like Arabidopsis thaliana and lupin [29]. As a confirmation of our findings, according to Zhang et al. [30] melatonin application results in an increased number of fruits, presumably as a consequence of signal enhancement of hormones such as ethylene, ADA, JA, SA, and ET [31]. Moreover, Kolář et al. [29] observed that melatonin treatment considerably affected plant growth and yield, maybe due to the low plant endogenous content of this substance. In this respect, the exogenously supplied melatonin is permeable in the plasma membrane, thus increasing its endogenous level, which promoted plant growth and seed output in soybean [32]. Notably, melatonin can either up- or down-regulate genes and, therefore, the effects of its application are genotype-dependant [33] and the most efficient exogenous concentration relates to plant species and cultivar [34]. In the study of Abbas and Sarhan [34] on faba bean, it arose that genotype and melatonin dose interacted on yield and plant growth indices, particularly increasing leaf area. The latter increase may enhance chlorophyll accumulation and photosynthetic efficiency [35], with a consequent bigger and heavier fruits and higher yield [36].
Melatonin may serve as a stress defence, but it is increasingly recognised as a biostimulator of seed germination, plant growth, photosynthesis, and other physiological and biochemical activities [37]. In fact, in our research at any dose of melatonin application, fruit number and yield were significantly higher than the untreated control, presumably as a consequence of the increased photosynthetic activity due to the hormonal effect. Moreover, strawberry yield was more than double compared to that recorded by Saini et al. [38], with the mean fruit weight ranging from 10.6 g to 15.5 g among the different cultivars. In this respect, Kim et al. [37] stated that the size of strawberry fruits is widely genotype-dependent and, indeed, Gasperotti et al. [39] reported that the fruit weight varied from 10.4 g of cultivar Clery to 15.8 g of Portola, Zeliou et al. [7] found a fruit weight range between 23.8 g to 27.27 g, and Liu et al. [40] recorded a fruit weight ranging from 21.5 g of cultivar Tochiotome to 35.0 g of Benihoppe.
In this research, the higher amount of plant dry matter is a representation of how melatonin application can promote the concentration increase of carbohydrates and of plant structural elements [41]. Differently, a lower fruit dry matter was recorded under melatonin application, which was explained by Altaf et al. [36], in a tomato investigation, as the modification of root architecture under this hormone treatment, resulting in uptake differences among the nutrients (i.e., N, P, K, Mn, Ca, and Fe).
Wei et al. (2014) [18] investigated the role of melatonin on transcription genes both in tree and vegetable species and confirmed the importance of this hormone in glucose metabolism. In addition, several genes linked to photosynthesis, starch, sucrose, glycolysis, fermentation, the Krebs cycle, and other metabolic processes were evidently overexpressed by melatonin [42,43]. Contrary to the latter, in the present research, most of the quality parameters were not significantly affected by melatonin application, assuming that it did not affect the transcription of genes involved in carbohydrate synthesis. Moreover, we did not find the positive effects recorded by Li et al. [41] on the quality of tea (Camellia sinensis L.).
The soluble solids content ranged from 9.1% to 9.8%, i.e., in a narrower interval compared to that reported by Voca et al. [44], varying from 6% to 10%.
In the work conducted by Arnao et al. (2021) [45], the melatonin application to various vegetable species led to the increase of soluble solids content, represented by carbohydrates, but in our study, no variation occurred.
Differently from Bhardwaj et al. [46], we did not find a relationship between titratable acidity and soluble solids content, whose ratio is considered a ripeness index.
In a study on wheat seedlings [33], melatonin application resulted in a different modulation of the antioxidant synthesis pathways, through the regulation of genes responsible for producing enzymes such as CAT, POD, APX, SOD, and GR; subsequently, antioxidant activities and molecules were enhanced, particularly the concentration of total polyphenols and ascorbic acid, as probably occurred in the present study.
5. Conclusions
The results of the present research suggest that melatonin application significantly improved yield, quality, and antioxidant compounds and activity of strawberry fruits, despite the variability of the cultivar response, also depending on the growing conditions. Cultivar Kabarla was generally the most productive, and Fortuna exceeded in yield Sweet Ann and Festival at 5 ppm melatonin dose. Interestingly, the fructose content only increased in Festival at the melatonin level of 1 ppm. In relation to the crop system, melatonin treatment had different effects on the total phenolics content, with the open field system benefiting more from 10 ppm. This study provides knowledge regarding the use of melatonin on strawberry, with particular reference to the relation between the application dose, cultivar and crop system. However, future research would be useful to investigate the interaction of melatonin dose with other crop management components.
Author Contributions
Conceptualization, V.O., M.A.A. and G.C.; data curation, M.P.; formal analysis, I.B., A.M.Ç. and A.V.T.; investigation, A.M.Ç., S.F.G. and I.B.; methodology, I.B., S.F.G. and İ.K.; supervision, V.O., M.A.A. and G.C.; validation, V.O. and G.C.; draft manuscript writing, V.O., M.A.A., A.V.T. and G.C.; manuscript revision and final editing, A.V.T., V.O. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported with a grant from the Uşak University Scientific Research Projects Coordination Unit (Project Number: 2017/MF002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Özgüven, A.I.; Yilmaz, C. Bazı Çilek Çeşitlerinin Adana Ekolojik Koşullarındaki Morfolojik ve Pomolojik Özellikleri Alatarım. Eskiseh. Osman. Univ. Res. Inf. Syst. 2009, 8, 17–21. [Google Scholar]
- Darrow, G.M. The strawberry. History, breeding and physiology. In The Strawberry. History, Breeding and Physiology; Holt, Rinehart & Winston: New York, NY, USA, 1966. [Google Scholar]
- Padmanabhan, P.; Mizran, A.; Sullivan, J.A.; Paliyath, G. Strawberries’ Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 193–198. [Google Scholar]
- Kader, A.A. Quality and its maintenance in relation to the postharvest physiology of strawberry. In The Strawberry into the 21st Century; Timber Press: Portland, Oregon, 1991; pp. 145–152. [Google Scholar]
- Salamé-Donoso, T.P.; Santos, B.M.; Chandler, C.K.; Sargent, S.A. Effect of high tunnels on the growth, yields, and soluble solids of strawberry cultivars in Florida. Int. J. Fruit Sci. 2010, 10, 249–263. [Google Scholar] [CrossRef]
- Naumann, W.D.; Seipp, D. Grundlagen für anbau und vermarktung. In Erdbeeren; Ulmer Fachbuch: Stuttgart, Germany, 1990; p. 256. [Google Scholar]
- Kahramanoğlu, İ. Effects of lemongrass oil application and modified atmosphere packaging on the postharvest life and quality of strawberry fruits. Sci. Hortic. 2019, 256, 108527. [Google Scholar] [CrossRef]
- Chandler, C.K.; Herrington, M.; Slade, A. Effect of harvest date on soluble solids and titratable acidity in fruit of strawberry grown in winter, annual hill production system. In XXVI International Horticultural Congress: Berry Crop Breeding, Production and Utilization for a New Century; Toronto, Canada, 2002; Volume 626, pp. 345–346. [Google Scholar]
- Türemiş, N.; Özgüven, A.I.; Paydaş, S. Güneydoğu Anadolu Bölgesinde Çilek Yetiştiriciliği. Türkiye Bilimsel ve Teknik Araştırma Kurumu. In Türkiye Tarımsal Araştırma Projesi Yay; Adana, Turkey, 2000; p. 36. [Google Scholar]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2017, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A. Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur. Food Res. Technol. 2009, 228, 623–631. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.P. In Vitro methods of assay of antioxidants: An overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- MoA. Turkey. Alata Bahçe Kültürleri Araştırma İstasyonu. Çilek yetiştirciliği. 2012. Available online: https://arastirma.tarimorman.gov.tr/alata/Belgeler/Diger-belgeler/%C3%87ilekYeti%C5%9Ftiricili%C4%9Fi%C3%87Nacar.pdf (accessed on 15 January 2022).
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Arnao, M.B. Phytomelatonin: Discovery, content, and role in plants. In Advances in Botany; Hindawi Publishing Corporation: London, UK, 2014. [Google Scholar]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.E.; Jang, H.; Byeon, Y.; Kim, Y.S.; Back, K. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef]
- Agathokleous, E.; Zhou, B.; Xu, J.; Ioannou, A.; Feng, Z.; Saitanis, C.J.; Frei, M.; Calabrese, E.J.; Fotopoulos, V. Exogenous application of melatonin to plants, algae, and harvested products to sustain agricultural productivity and enhance nutritional and nutraceutical value: A meta-analysis. Environ. Res. 2021, 200, 111746. [Google Scholar] [CrossRef]
- Hışıl, Y. Enstrümantal Gıda Analizleri Laboratuar Kılavuzu Ege Üniversitesi Yayınları; 1993; Volume 55. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Boskou, D.; Tsimidou, M.; Blekas, G. Polar phenolic compounds. In Olive Oil, 2nd ed.; Boskou, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 73–92. [Google Scholar]
- Kolář, J.; Macháčková, I.; Eder, J.; Prinsen, E.; Van Dongen, W.; Van Onckelen, H.; Illnerová, H. Melatonin: Occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 1997, 44, 1407–1413. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J. Soil Sci. Plant Nutr. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.T.; Pessarakli, M.; Sekara, A.; Caruso, G.; Rady, M.M.; El-Metwally, I.M. Role of melatonin in improving the tolerance of plants to salinity stress. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 158–173. [Google Scholar]
- Abbas, S.M.; Sarhan, I.A. The role of melatonin in improving the vegetative growth characteristics of four genotypes of Faba Bean (Vicia faba L.). In IOP Conference Series: Earth and Environmental Science (Vol. 904, No. 1, p. 012028); IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Ren, M.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Shahid, S.; Shakoor, A.; Sohail, H.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2020, 172, 820–846. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Kim, S.K.; Bae, R.N.; Na, H.; Dal Ko, K.; Chun, C. Changes in physicochemical characteristics during fruit development in June-bearing strawberry cultivars. Hortic. Environ. Biotechnol. 2013, 54, 44–51. [Google Scholar] [CrossRef]
- Saini, S.; Kumar, P.; Sharma, N.C.; Sharma, N.; Balachandar, D. Nano-enabled Zn fertilization against conventional Zn analogues in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2021, 282, 110016. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Palmieri, L.; Martinatti, P.; Pojer, E.; Mattivi, F.; Vrhovsek, U. Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp. J. Agric. Food Chem. 2013, 61, 8597–8607. [Google Scholar] [CrossRef]
- Liu, L.; Ji, M.L.; Chen, M.; Sun, M.Y.; Fu, X.L.; Li, L.; Gao, D.S.; Zhu, C.Y. The flavor and nutritional characteristic of four strawberry varieties cultured in soilless system. Food Sci. Nutr. 2016, 4, 858–868. [Google Scholar] [CrossRef]
- Li, X.; Li, M.H.; Deng, W.W.; Ahammed, G.J.; Wei, J.P.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.D.; Buck, G.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef] [PubMed]
- Voća, S.; Dobričević, N.; Dragović-Uzelac, V.; Duralija, B.; Družić, J.; Čmelik, Z.; Skendrović Babojelić, M. Fruit quality of new early ripening strawberry cultivars in Croatia. Food Technol. Biotechnol. 2008, 46, 292–298. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Pareek, S.; González-Aguilar, G.A.; Domínguez-Avila, J.A. Changes in the activity of proline-metabolising enzymes is associated with increased cultivar-dependent chilling tolerance in mangos, in response to pre-storage melatonin application. Postharvest Biol. Technol. 2021, 182, 111702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).