Starch-Based Superabsorbent Enhances the Growth and Physiological Traits of Ornamental Shrubs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Pattern of Conducted Experiment
2.2. Growth Parameters of Plants
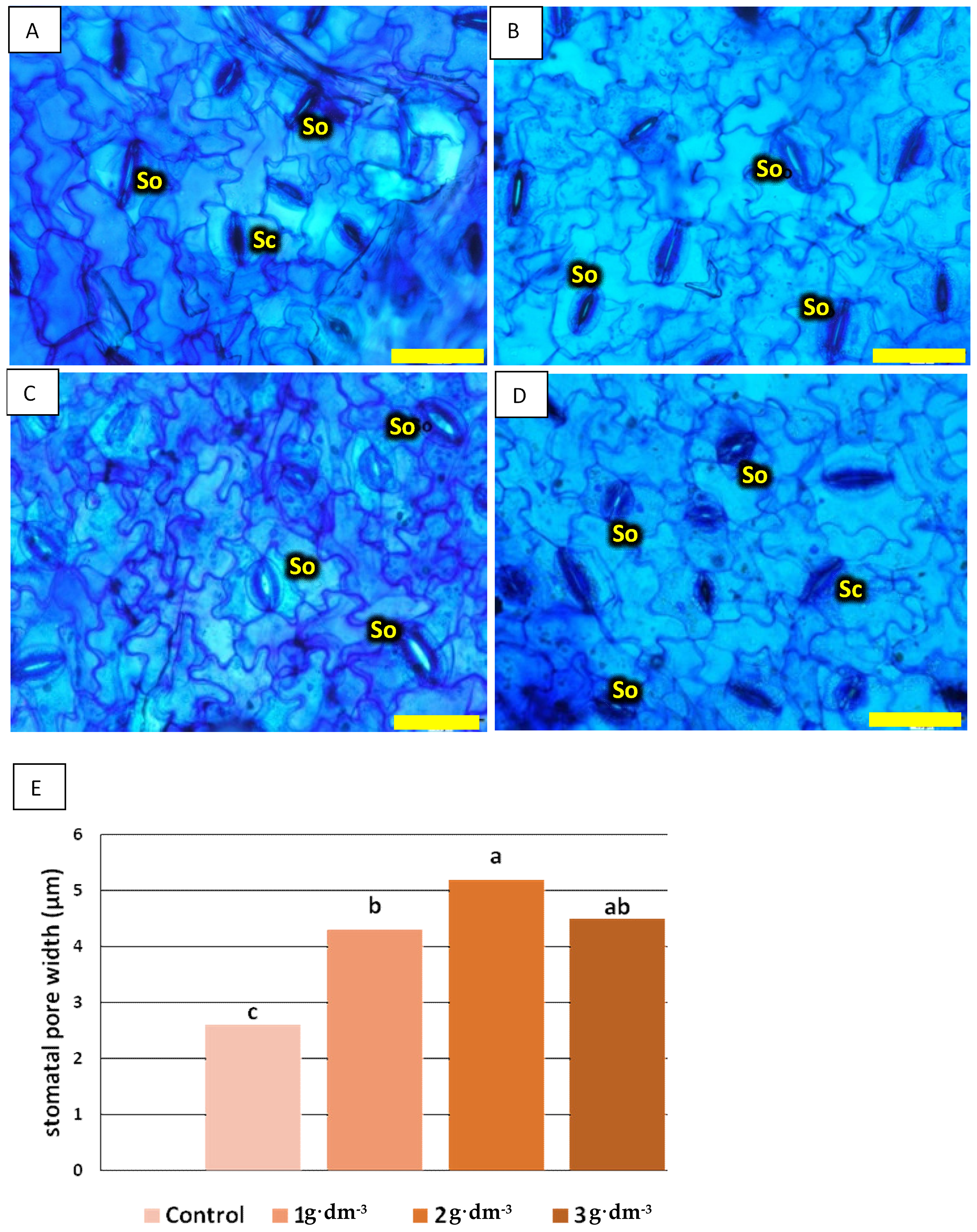
2.3. Epidermis Stomata
2.4. Photosynthetic Rate and Stomatal Conductance
2.5. Determination of Chlorophylls and Carotenoids
2.6. Determination of Bioactive Components
2.7. Statistical Analysis
3. Results
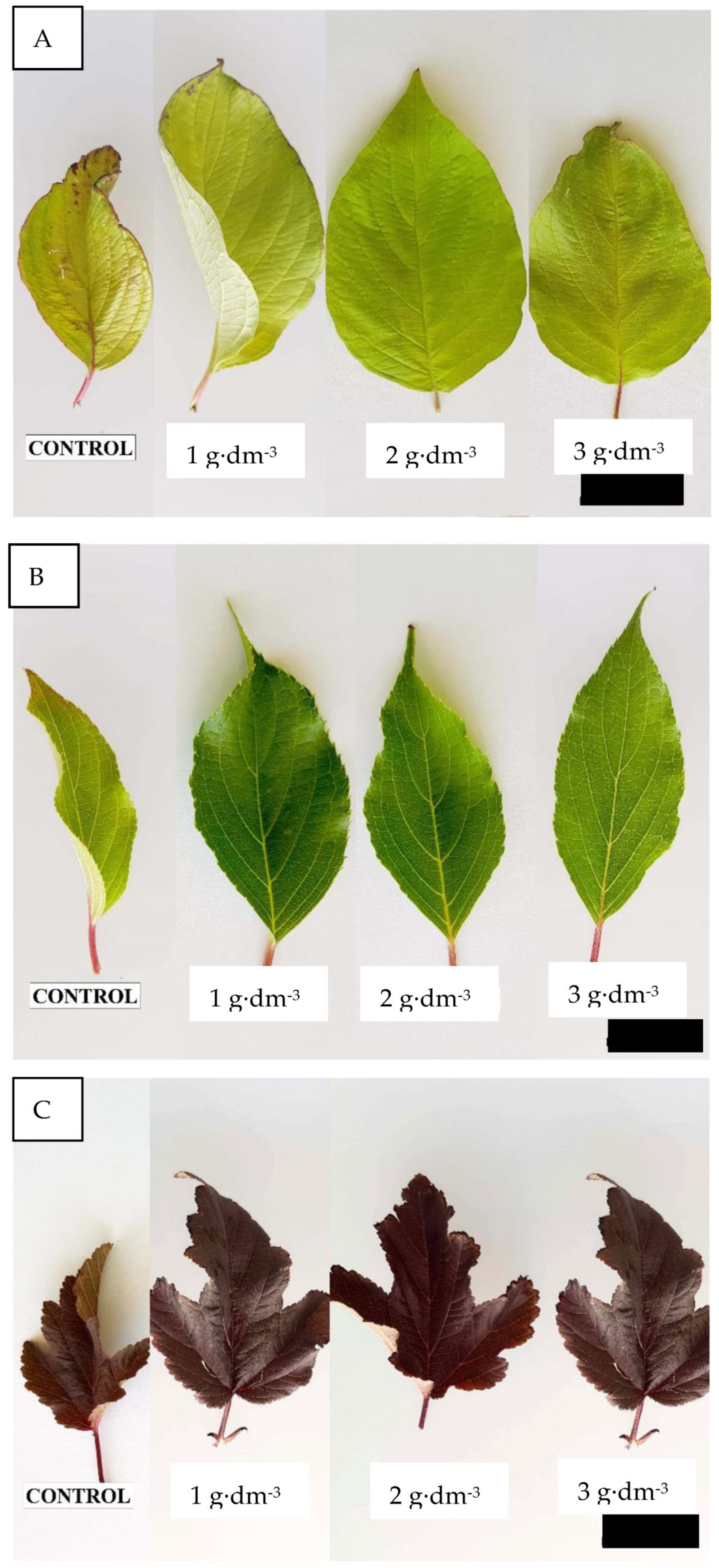
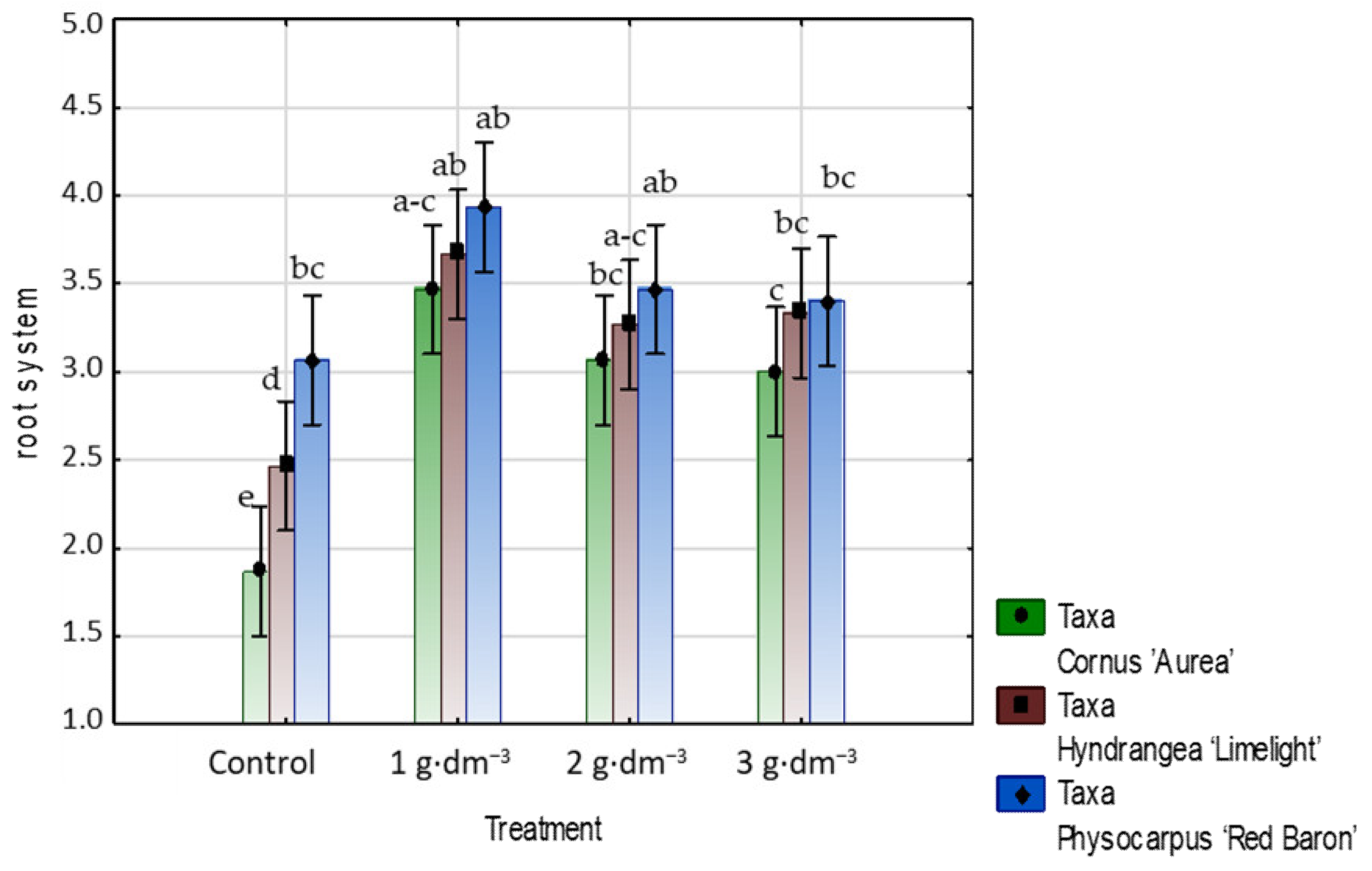
3.1. Starch-Based Superabsorbent Added to Medium Encourages Woody Plant Growth
3.2. Starch-Based Superabsorbent Added to Medium Differentially Affects Changes in Photosynthetic Rate and Stomatal Conductance
3.3. Starch-Based Superabsorbent Added to Medium Changes the Content of Photosynthetically Active Pigments and Carbohydrates in Leaves of Woody Plants
3.4. Starch-Based Superabsorbent Added to Medium Differentially Determines the Level of Hydrogen Peroxide in Leaves
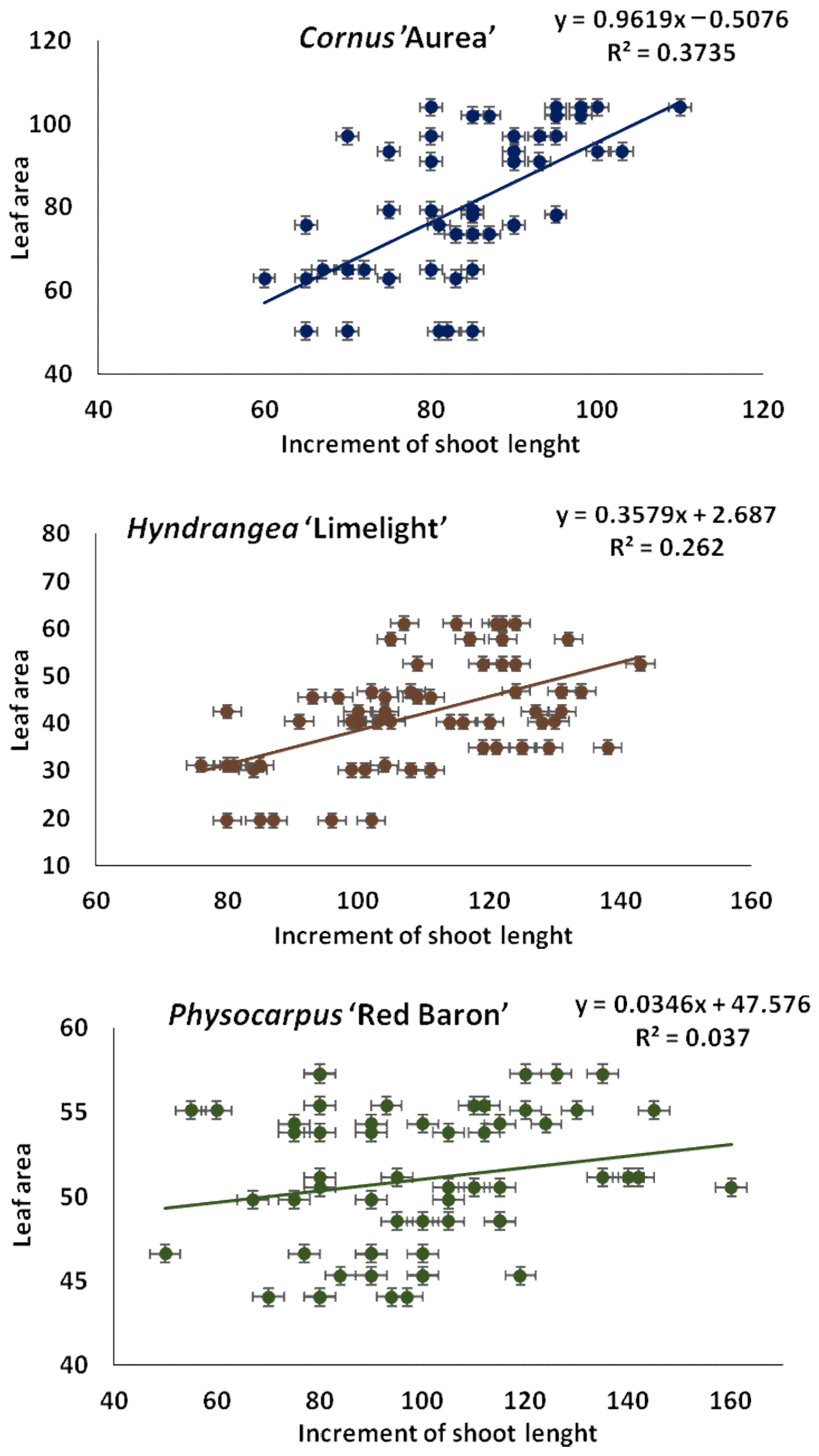
3.5. Correlations between Biologically Active Components Change with the Use of Various Doses of Starch-Based Superabsorbent
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Hanel, M.; Rakovec, O.; Markonis, Y.; Máca, P.; Samaniego, L.; Kyselý, J.; Kumar, R. Revisiting the recent European droughts from a long-term perspective. Sci. Rep. 2018, 1, 9499. [Google Scholar] [CrossRef] [PubMed]
- Rydlová, J.; Püschel, D. Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 2020, 146, 103394. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.; Zhao, C.X. Water deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Yadav, N. Stress-adaptive microbes for plant growth promotion and alleviation of drought stress in plants. Acta Sci. Agric. 2018, 2, 85–88. [Google Scholar]
- Elansary, H.O.; Salem, M.Z.M. Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac-ethyl application. Sci. Hort. 2015, 189, 1–11. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Ghebru, G.M.; Du Toit, E.S.; Steyn, M.J. Water and nutrient retention by Aquasoil and Stockosorb polymers. S. Afr. J. Plant Soil 2007, 24, 32–36. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, I. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Synthesis of starch-based smart hydrogel derived from rice-cooked wastewater for agricultural use. Int. J. Biol. Macromol. 2022, 226, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Chiorescu, E.; Clinciu-Radu, A.R.; Chiorescu, D.; Teliban, G.C.; Robu, T. Research on the influence of hydrogels Zeba SP and Terracottem on the development of some aromatic plant species. Res. J. Agric. Sci. 2017, 49, 385–391. [Google Scholar]
- Mazen, M.M.; Eldeen, D.; Radwan, M.; Ahmed, F.A. Growth responses of maize plants cultivated in sandy soil amended by different superabsorbant hydrogels. J. Plant Nutr. 2015, 38, 325–337. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of direct chemical and biochemical transformations of starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef]
- Luo, H.; Dong, F.; Wang, Q.; Li, Y.; Xiong, Y. Construction of porous starch-based hydrogel via regulating the ratio of amylopectin/amylose for enhanced water-retention. Molecules 2021, 26, 3999. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Ostrand, M.S.; DeSutter, T.M.; Daigh, A.L.M.; Limb, R.F.; Steele, D.D. Superabsorbent polymer characteristics, properties and applications. Agrosyst. Geosci. Environ. 2020, 3, e20074. [Google Scholar] [CrossRef]
- Luo, Z.; Li, K.; Jiang, X.; Polle, A. Ectomycorrhizal fungus (Paxillus involutus) and hydrogels affect performance of Populus euphratica exposed to drought stress. Ann. For. Sci. 2009, 66, 106–116. [Google Scholar] [CrossRef]
- Moallemi, N.; Khaleghii, E.; Danaeifar, A. Investigating the effect of using three types of superabsorbent polymers on N.P.K absorption by acacia under drought stress conditions. Agric. Eng. 2022, 2, 153–167. [Google Scholar]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Cellulose-based hydrogels for agricultures. In Polymers and Polymeric Composites: A Reference Series; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–21. [Google Scholar]
- Official Journal of the European Union. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- Lertsarawut, P.; Rattanawongwiboon, T.; Tangthong, T.; Laksee, S.; Kwamman, T.; Phuttharak, B.; Romruensukharom, P.; Suwanmala, P.; Hemvichian, K. Starch-based super water absorbent: A promising and sustainable way to increase survival rate of trees planted in arid areas. Polymers 2021, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Monder, M.J.; Bąbelewski, P.; Szperlik, J. The adjustment of China endemic Heptacodium miconioides Rehd. to temperate zone of Poland. BMC Plant Biol. 2023, 23, 184. [Google Scholar] [CrossRef] [PubMed]
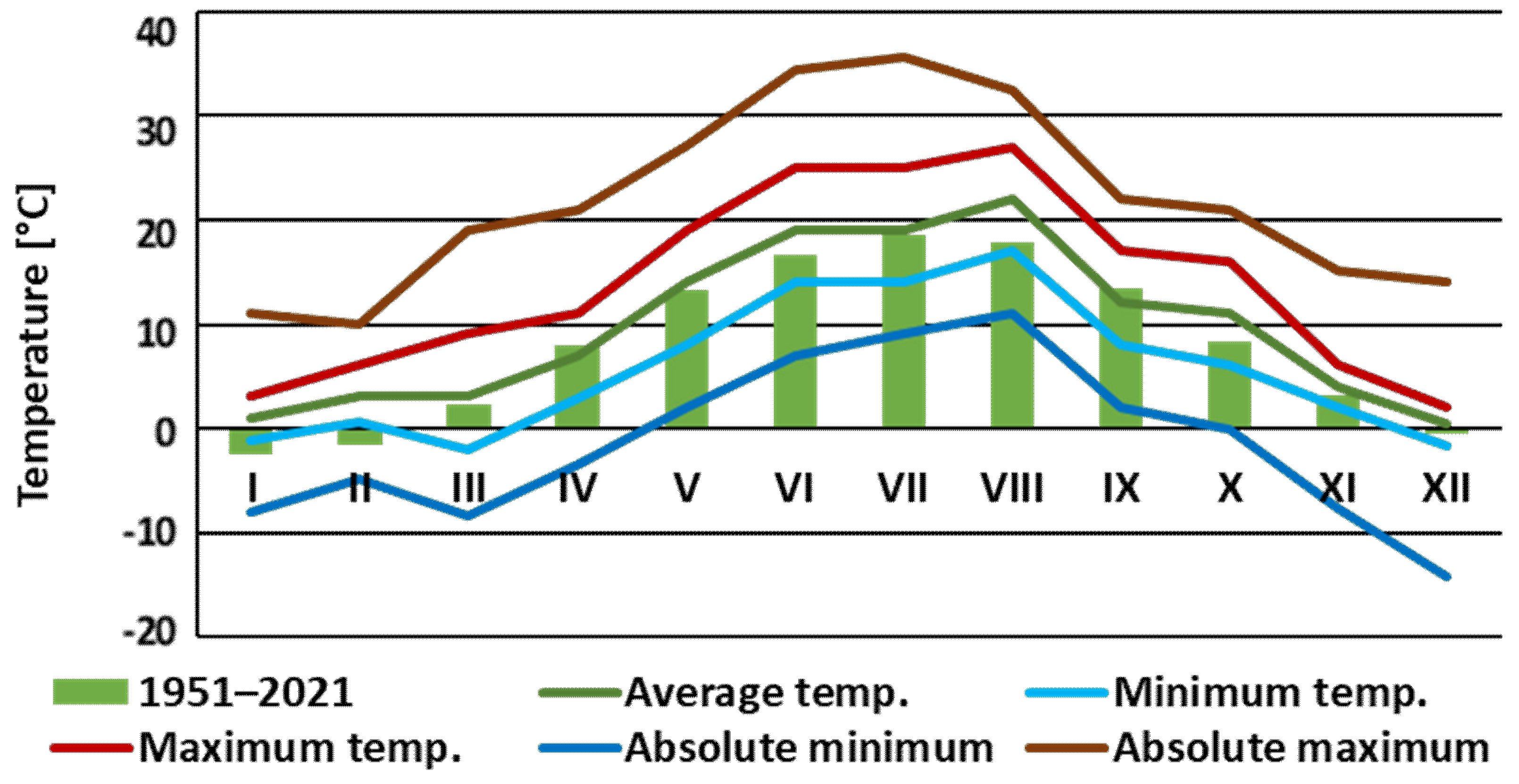
- Climate. 2022. Available online: https://meteomodel.pl/dane/srednie-miesieczne/?imgwid=352200375&par=%28tx%2Btn%29%2F2&max_empty=2 (accessed on 1 June 2023).
- Lichtenthaler, K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Strzelecka, H.; Kamińska, J.; Kowalski, J. Chemiczne Metody Badań Roślinnych Surowców Leczniczych; PZWL: Warsaw, Poland, 1982; pp. 56–57. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 35–356. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y.A. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Laudański, Z.; Mańkowski, D.R. Statistical Methods for the Preparation of Results; IHAR: Radzików, Poland, 2007; Chapter 5; pp. 126–140. [Google Scholar]
- Compton, M.E. Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult. 1994, 37, 217–242. [Google Scholar] [CrossRef]
- Torrecillas, A.; Rodríguez, P.; Sánchez-Blanco, M.J. Comparison of growth, leaf water relations and gas exchange of Cistus albidus and Cistus monspeliensis plants irrigated with water of different NaCl salinity levels. Sci. Hort. 2003, 97, 353–368. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.-G.; Yoon, T.-M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hort. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef]
- El-Asmar, J.; Jaafar, H.; Bash, I. Hydrogel banding improves plant, survival, and water use efficiency in two calcareous soils. Clean Soil Air Water 2017, 47, 1700251. [Google Scholar] [CrossRef]
- Farzi, R.; Gholami, M.; Baninasab, B. Water-retention additives’ effects on plant water status and some physiological parameters of two olive cultivars under reduced irrigation regimes. Acta Physiol. Plant. 2017, 3, 126. [Google Scholar] [CrossRef]
- Hadam, A.; Wrochna, M.; Karaczun, Z. Effect of hydrogel on the turf grass species growing under salt stress. Ann. WULS 2011, 43, 47–55. [Google Scholar]
- Barakat, M.R.; El-Kosary, S.; Borham, T.I. Effect of hydrogel soil addition under different irrigation levels on Grand Nain banana plants. J. Hort. Sci. Ornam. Plants 2015, 7, 19–28. [Google Scholar]
- Jamnická, G.; Ditmarová, L.; Kurjak, D.; Kme, J.; Pšidová, E.; Macková, M.; Gömöry, D.; Střelcová, K. The soil hydrogel improved photosynthetic performance of beech seedlings treated under drought. Plant Soil Environ. 2013, 59, 446–451. [Google Scholar] [CrossRef]
- Woodward, A.J.; Bennett, I.J. The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult. 2005, 82, 189–200. [Google Scholar] [CrossRef]
- Kenawy, E.; Rashad, M.; Hosny, A.; Shendy, S.; Gad, D.; Saad-Allah, K.M. Enhancement of growth and physiological traits under drought stress in Faba bean (Vicia faba L.) using nanocomposite. J. Plant Interact. 2022, 17, 404–418. [Google Scholar] [CrossRef]
- Sayed, E.H.; Kirkwood, C.R.; Graham, B.N. Studies on the effect of salinity and hydrogel polymer treatments on the growth. yield production and solute accumulation in cotton and maize. Agric. Sci. 1995, 7, 129–333. [Google Scholar]
- Sayed, H.; Sayed, A. Influence of salinity stress on growth parameters photosynthetic activity and cytological studies of Zea mays. L. plant using hydrogel polymer. Agric. Biol. J. N. Am. ABJNA 2011, 2, 907–920. [Google Scholar]
- Essmann, J.; Bones, P.; Weis, E.; Scharte, J. Leaf carbohydrate metabolism during defense. Intracellular sucrose-cleaving enzymes do not compensate repression of cell wall invertase. Plant Signal. Behav. 2008, 3, 885–887. [Google Scholar] [CrossRef]
- Šarapatka, B.; Rak, I.; Bubenikova, I. The effect of hydroabsorbent on selected soil biological and biochemical characteristics and its possible use in revitalization. Ekológia 2006, 25, 422–429. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species. oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species. abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An introduction to antioxidants and their roles in plant stress tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M., Khan, N., Eds.; Springer: Singapore, 2017; pp. 1–23. [Google Scholar]
- Lee, S.; Seo, P.J.; Lee, H.-J.; Park, C.-M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012, 70, 831–844. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Xi, Y.; Kong, F.; Chi, Z. ROS induce β-carotene biosynthesis caused by changes of photosynthesis efficiency and energy metabolism in Dunaliella salina under stress conditions. Front. Bioeng. Biotechnol. 2021, 8, 613768. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Sayed, E.H.; Kirkwood, C.R.; Graham, B.N. The effects of a hydrogel polymer on the growth of certain horticultural crops under saline conditions. J. Exp. Bot. 1991, 42, 891–899. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 69–777. [Google Scholar] [CrossRef]













| Variable | Mean | SD | Stomatal Conductance | Photosynthetic Rate |
|---|---|---|---|---|
| Control | ||||
| Stomatal conductance | 0.3 | 0.02 | 1.000 | |
| Photosynthetic rate | 2.3 | 0.23 | −0.945 | 1.000 |
| Width of stomata | 2.5 | 1.26 | 0.177 | −0.194 |
| Starch-based superabsorbent 1 g·dm−3 | ||||
| Stomatal conductance | 0.4 | 0.23 | 1.000 | |
| Photosynthetic rate | 3.1 | 0.52 | −0.866 | 1.000 |
| Width of stomata | 5.2 | 0.45 | −0.592 | 0.712 |
| Starch-based superabsorbent 2 g·dm−3 | ||||
| Stomatal conductance | 0.4 | 0.01 | 1.000 | |
| Photosynthetic rate | 3.1 | 0.31 | 0.404 | 1.000 |
| Width of stomata | 4.5 | 0.69 | 0.861 | 0.201 |
| Starch-based superabsorbent 3 g·dm−3 | ||||
| Stomatal conductance | 0.4 | 0.01 | 1.000 | |
| Photosynthetic rate | 4.4 | 0.24 | 0.945 | 1.000 |
| Width of stomata | 4.2 | 0.47 | −0.144 | −0.398 |
 | ||||
| Variable Cornus ‘Aurea’ | Mean | SD | Chlorophylls | Carotenoids | Total Carbohydrates | Reducing Carbohydrates |
|---|---|---|---|---|---|---|
| Control | ||||||
| Chlorophylls | 1.83 | 0.06 | 1.000 | |||
| Carotenoids | 0.45 | 0.01 | −0.835 | 1.000 | ||
| Total carbohydrates | 35.58 | 0.82 | −0.936 | 0.975 | 1.000 | |
| Reducing carbohydrates | 31.27 | 1.84 | −0.277 | 0.761 | 0.597 | 1.000 |
| H2O2 | 333.18 | 3.42 | 0.555 | −0.921 | −0.811 | −0.953 |
| Starch-based superabsorbent 1 g·dm−3 | ||||||
| Chlorophylls | 1.99 | 0.07 | 1.000 | |||
| Carotenoids | 0.49 | 0.02 | 0.994 | 1.000 | ||
| Total carbohydrates | 41.15 | 0.48 | 0.883 | 0.828 | 1.000 | |
| Reducing carbohydrates | 30.07 | 1.46 | −0.698 | −0.771 | −0.282 | 1.000 |
| H2O2 | 323.30 | 3.97 | 0.665 | 0.581 | 0.937 | 0.071 |
| Starch-based superabsorbent 2 g·dm−3 | ||||||
| Chlorophylls | 1.94 | 0.03 | 1.000 | |||
| Carotenoids | 0.42 | 0.02 | 0.982 | 1.000 | ||
| Total carbohydrates | 37.90 | 0.64 | −0.997 | −0.994 | 1.000 | |
| Reducing carbohydrates | 20.58 | 4.55 | 0.311 | 0.485 | −0.383 | 1.000 |
| H2O2 | 340.38 | 1.50 | 0.655 | 0.500 | −0.595 | −0.515 |
| Starch-based superabsorbent 3 g·dm−3 | ||||||
| Chlorophylls | 1.79 | 0.06 | 1.000 | |||
| Carotenoids | 0.43 | 0.02 | −0.260 | 1.000 | ||
| Total carbohydrates | 34.46 | 1.19 | 0.950 | −0.549 | 1.000 | |
| Reducing carbohydrates | 20.52 | 2.21 | 0.066 | −0.981 | 0.375 | 1.000 |
| H2O2 | 344.37 | 1.11 | −0.141 | 0.993 | −0.444 | −0.997 |
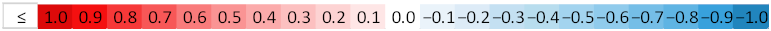 | ||||||
| Variable Hydrangea ‘Limelight’ | Mean | SD | Chlorophylls | Carotenoids | Total Carbohydrates | Reducing Carbohydrates |
|---|---|---|---|---|---|---|
| Control | ||||||
| Chlorophylls | 1.74 | 0.03 | 1.000 | |||
| Carotenoids | 0.48 | 0.03 | −0.342 | 1.000 | ||
| Total carbohydrates | 61.28 | 0.47 | 0.119 | −0.974 | 1.000 | |
| Reducing carbohydrates | 56.82 | 0.17 | −0.980 | 0.150 | 0.080 | 1.000 |
| H2O2 | 209.52 | 3.65 | −0.713 | −0.415 | 0.612 | 0.838 |
| Starch-based superabsorbent 1 g·dm−3 | ||||||
| Chlorophylls | 1.88 | 0.06 | 1.000 | |||
| Carotenoids | 0.49 | 0.01 | −0.604 | 1.000 | ||
| Total carbohydrates | 60.89 | 3.21 | 0.413 | 0.476 | 1.000 | |
| Reducing carbohydrates | 55.26 | 1.18 | 0.847 | −0.088 | 0.834 | 1.000 |
| H2O2 | 128.83 | 1.81 | −0.189 | 0.897 | 0.816 | 0.362 |
| Starch-based superabsorbent 2 g·dm−3 | ||||||
| Chlorophylls | 1.64 | 0.09 | 1.000 | |||
| Carotenoids | 0.51 | 0.01 | 0.661 | 1.000 | ||
| Total carbohydrates | 68.84 | 2.94 | 0.699 | −0.074 | 1.000 | |
| Reducing carbohydrates | 77.35 | 1.13 | 0.999 | 0.631 | 0.728 | 1.000 |
| H2O2 | 189.30 | 1.67 | 0.074 | 0.797 | −0.661 | 0.034 |
| Starch-based superabsorbent 3 g·dm−3 | ||||||
| Chlorophylls | 1.59 | 0.05 | 1.000 | |||
| Carotenoids | 0.46 | 0.03 | −0.538 | 1.000 | ||
| Total carbohydrates | 68.68 | 0.82 | −0.779 | −0.109 | 1.000 | |
| Reducing carbohydrates | 71.22 | 2.78 | −0.870 | 0.884 | 0.368 | 1.000 |
| H2O2 | 171.78 | 0.98 | 0.001 | −0.843 | 0.627 | −0.494 |
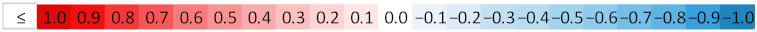 | ||||||
| Variable P. ‘Red Baron’ | Mean | SD | Chlorophylls | Carotenoids | Total Carbohydrates | Reducing Carbohydrates |
|---|---|---|---|---|---|---|
| Control | ||||||
| Chlorophylls | 2.09 | 0.02 | 1.000 | |||
| Carotenoids | 0.74 | 0.00 | −0.971 | 1.000 | ||
| Total carbohydrates | 140.75 | 1.98 | 0.017 | −0.257 | 1.000 | |
| Reducing carbohydrates | 74.39 | 0.84 | 0.888 | −0.973 | 0.474 | 1.000 |
| H2O2 | 363.43 | 3.27 | 0.996 | −0.945 | −0.073 | 0.843 |
| Starch-based superabsorbent 1 g·dm−3 | ||||||
| Chlorophylls | 2.38 | 0.08 | 1.000 | |||
| Carotenoids | 0.78 | 0.01 | −0.977 | 1.000 | ||
| Total carbohydrates | 157.51 | 0.73 | −0.452 | 0.631 | 1.000 | |
| Reducing carbohydrates | 82.80 | 1.26 | 0.645 | −0.468 | 0.390 | 1.000 |
| H2O2 | 313.64 | 1.32 | −0.351 | 0.144 | −0.677 | −0.942 |
| Starch-based superabsorbent 2 g·dm−3 | ||||||
| Chlorophylls | 1.94 | 0.01 | 1.000 | |||
| Carotenoids | 0.71 | 0.03 | 0.998 | 1.000 | ||
| Total carbohydrates | 145.87 | 4.24 | −0.020 | 0.036 | 1.000 | |
| Reducing carbohydrates | 79.33 | 2.14 | −0.996 | −0.989 | 0.112 | 1.000 |
| H2O2 | 386.23 | 7.02 | 0.851 | 0.879 | 0.507 | −0.800 |
| Starch-based superabsorbent 3 g·dm−3 | ||||||
| Chlorophylls | 2.04 | 0.04 | 1.000 | |||
| Carotenoids | 0.73 | 0.04 | 0.939 | 1.000 | ||
| Total carbohydrates | 133.66 | 1.42 | 0.561 | 0.813 | 1.000 | |
| Reducing carbohydrates | 75.66 | 0.55 | −0.963 | −0.997 | −0.763 | 1.000 |
| H2O2 | 383.98 | 1.25 | −0.992 | −0.887 | −0.452 | 0.922 |
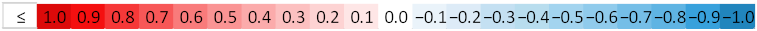 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacholczak, A.; Nowakowska, K.; Monder, M.J. Starch-Based Superabsorbent Enhances the Growth and Physiological Traits of Ornamental Shrubs. Agriculture 2023, 13, 1893. https://doi.org/10.3390/agriculture13101893
Pacholczak A, Nowakowska K, Monder MJ. Starch-Based Superabsorbent Enhances the Growth and Physiological Traits of Ornamental Shrubs. Agriculture. 2023; 13(10):1893. https://doi.org/10.3390/agriculture13101893
Chicago/Turabian StylePacholczak, Andrzej, Karolina Nowakowska, and Marta Joanna Monder. 2023. "Starch-Based Superabsorbent Enhances the Growth and Physiological Traits of Ornamental Shrubs" Agriculture 13, no. 10: 1893. https://doi.org/10.3390/agriculture13101893





