Physiological Response of Miscanthus sinensis (Anderss.) to Biostimulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pot Experiment Design
- Control
- S1—200 mM NaCl
- S2—400 mM NaCl
- B1—Bombardino
- B2—Quantis
- B3—BiofolPlex
- B4—Megafol
- S1 + B1—200 mM NaCl + BioFol Bombardino
- S2 + B1—400 mM NaCl + BioFol Bombardino
- S1 + B2—200 mM NaCl + Quantis®
- S2 + B2—400 mM NaCl + Quantis®
- S1 + B3—200 mM NaCl + BiofolPlex
- S2 + B3—400 mM NaCl + BiofolPlex
- S1 + B4—200 mM NaCl + Megafol
- S2 + B4—400 mM NaCl + Megafol
2.2. Physiological Measurement
2.2.1. Relative Chlorophyll Content
2.2.2. Chlorophyll Fluorescence
2.2.3. Gas Exchange
2.3. Statistical Analysis
3. Results
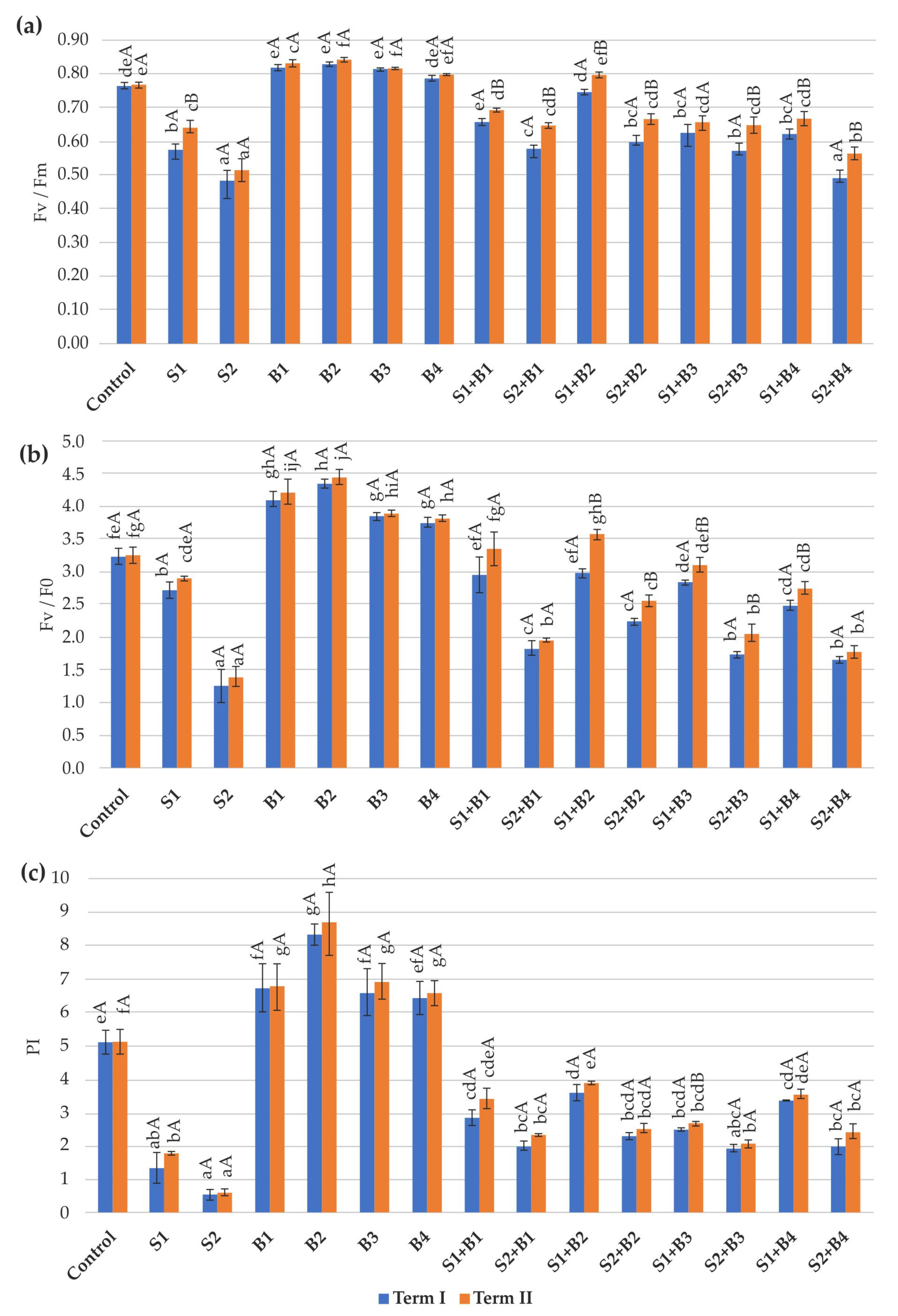
3.1. Relative Chlorophyll Content
3.2. Chlorophyll Fluorescence
3.3. Gas Exchange
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alemán-Ramirez, J.L.; Okoye, P.U.; Torres-Arellano, S.; Mejía-Lopez, M.; Sebastian, P.J. A review on bioenergetic applications of Leucaena leucocephala. Ind. Crops Prod. 2022, 182, 114847. [Google Scholar] [CrossRef]
- Núñez-Regueiro, M.M.; Siddiqui, S.F.; Fletcher, R.J., Jr. Effects of bioenergy on biodiversity arising from land-use change and crop type. Conserv. Biol. 2019, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.; Boscaro, D.; Dalla Venezia, F.; Santacroce, F.C.; Pezzuolo, A.; Sartori, L.; Bolzonella, D. Biogas from residual grass: A territorial approach for sustainable bioenergy production. Waste Biomass Valoriz. 2017, 8, 2747–2756. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; Da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from fresh spring and summer grass: Effect of the harvesting period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef]
- Ferrari, G.; Pezzuolo, A.; Nizami, A.-S.; Marinello, F. Bibliometric analysis of trends in biomass for bioenergy research. Energies 2020, 13, 3714. [Google Scholar] [CrossRef]
- Oleszek, M.; Matyka, M. Nitrogen fertilization level and cutting affected lignocellulosic crops properties important for biogas production. BioResources 2017, 12, 8565–8580. [Google Scholar] [CrossRef]
- Helis, M.; Strzelczyk, M.; Golimowski, W.; Steinhoff-Wrześniewska, A.; Paszkiewicz-Jasińska, A.; Hawrot-Paw, M.; Koniuszy, A.; Hryniewicz, M. Biomass potential of the marginal land of the polish Sudetes mountain range. Energies 2021, 14, 7156. [Google Scholar] [CrossRef]
- Khanna, M.; Chen, L.; Basso, B.; Cai, X.; Field, J.L.; Guan, K.; Jiang, C.; Lark, T.J.; Richard, T.L.; Spawn-Lee, S.A.; et al. Redefining marginal land for bioenergy crop production. GCB Bioenergy 2021, 13, 1590–1609. [Google Scholar] [CrossRef]
- Li, W.; Ciais, P.; Stehfest, E.; van Vuuren, D.; Popp, A.; Arneth, A.; Di Fulvio, F.; Doelman, J.; Humpenöder, F.; Harper, A.B.; et al. Mapping the yields of lignocellulosic bioenergy crops from observations at the global scale. Earth Syst. Sci. 2020, 12, 789–804. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Stewart, J.R.; Toma, Y.; Fernández, F.G.; Nishiwaki, A.; Yamada, T.; Bollero, G. The ecology and agronomy of Miscanthus sinensis, a species important to bioenergy crop development, in its native range in Japan: A review. GCB Bioenergy 2009, 1, 126–153. [Google Scholar] [CrossRef]
- Quinn, L.D.; Allen, D.J.; Stewart, J.R. Invasiveness potential of Miscanthus sinensis: Implications for bioenergy production in the United States. GCB Bioenergy 2010, 2, 310–320. [Google Scholar] [CrossRef]
- Sun, Q.; Yamada, T.; Han, Y.; Takano, T. Influence of salt stress on C4 photosynthesis in Miscanthus sinensis Anderss. Plant Biol. 2020, 23, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Zhong, M.; Cai, J.; Yang, X.; Zhou, J.; Appiah, C.; Tang, M.; Wang, X.; Feng, G.; Huang, L.; et al. Transcriptome characterization of candidate genes related to chromium uptake, transport and accumulation in Miscanthus sinensis. Ecotoxicol. Environ. Saf. 2021, 221, 112445. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Katul, G.G.; Manzoni, S.; Vico, G. Increasing water use efficiency along the C3 to C4 evolutionary pathway: A stomatal optimization perspective. J. Exp. Bot. 2014, 65, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Ren, L.L.; Zhang, L.T.; Gao, H.Y. Inhibition effects of salt stress on photosynthetic activity of RumexK-1. Chin. J. Appl. Ecol. 2008, 10, 2137–2142. [Google Scholar]
- Zhang, H.; Xu, N.; Wu, X.; Wang, J.; Ma, S.; Li, X.; Sun, G. Effects of four types of sodium salt stress on plant growth and photosynthetic apparatus in sorghum leaves. J. Plant Interact. 2018, 13, 506–513. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Oyiga, B.C.; Sharma, R.C.; Shen, J.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Identification and Characterization of Salt Tolerance of Wheat Germplasm Using a Multivariable Screening Approach. J. Agron. Crop Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- Desoky, E.M.; EL-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defense system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Blasco, B.; Martos, V. Combating salinity through natural plant extracts based biostimulants: A Review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. International soil classification system for naming soils and creating legends for soil maps. In Word Reference Base for Soil Resources 2014, Update 2015; Word Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; pp. 172–173. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Chen, K.-C.; Cheng, T.-S.; Lee, C.; Lin, S.-H.; Tung, C.-W. Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol. 2019, 19, 403. [Google Scholar] [CrossRef]
- Tobiasz-Salach, R.; Mazurek, M.; Jacek, B. Physiological, Biochemical, and Epigenetic Reaction of Maize (Zea mays L.) to cultivation in conditions of varying soil salinity and foliar application of silicon. Int. J. Mol. Sci. 2023, 24, 1141. [Google Scholar] [CrossRef]
- Abdelkader, A.F.; Aronsson, H.; Sundqvist, C. High salt stress in wheat leaves causes retardation of chlorophyll accumulation due to a limited rate of protochlorophyllide formation. Physiol. Plant. 2007, 130, 157–166. [Google Scholar] [CrossRef]
- Abdeshahian, M.; Nabipour, M.; Meskarbashee, M. Chlorophyll fluorescence as criterion for the diagnosis salt stress in wheat (Triticum aestivum) plants. World Acad. Sci. Eng. Technol. 2010, 71, 569–571. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Balawejder, M. Assessment of the impact of the application of a quercetin—Copper complex on the course of physiological and biochemical processes in wheat plants (Triticum aestivum L.) growing under saline conditions. Cells 2022, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqbool, R.; Chattha, M.U.; Khan, I.; Gitari, H.I.; Uslu, O.S.; Roy, R.; et al. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton-Int. J. Exp. Bot. 2021, 91, 337. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Wang, R.L.; Hua, C.; Zhou, F.; Zhou, Q.-C. Effects of NaCl stress on photochemical activity and thylakoid membrane polypeptide composition of a salt-tolerant and a salt-sensitive rice cultivar. Photosynthetica 2009, 47, 125–127. [Google Scholar] [CrossRef]
- Zw, S.; Lk, R.; Jw, F.; Li, Q.; Kj, W.; Mm, G.; Wang, L.; Li, J.; Gx, Z.; Zy, Y.; et al. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ. 2016, 62, 515–521. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- De Freitas, P.A.F.; De Carvalho, H.H.; Costa, J.H.; Miranda, R.D.S.; Saraiva, K.D.D.C.; De Oliveira, F.D.B.; Coelho, D.G.; Prisco, J.T.; Gomes-Filho, E. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stevanato, P.; Yu, L.; Zhao, H.; Sun, X.; Sun, F.; Li, J.; Geng, G. The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. J. Plant Res. 2017, 130, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Guidi, L.; Soldatini, G. Effect of Chronic O3 Fumigation on the Activity of Some Calvin Cycle Enzymes in Two Poplar Clones. Photosynthetica 2002, 40, 121–126. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Migut, D.; Skrobacz, K.; Piechowiak, T.; Pawlak, R.; Balawejder, M. Physiological and biochemical properties of potato (Solanum tuberosum L.) in response to ozone-induced oxidative stress. Agronomy 2020, 10, 1745. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of potato (Solanum tuberosum L.) plants to spraying by hydrogen peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef]
- Stael, S.; Rocha, A.G.; Robinson, A.J.; Kmiecik, P.; Vothknecht, U.C.; Teige, M. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Lett. 2011, 585, 3935–3940. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The “prime-ome”: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef] [PubMed]
- Westman, S.M.; Kloth, K.J.; Hanson, J.; Ohlsson, A.B.; Albrectsen, B.R. Defence priming in Arabidopsis—A Meta-Analysis. Sci. Rep. 2019, 9, 13309. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Depciuch, J.; Drygaś, B.; Jańczak-Pieniążek, M.; Mazurek, K.; Pawlak, R. The influence of biostimulants used in sustainable agriculture for antifungal protection on the chemical composition of winter wheat grain. Int. J. Environ. Res. Public Health. 2022, 19, 12998. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef]
- Butt, M.; Ayyub, C.M.; Amjad, M.; Ahmad, R. Proline application enhances growth of chilli by improving physiological and biochemical attributes under salt stress. Pak. J. Agric. Sci. 2016, 53, 43–49. [Google Scholar] [CrossRef]


| Foliar Fertilizers | Producer | Fertilizer Characteristics | Dose (per 1000 mL of Water) |
|---|---|---|---|
| BioFol Bombardino | Biostyma Sp. z o.o. (Poland) | 70.0% organic matter content, 35.0% seaweed concentrate, 30.0% organic carbon, 5.0% free L-amino acids, polysaccharides, phosphorus, potassium, magnesium, iron, calcium, copper, vitamins B1, B2, B3, B6, B9; | 5 mL |
| Quantis® | SAF Argentina S.A. (Argentina) | 1% total nitrogen (N), 0.9% organic nitrogen (Norg), 9.3% potassium (in conversion to K2O), 4.6% calcium (in conversion to CaO), dry matter 52%, organic matter 26% | 10 mL |
| BioFol Plex | Biostyma Sp. Z o.o. (Poland) | 2.0% Ntot; 0.3% Mg; 5.0% S; 0.15% B; 0.05% Cu; 0.20% Fe; 0.10% Mn; 0.50% Zn; 1.25% C; 5.0% extract from algae; traces of plant hormones, betaine (C5H11NO2), amino acids, thiamine | 7.5 mL |
| Megafol | VALAGRO (Italy) | amino acids (proline and tryptophan), glycosides, polysaccharides, organic nitrogen and organic carbon | 10 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jańczak-Pieniążek, M.; Pikuła, W.; Pawlak, R.; Drygaś, B.; Szpunar-Krok, E. Physiological Response of Miscanthus sinensis (Anderss.) to Biostimulants. Agriculture 2024, 14, 33. https://doi.org/10.3390/agriculture14010033
Jańczak-Pieniążek M, Pikuła W, Pawlak R, Drygaś B, Szpunar-Krok E. Physiological Response of Miscanthus sinensis (Anderss.) to Biostimulants. Agriculture. 2024; 14(1):33. https://doi.org/10.3390/agriculture14010033
Chicago/Turabian StyleJańczak-Pieniążek, Marta, Wojciech Pikuła, Renata Pawlak, Barbara Drygaś, and Ewa Szpunar-Krok. 2024. "Physiological Response of Miscanthus sinensis (Anderss.) to Biostimulants" Agriculture 14, no. 1: 33. https://doi.org/10.3390/agriculture14010033
APA StyleJańczak-Pieniążek, M., Pikuła, W., Pawlak, R., Drygaś, B., & Szpunar-Krok, E. (2024). Physiological Response of Miscanthus sinensis (Anderss.) to Biostimulants. Agriculture, 14(1), 33. https://doi.org/10.3390/agriculture14010033









