Bioactive Compounds of Endemic Medicinal Plants (Cuphea spp.) Cultured in Aquaponic Systems: A Short Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Setup
2.1.1. Aquatic Species
2.1.2. Plant Species
2.2. Samples and Treatment of Cuphea spp.
2.2.1. Pre-Treatment of Samples and Monitoring
2.2.2. Determination of Total Phenolic Compounds
2.2.3. Determination of Total Flavonoids
2.2.4. Determination of Antioxidant Activity,1-Diphenyl-2-picrylhydrazyl Radical (DPPH) Inhibition Assay
2.2.5. Determination of Antioxidant Activity Ferric-Reducing/Antioxidant Power (FRAP) Assay
2.2.6. Ultra-Performance Convergence Chromatography
Extraction for Identification and Quantification
Analysis of Phenolic Compounds
2.3. Statistical Analysis
3. Results
3.1. Integrated Agri-Aquaculture System Performance
3.1.1. Water Quality in the Integrated Agri-Aquaculture System
3.1.2. Growth and Development of Cuphea spp.
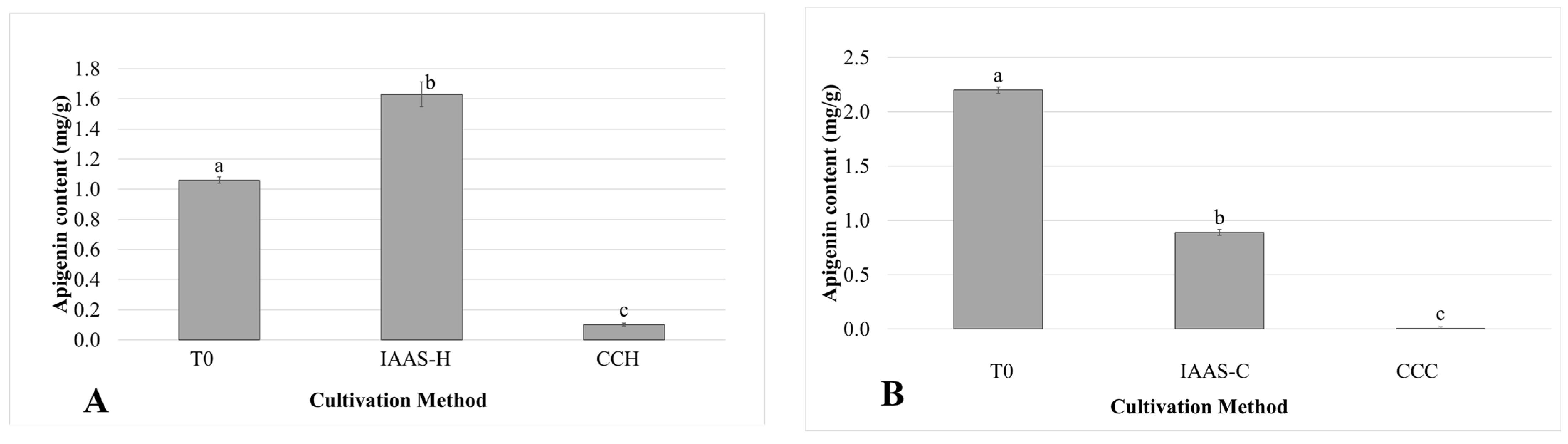
3.2. Bioactive Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Palm, H.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Haïssam Jijakli, M.; Kotzen, B. Towards Commercial Aquaponics: A Review of Systems, Designs, Scales and Nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H. Comparisons of Nitrogen and Phosphorus Mass Balance for Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Gott, J.; Morgenstern, R.; Turnšek, M. Aquaponics for the Anthropocene: Towards a ‘Sustainability First’ Agenda. In Aquaponics Food Production Systems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 393–432. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic Trends and Challenges—A Review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Oladimeji, A.S.; Olufeagba, S.O.; Ayuba, V.O.; Sololmon, S.G.; Okomoda, V.T. Effects of Different Growth Media on Water Quality and Plant Yield in a Catfish-Pumpkin Aquaponics System. J. King Saud. Univ. Sci. 2020, 32, 60–66. [Google Scholar] [CrossRef]
- Bartelme, R.P.; Oyserman, B.O.; Blom, J.E.; Sepulveda-Villet, O.J.; Newton, R.J. Stripping Away the Soil: Plant Growth Promoting Microbiology Opportunities in Aquaponics. Front. Microbiol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Delaide, B.; Teerlinck, S.; Decombel, A.; Bleyaert, P. Effect of Wastewater from a Pikeperch (Sander lucioperca L.) Recirculated Aquaculture System on Hydroponic Tomato Production and Quality. Agric. Water Manag. 2019, 226, 105814. [Google Scholar] [CrossRef]
- Braglia, R.; Costa, P.; di Marco, G.; D’Agostino, A.; Redi, E.L.; Scuderi, F.; Gismondi, A.; Canini, A. Phytochemicals and Quality Level of Food Plants Grown in an Aquaponics System. J. Sci. Food Agric. 2022, 102, 844–850. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced Aquaponics: Evaluation of Intensive Tomato Production in Aquaponics vs. Conventional Hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Graham, S.A.; Sosa, V. Flora de Veracruz. “Lytraceae”. Fascículo 1991, 66, 15–24. [Google Scholar]
- Santos, M.C.; Henriques, A.T.; Mendez, A.S.L. Analytical Methods of Phytochemicals from the Cuphea Genus—A Review. Drug Anal. Res. 2021, 5, 4–10. [Google Scholar] [CrossRef]
- Graham, S.A.; Kleiman, R. Composition of Seed Oils in Some Latin American Cuphea (Lythraceae). Ind. Crop. Prod. 1992, 1, 31–34. [Google Scholar] [CrossRef]
- Gesch, R.; Forcella, F.; Olness, A.; Archer, D.; Hebard, A. Agricultural Management of Cuphea and Potential for Commercial Production in the Northern Corn Belt. Ind. Crop. Prod. 2006, 24, 300–306. [Google Scholar] [CrossRef]
- Elgindi, M.; Ayoub, N.; Milad, R.; Hassan, R. A Comprehensive Review of Cuphea (Lythraceae). Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 847–856. [Google Scholar]
- Tiwari, D.K.; Coria-Tellez, A.V.; Tripathi, D.; Alonso-Perez, M.O.; Navarro-Pérez, A.D. Extraction and Characterization of Chemical Constituents Present in Cuphea Aequipetala and Their Properties. Microsc. Microanal. 2021, 27, 2278–2282. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Palm, H.; Knaus, U.; Appelbaum, S.; Strauch, S.M.; Kotzen, B. Coupled Aquaponics Systems. In Aquaponics Food Production Systems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–199. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient Management in Aquaponics: Comparison of Three Approaches for Cultivating Lettuce, Mint and Mushroom Herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Kloas, W.; Groß, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Staaks, G.; Suhl, J.; Tschirner, M.; Wittstock, B.; et al. A New Concept for Aquaponic Systems to Improve Sustainability, Increase Productivity, and Reduce Environmental Impacts. Aquac. Environ. Interact. 2015, 7, 179–192. [Google Scholar] [CrossRef]
- Rakocy, J.; Masser, M.; Losordo, T. Recirculating Aquaculture Tank Production Systems: Aquaponics-Integrating Fish and Plant Culture; Oklahoma Cooperative Extension Service: Stillwater, OK, USA, 2016. [Google Scholar]
- Vazquez-Cruz, M.A.; Luna-Rubio, R.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Guevara-Gonzalez, R.G. Estimating the Response of Tomato (Solanum lycopersicum) Leaf Area to Changes in Climate and Salicylic Acid Applications by Means of Artificial Neural Networks. Biosyst. Eng. 2012, 112, 319–327. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and Antioxidative Activities in Common Beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, J.K.; Hu, L.; Dong, Z.J.; Hu, Q. DPPH Radical Scavenging Activity of Ten Natural P-Terphenyl Derivatives Obtained from Three Edible Mushrooms Indigenous to China. Chem. Biodivers. 2004, 1, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Graham, S. Lythraceae*; Flora del Bajio y de Regiones Adyacentes; Instituto de Ecologia: Kent, OH, USA, 1994; Volume 24, pp. 1–62. [Google Scholar]
- Abdel-Rahim, M.M.; Awad, Y.M.; Abdallah, Y.A.; Radwan, S.M. Effects of Four Medicinal Plants on the Bioeconomic Analysis and Water-Use Efficiency of Nile Tilapia, Oreochromis Niloticus Fry Nursed under a Small-Scale Aquaponics System. AACL Bioflux 2019, 12, 851–866. [Google Scholar]
- Berti, M.T.; Johnson, B.L. Growth and Development of Cuphea. Ind. Crop. Prod. 2008, 27, 265–271. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Nutrient Management Regime Affects Water Quality, Crop Growth, and Nitrogen Use Efficiency of Aquaponic Systems. Sci. Hortic. 2019, 256, 108619. [Google Scholar] [CrossRef]
- Elgindi, M.; Ayoub, N.; Milad, R.; Mekky, R. Antioxidant and Cytotoxic Activities of Cuphea Hyssopifolia Kunth (Lythraceae) Cultivated in Egypt. J. Pharmacogn. Phytochem. 2012, 1, 68–78. [Google Scholar]
- Moke, E.G.; Mordi, J.C.; Umukoro, E.K. Effects of Methanol Leaf Extract of Cuphea Hyssopifolia Kunth on Liver Enzymes Activity and Antioxidant Indices of Paracetamol-Induced Hepatotoxicity in Wistar Rats. Afr. J. Biomed. Res. 2020, 23, 123–126. [Google Scholar]
- Flanigan, P.M.; Niemeyer, E.D. Effect of Cultivar on Phenolic Levels, Anthocyanin Composition, and Antioxidant Properties in Purple Basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and Molecular Regulation of Phenylpropanoids Pathway Under Abiotic Stresses. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Elsevier: Amsterdam, The Netherlands, 2019; pp. 183–192. [Google Scholar] [CrossRef]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and Phenylpropanoids Are Key Components of the Antioxidant Defense System of Plants Facing Severe Excess Light Stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Khandaker, M.; Kotzen, B. The Potential for Combining Living Wall and Vertical Farming Systems with Aquaponics with Special Emphasis on Substrates. Aquac. Res. 2018, 49, 1454–1468. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental Hormesis of Non-Specific and Specific Adaptive Mechanisms in Plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Plant Hormesis: Revising of the Concepts of Biostimulation, Elicitation and Their Application in a Sustainable Agricultural Production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef] [PubMed]
- González, R.G.G.; Pacheco, I.T. Manejo del Estrés Vegetal Como una Estrategia Para una Agricultura Sostenible; Universidad Almería: Almería, Spain, 2022; Volume 3, ISBN 8413511534. [Google Scholar]
- Rakocy, J. Aquaponics-Integrating Fish and Plant Culture; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Olness, A.; Gesch, R.; Forcella, F.; Archer, D.; Rinke, J. Importance of Vanadium and Nutrient Ionic Ratios on the Development of Hydroponically Grown Cuphea. Ind. Crop. Prod. 2005, 21, 165–171. [Google Scholar] [CrossRef]
- Ferreira, F.S.; de Oliveira, V.S.; Chávez, D.W.H.; Chaves, D.S.; Riger, C.J.; Sawaya, A.C.H.F.; Guizellini, G.M.; Sampaio, G.R.; Torres, E.A.F.d.S.; Saldanha, T. Bioactive Compounds of Parsley (Petroselinum crispum), Chives (Allium schoenoprasum L.) and Their Mixture (Brazilian Cheiro-Verde) as Promising Antioxidant and Anti-Cholesterol Oxidation Agents in a Food System. Food Res. Int. 2022, 151, 110864. [Google Scholar] [CrossRef]
- Hernández, M.V.; Jiménez, N.I.F. Introducción a La Teoría Del Estrés e Inmunología Vegetal. In Manejo del Estrés Vegetal Como una Estrategia Para una Agricultura Sostenible; Editorial Universidad de Almería: Almería, Spain, 2022; pp. 53–102. [Google Scholar]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A Current Review on Its Beneficial Biological Activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2016, 20, 1197–1238. [Google Scholar] [CrossRef]

| Variables A | Data |
|---|---|
| Temperature greenhouse (°C) | 29.02 ± 9.48 |
| Radiation * (W/m2) | 163.6 ± 42.06 |
| Relative Humidity (%) | 53 ± 8.05 |
| Variables B | Data |
| Temperature in the fish tank (°C) | 24.62 ± 5.04 |
| pH | 8.95 ± 0.20 |
| Oxygen (mg L−1) | 7.23 ± 0.88 |
| Conductivity (µS cm−1) | 248.52 ± 14.22 |
| Water Quality (mg L−1) * | Min | Max |
|---|---|---|
| NH4-N | 0.15 | 1.00 |
| NO3-N | 5.00 | 22.0 |
| NO2-N | 0.04 | 0.045 |
| PO4-P | 0.50 | 3.00 |
| SO4-S | 1.50 | 24.0 |
| Ca | 10.0 | 90.5 |
| Mg | 11.0 | 41.0 |
| Cl | 0.02 | 0.024 |
| K | 26.0 | 28.0 |
| Time (Min) | * CO2 (%) | Methanol (%) |
|---|---|---|
| 0 | 95 | 5 |
| 8 | 70 | 30 |
| 9 | 70 | 30 |
| 10 | 95 | 5 |
| 11 | 95 | 5 |
| Plant Species | Maximum Branch Height | Leaf Area | |
|---|---|---|---|
| C. hyssopifolia b | T0 | 29.71 ± 8.1 | 1.42 ± 0.7 |
| IAAS-H | 39.98 ± 10.3 | 1.86 ± 0.5 | |
| CCH | 30.05 ± 5.2 | 1.87 ± 0.2 | |
| C. cyanea a | T0 | 77.76 ± 40.1 | 19.16 ± 1.4 |
| IAAS-C | 69.78 ± 32.9 | 22.07 ± 3.8 | |
| CCC | 65.04 ± 19.7 | 23.74 ± 8.5 |
| C. hyssopifolia a | C. cyanea b | |||||
|---|---|---|---|---|---|---|
| T0 | IAAS-H | CCH | T0 | IAAS-C | CCC | |
| Total phenolic content (mg g−1 GAEq) | 80.39 ± 9.9 a | 61.08 ± 7.2 b | 16.99 ± 0.4 c | 17.06 ± 0.8 a | 15.36 ± 0.8 b | 14.11 ± 1.3 c |
| Total flavonoid content (mg g−1 CAEq) | 10.71 ± 1.0 a | 5.62 ± 0.5 c | 8.19 ± 1.6 b | 7.456 ± 0.8 a | 3.52 ± 0.6 b | 1.79 ± 0.1 c |
| DPPH (mg g−1 TEq DW) | 125.73 ± 3.4 a | 114.82 ± 6.0 b | 96.92 ± 12.1 c | 11.05 ± 0.9 b | 15.16 ± 0.5 a | 7.22 ± 0.6 c |
| FRAP (mg g−1 TEq DW) | 133.05 ± 9.0 a | 134.53 ± 14.1 a | 114.878 ± 16.3 b | 13.34 ± 0.9 a | 13.35 ± 1.2 a | 7.37 ± 0.4 b |
| Cultivation Method | Total Phenolic Content (mg g−1 GAEq) | Total Flavonoid Content (mg g−1 CAEq) | Apigenin (mg g−1) | DPPH (mg g−1 TEq DW) | FRAP (mg g−1 TEq DW) |
|---|---|---|---|---|---|
| T0 | 48.7 ± 1.2 a | 9.08 ± 0.2 a | 1.63 ± 0.01 a | 68.39 ± 1.3 a | 73.93 ± 2.3 a |
| IAA | 38.2 ± 1.2 b | 4.99 ± 0.2 b | 1.26 ± 0.01 b | 64.99 ± 1.3 a | 73.19 ± 2.3 a |
| CC | 15.5 ± 1.2 c | 4.56 ± 0.2 b | 0.05 ± 0.01 c | 52.06 ± 1.3 b | 61.12 ± 2.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Aguilar, P.S.; Rico-Chávez, A.K.; Rodriguez-deLeón, E.; Aguirre-Becerra, H.; Zamora-Castro, S.A.; Soto-Zarazúa, G.M. Bioactive Compounds of Endemic Medicinal Plants (Cuphea spp.) Cultured in Aquaponic Systems: A Short Study. Agriculture 2023, 13, 2018. https://doi.org/10.3390/agriculture13102018
Flores-Aguilar PS, Rico-Chávez AK, Rodriguez-deLeón E, Aguirre-Becerra H, Zamora-Castro SA, Soto-Zarazúa GM. Bioactive Compounds of Endemic Medicinal Plants (Cuphea spp.) Cultured in Aquaponic Systems: A Short Study. Agriculture. 2023; 13(10):2018. https://doi.org/10.3390/agriculture13102018
Chicago/Turabian StyleFlores-Aguilar, Priscila Sarai, Amanda Kim Rico-Chávez, Eloy Rodriguez-deLeón, Humberto Aguirre-Becerra, Sergio Aurelio Zamora-Castro, and Genaro Martín Soto-Zarazúa. 2023. "Bioactive Compounds of Endemic Medicinal Plants (Cuphea spp.) Cultured in Aquaponic Systems: A Short Study" Agriculture 13, no. 10: 2018. https://doi.org/10.3390/agriculture13102018
APA StyleFlores-Aguilar, P. S., Rico-Chávez, A. K., Rodriguez-deLeón, E., Aguirre-Becerra, H., Zamora-Castro, S. A., & Soto-Zarazúa, G. M. (2023). Bioactive Compounds of Endemic Medicinal Plants (Cuphea spp.) Cultured in Aquaponic Systems: A Short Study. Agriculture, 13(10), 2018. https://doi.org/10.3390/agriculture13102018








