The Evaluation of the Water Consumption and the Productive Parameters of a Table Grapevine, Cardinal Cultivar, Grafted onto Two Rootstocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Material and Experimental Design
2.2. Phenological Surveys
2.3. Gas Exchange and Radiometric Measurements
2.4. Tomography Measurements
2.5. Leaf Water Potential
2.6. Sap Flow–HFD
2.7. Total Sap Flow Measurement
2.8. Harvest
2.9. Winter Pruned
2.10. Biometric Measurements, Maturation Indexes, and Nutraceutical Parameters
2.11. Statistical Analysis
3. Results
3.1. Conductive Xylem Area
3.2. Phenophase Evolution
3.3. Vegetative Growth
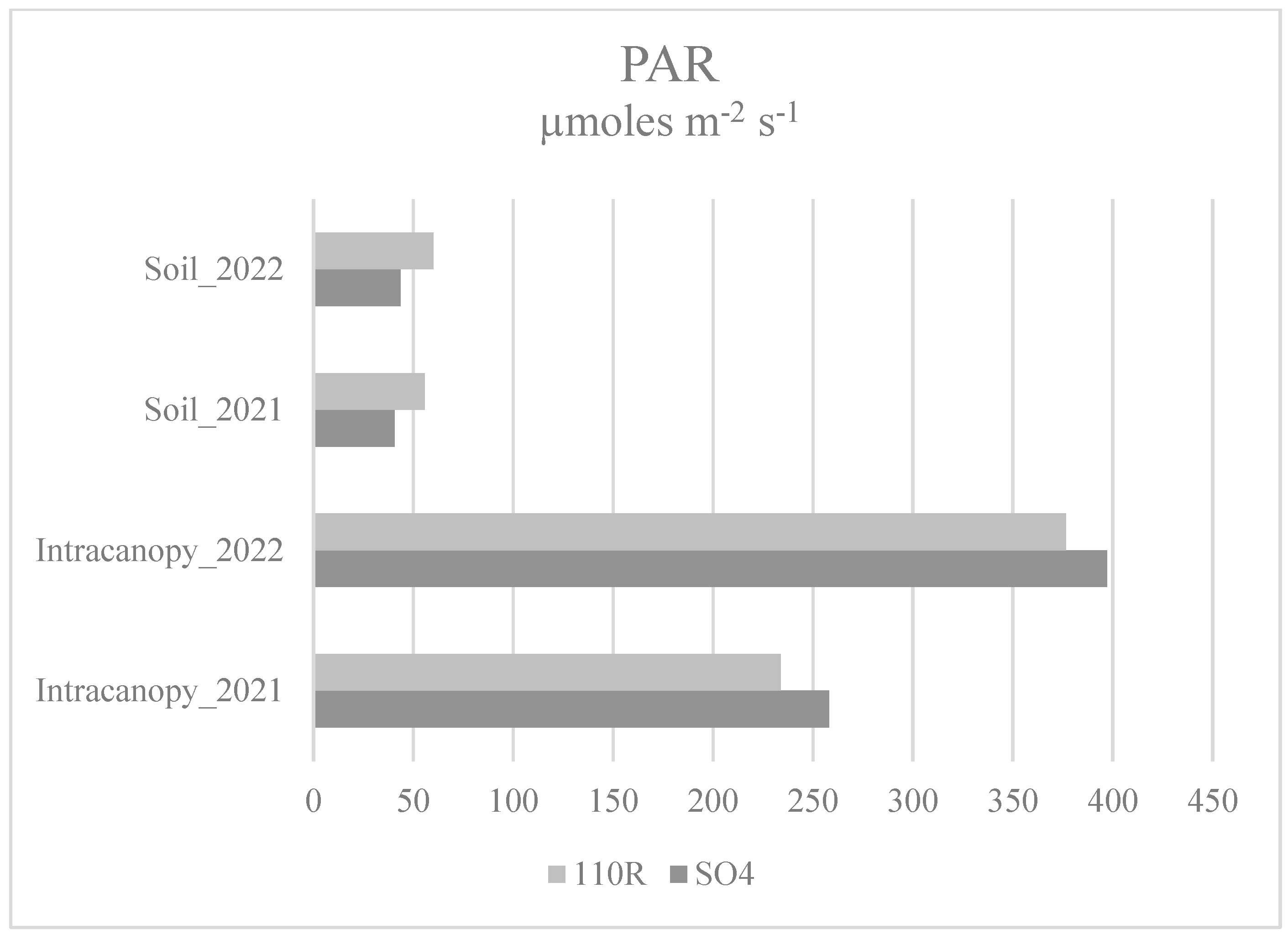
3.4. Light Distribution
3.5. Gas Exchange Measurement
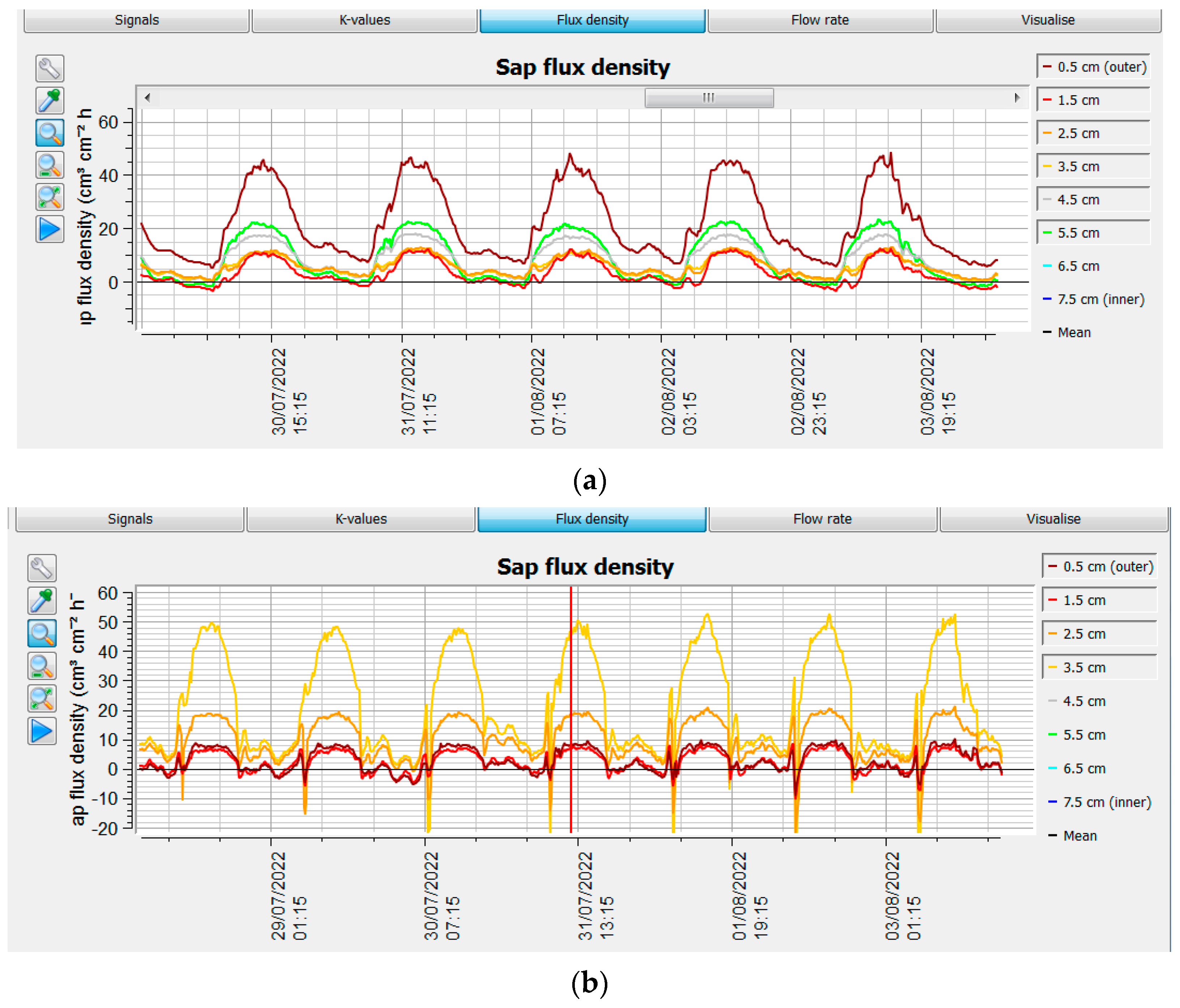
3.6. Sap Flow Measurements
3.7. Yield and Quality Parameters
3.8. Nutraceutical Parameters
4. Discussion
4.1. Phenophase Evolution
4.2. Conductive Xylem Area
4.3. Vegetative Growth
4.4. Light Distribution
4.5. Gas Exchange
4.6. Quality Parameters
4.7. Sap Flow
4.8. Biomass Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A history of grafting. Hortic. Rev. 2009, 35, 437–493. [Google Scholar]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Granett, J.; Walker, A.M.; Kocsis, L.; Omer, A.D. Biology and management of grape Phylloxera. Annu. Rev. Entomol. 2001, 46, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Galet, P.; Smith, J. Grape Varieties and Rootstock Varieties; Collection Avenir oenologie; Oenoplurimédia: Chaintré, France, 1998. [Google Scholar]
- Janott, M.; Gayler, S.; Gessler, A.; Javaux, M.; Klier, C.; Priesacket, E. A one-dimensional model of water flow in soil–plant systems based on plant architecture. Plant Soil 2011, 341, 233–256. [Google Scholar] [CrossRef]
- Jones, G.V. Climate, grapes, and wine: Structure and suitability in a changing climate. Acta Hortic. 2012, 931, 19–28. [Google Scholar] [CrossRef]
- Pongrácz, D.P. Rootstocks for Grapevines; David Philip Publisher: Cape Town, South Africa, 1983. [Google Scholar]
- Martínez-Ballesta, M.C.; Alcaraz-Lopez, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock-scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Serra, I.; Strever, A.; Myburgh, P.; Deloire, A. The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Evaluation of water relation parameters in vitis rootstocks with different drought tolerance and their effects on growth of a grafted cultivar. J. Plant Physiol. 2018, 226, 172–178. [Google Scholar] [CrossRef]
- Sucu, S.; Yagcı, A. Determination of sapling yield and quality features in some rootstocks and Sultanı Çekırdeksız (Vitis vinifera L.) variety grafted on these rootstocks. Ege Üniv. Ziraat Fak. Derg. 2017, 54, 53–59. [Google Scholar] [CrossRef]
- Lider, L.L. A grape rootstock trial in the Napa Valley: A nine-year progress report of three stocks on bale loam soil. Am. J. Enol. Vitic. 1957, 8, 121–126. [Google Scholar] [CrossRef]
- Carbonneau, A. The early selection of grapevine rootstocks for resistance to drought conditions. Am. J. Enol. Vitic. 1985, 36, 195–198. [Google Scholar] [CrossRef]
- Iacono, F.; Peterlunger, E. Rootstock-scion interaction may affect drought tolerance in Vitis vinifera cultivars. Implications in selection programs. Acta Hortic. 2000, 528, 543–554. [Google Scholar] [CrossRef]
- Ezzahouani, A.; Williams, L.E. The influence of rootstock on leaf water potential, yield, and berry composition of Ruby Seedless grapevines. Am. J. Enol. Vitic. 1995, 46, 559–563. [Google Scholar] [CrossRef]
- Choné, X.; Van Leeuwen, C.; Dubourdieu, D.; Gaudillère, J. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Deloire, A.; Carbonneau, A.; Wang, Z.; Ojeda, H. Vine and water: A short review. J. Int. Sci. Vigne Vin. 2004, 38, 1–13. [Google Scholar] [CrossRef]
- Williams, L.E. Interaction of rootstock and applied water amounts at various fractions of estimated evapotranspiration (ETc) on productivity of Cabernet Sauvignon. Aust. J. Grape Wine Res. 2010, 16, 434–444. [Google Scholar] [CrossRef]
- Nadezhdina, N. A simplified equation for sap flow calculation based on the Heat-Field Deformation (HFD) measurements. Acta Hortic. 2012, 951, 11. [Google Scholar] [CrossRef]
- Howard, A.R.; Van Iersel, M.W.; Richards, J.H.; Donovan, L. Night-time transpiration can decrease hydraulic redistribution. Plant Cell Environ. 2009, 32, 1060–1070. [Google Scholar] [CrossRef]
- Antonacci, D.; Perniola, R. L’uva da Tavola, Collana Coltura e Cultura; Bayer CropScience: Milano, Italy, 2010; pp. 385–421. [Google Scholar]
- Galet, P. Cépages et Vignobles de France; Galet, P., Ed.; Dehan: Montpellier, France, 1988. [Google Scholar]
- Nadezhdina, N. Temperature gradients around a linear heater due to moving sap. In Proceedings of the 4th International Workshop on Measuring Sap Flow in Intact Plants, Zidlochovice, Czech Republic, 3–5 October 1998; Cermák, J., Nadezhdina, N., Eds.; Publishing House of Mendel University: Brno, Czech Republic, 1998; pp. 65–71. [Google Scholar]
- Nadezhdina, N.; Tributsch, H.; Èermák, J. Infra-red images of heat filed around a linear heater and sap flow in stems of lime trees under natural and experimental conditions. Ann. Sci. For. 2004, 61, 203–213. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Èermák, J.; Neruda, J.; Prax, A.; Ulrich, R.; Nadezhdin, V.J.; Gašpárek, J.; Pokorny, E. Roots under the load of heavy machinery in spruce trees. Eur. J. For. Res. 2006, 125, 111–128. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Vandegehuchte, M.; Steppe, K. Sap flux density measurements based on the heat field deformation method. Trees Struct. Funct. 2012, 26, 1439–1448. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying the ABTS+ radical cation decolorization assay. Methods Enzimol. 1999, 299, 589–603. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Holbrook, N.M.; Zimmermann, M.H.; Tyree, M.T. Spring filling of xylem vessels in wild grapevine. Plant Physiol. 1987, 83, 414–417. [Google Scholar] [CrossRef]
- Maurel, K.; Leite, G.B.; Bonhomme, M.; Guilliot, A.; Rageau, R.; Pétel, G.; Sakr, S. Trophic control of bud break in peach (Prunus persica) trees: A possible role of hexoses. Tree Physiol. 2004, 24, 579–588. [Google Scholar] [CrossRef]
- Bonhomme, M.; Peuch, M.; Ameglio, T.; Rageau, R.; Guilliot, A.; Decourteix, M.; Alves, G.; Sakr, S.; Lacointe, A. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiol. 2010, 30, 89–102. [Google Scholar] [CrossRef]
- Keller, M.; Zhang, Y.; Shrestha, P.M.; Biondi, M.; Bondada, B.R. Sugar demand of ripening grape berries leads to recycling of surplus phloem water via the xylem. Plant Cell Environ. 2015, 38, 1048–1059. [Google Scholar] [CrossRef]
- Rubio, S.; Noriega, X.; Pérez, F.J. ABA promotes starch synthesis and storage metabolism in dormant grapevine buds. J. Plant Physiol. 2019, 234–235, 1–8. [Google Scholar] [CrossRef]
- Van Staden, J.; Davey, J.G. The synthesis, transport and metabolism of endogenous cytokinins. Plant Cell Environ. 1979, 2, 93–106. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Christou, A.; Kitta, E.; Katsoulas, N. Implementing sustainable irrigation in water-scarce regions under the impact of climate change. Agronomy 2020, 10, 1120. [Google Scholar] [CrossRef]
- Jones, T.H.; Cullis, B.R.; Clingeleffer, P.R.; Rühl, E.H. Effects of novel hybrid and traditional rootstocks on vigour and yield components of Shiraz grapevines. Aust. J. Grape Wine Res. 2009, 15, 284–292. [Google Scholar] [CrossRef]
- Tandonnet, J.P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2010, 16, 290–300. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Pirie, A.; Mullins, M.G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol. 1976, 58, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Dokoozlian, N.K. Grape berry growth and development. In Raisin Production Manual; Christensen, L.P., Ed.; UCANR Publications: Oakland, CA, USA, 2000; pp. 30–37. [Google Scholar]
- Jogaiah, S.; Striegler, K.R.; Bergmeier, E.; Harris, J. Influence of cluster exposure to sun on fruit composition of ‘Norton’ Grapes (Vitis aestivalis Michx) in Missouri. Int. J. Fruit. Sci. 2012, 12, 410–426. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Richards, J.H.; Smart, D.R.; Eissenstat, D.M. Importance of internal hydraulic redistribution for prolonging lifespan of roots in dry soil. Plant Cell Environ. 2008, 31, 177–186. [Google Scholar] [CrossRef]

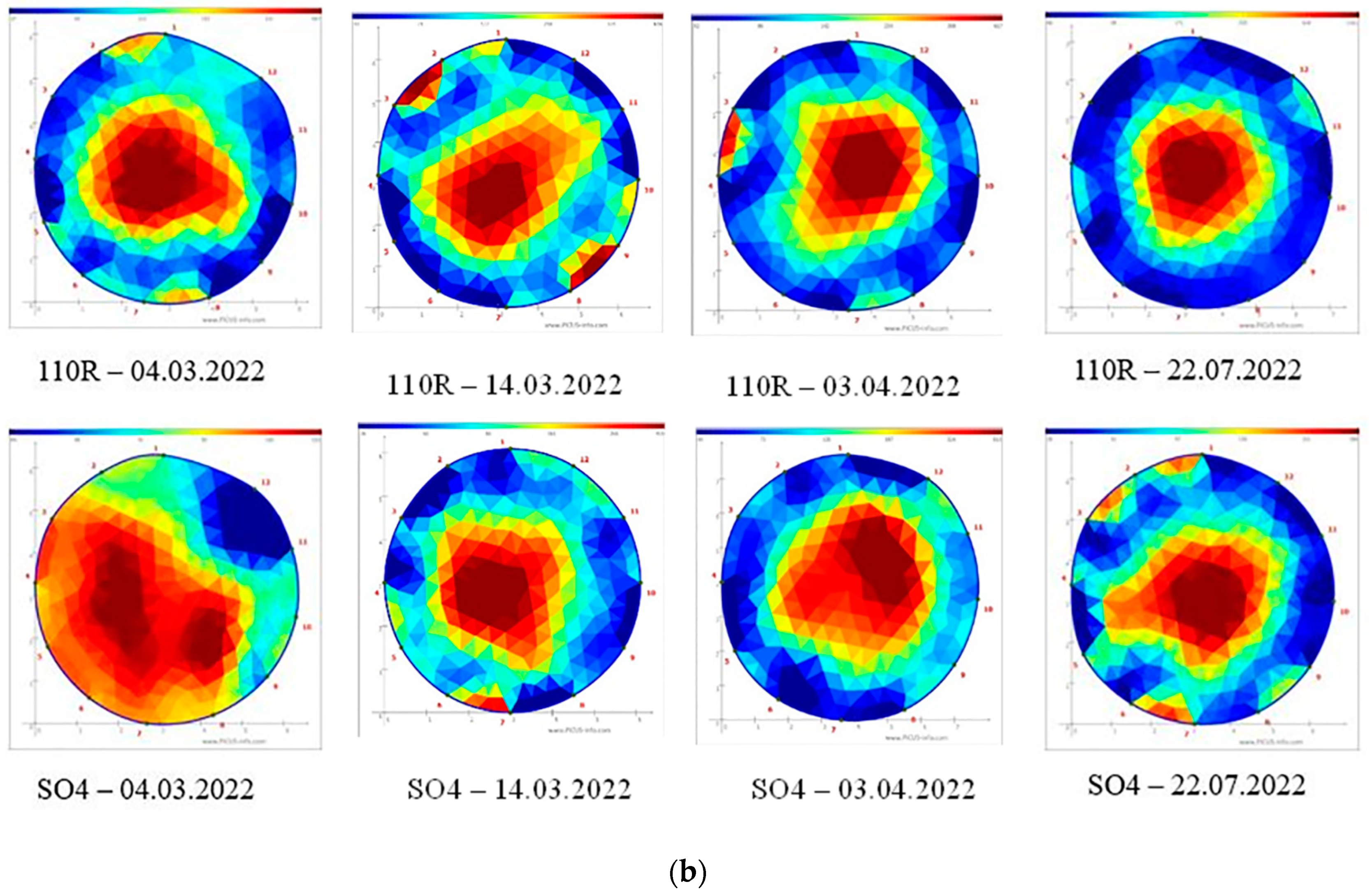
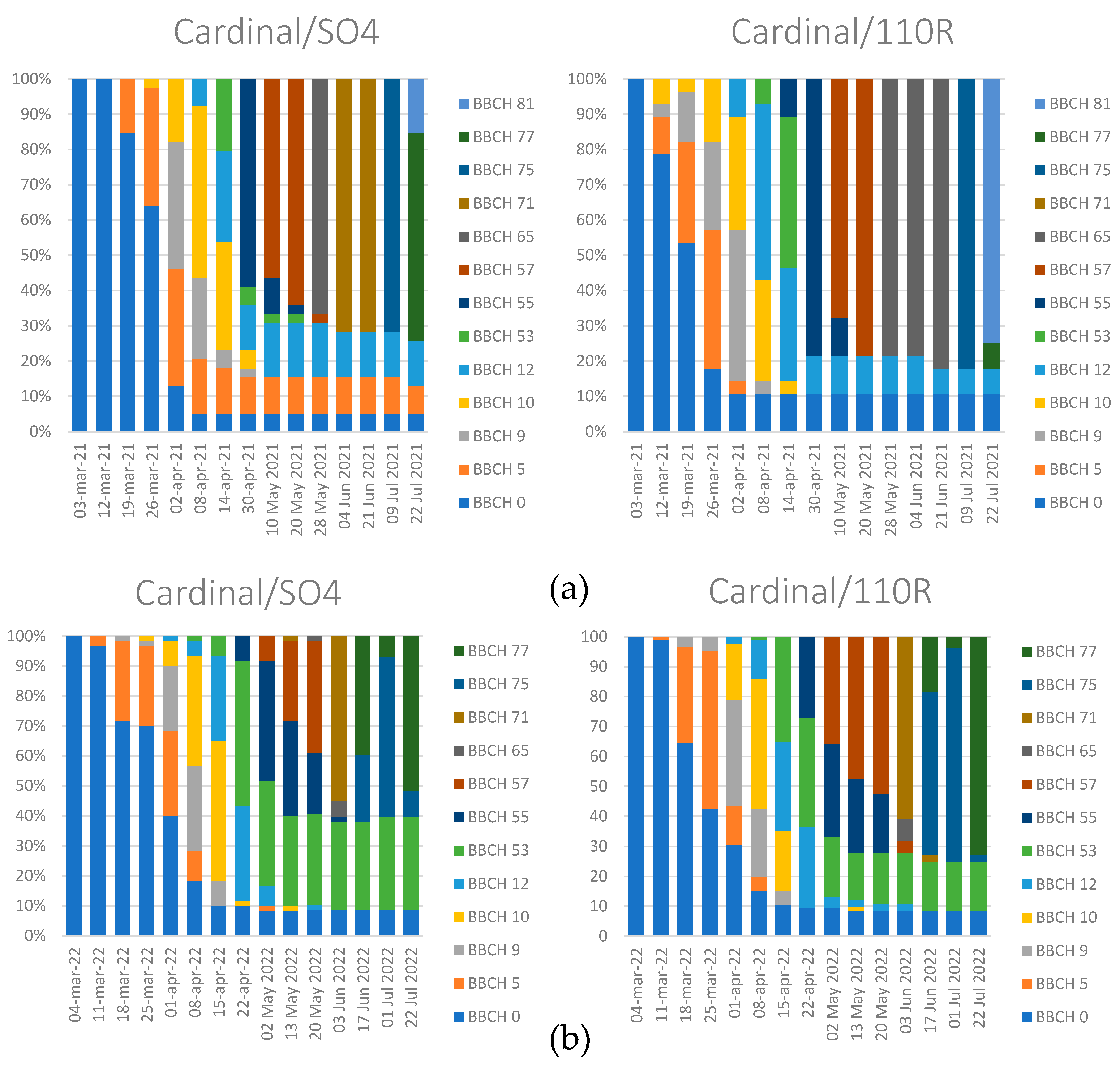
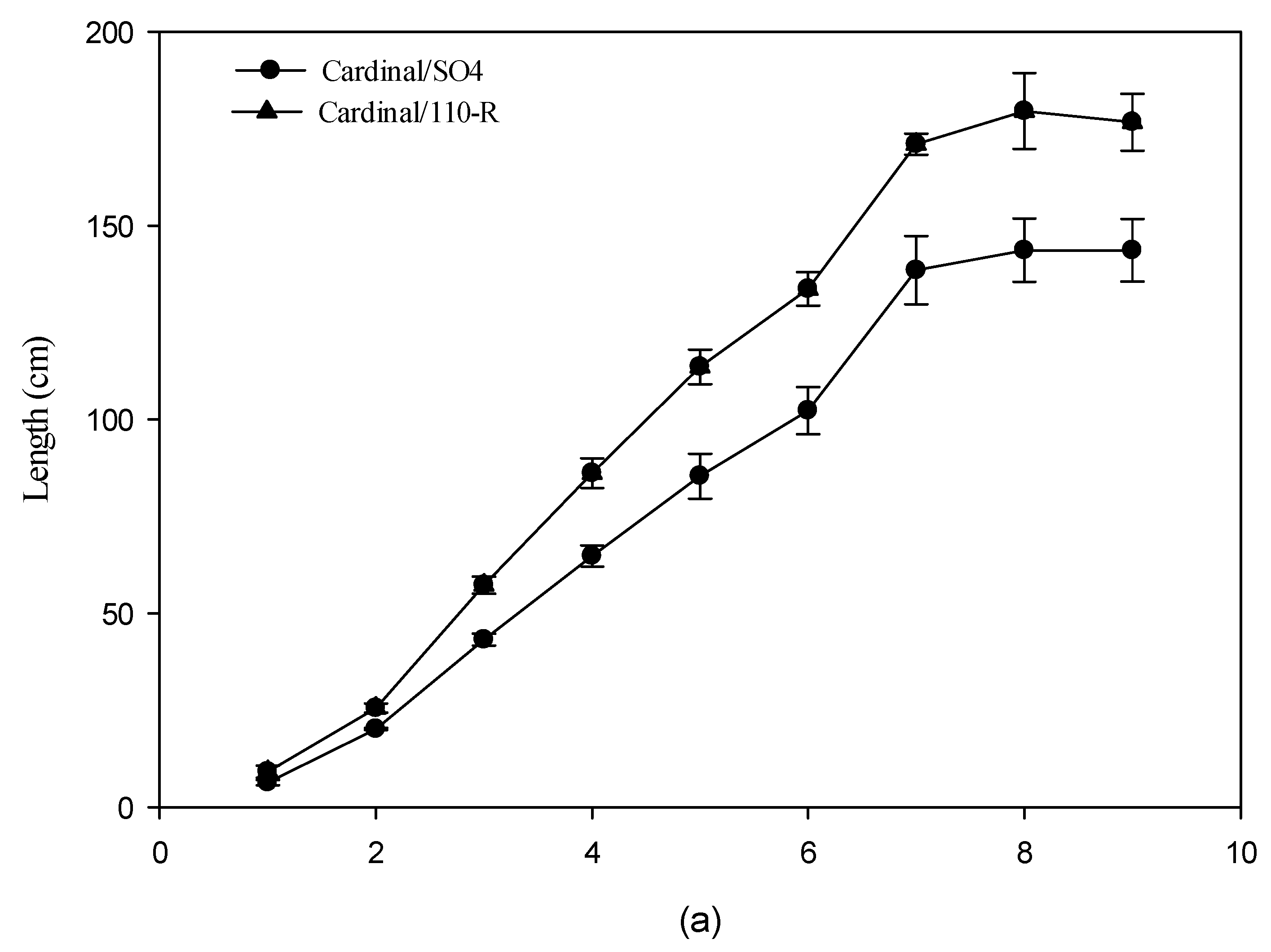
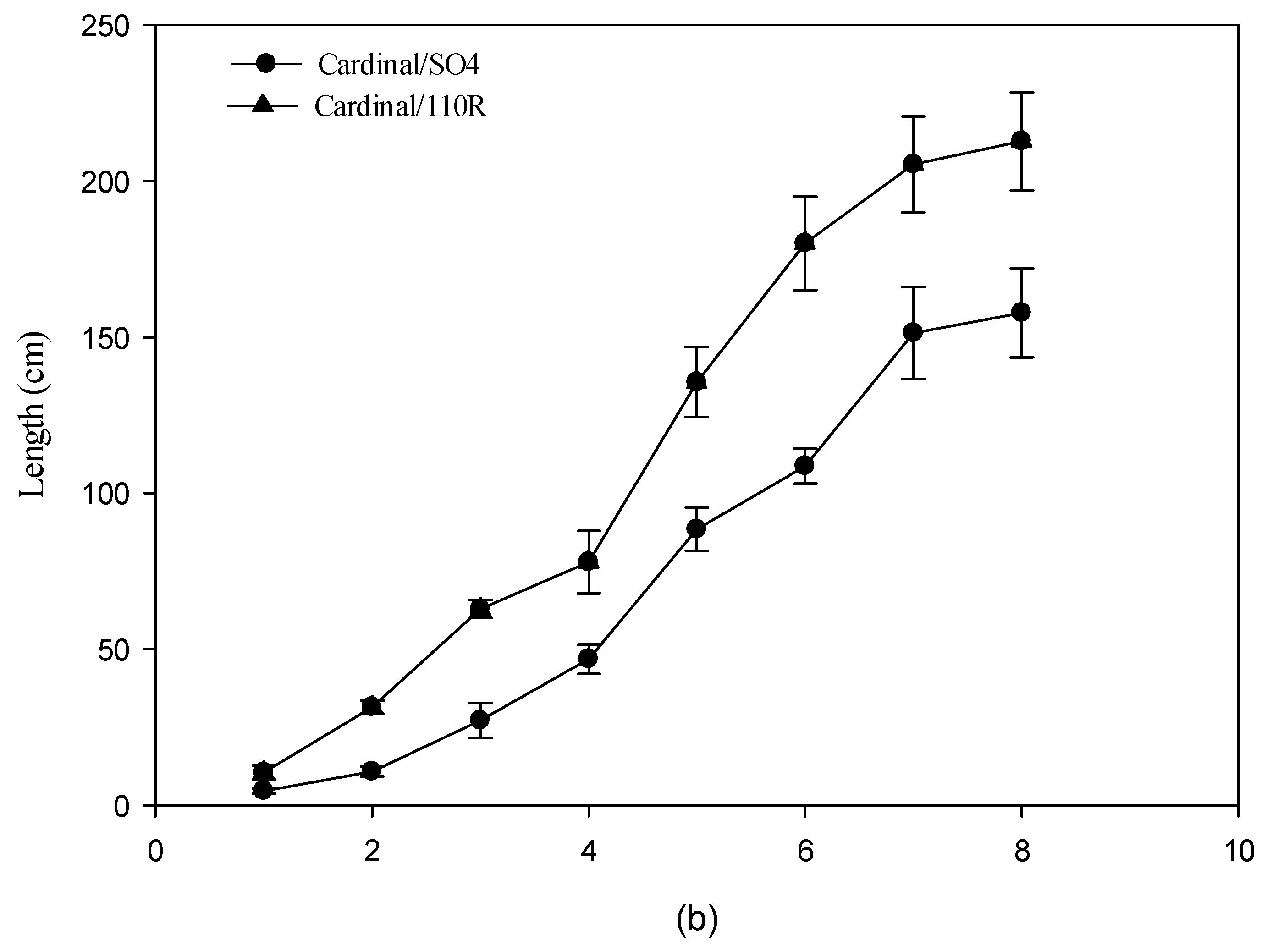
| Graft Combination | CXA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Ten Days March | Second Ten Days March | Third Ten Days April | Third Ten Days July | ||||||
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | ||
| C/110R | % | 66.47 a (±1.71) | 60.73 a (±0.44) | 68.61 a (±2.29) | 61.22 a (±0.47) | 67.72 a (±1.05) | 65.33 a (±1.56) | 65.03 n.s. (±2.60) | 70.88 n.s. (±1.26) |
| C/SO4 | 49.39 b (±2.33) | 44.12 b (±1.32) | 53.91 b (±2.17) | 56.15 b (±0.82) | 61.56 b (±2.80) | 62.33 b (±2.80) | 62.97 (±1.91) | 68.54 (±0.66) | |
| Graft Combination | Year | Number of Leaves per Principal Shoot (PS) n° | Total Leaf Area per Principal Shoot m2 | Number of Leaves per Lateral Shoot n° | Leaf Area per Lateral Shoot (cm2) | Total Number of Leaves per Shoot (PS + LS) n° | Total Leaf Area per Shoot (PS + LS) m2 | Area of Single Leaf cm2 | Leaf Area per Vine m2 | Lateral Shoot % | LAI | Internode Length (cm) | Winter Pruning Residue (kg pianta−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO4 | 21 | 16.30 ± 1.9 d | 0.12 ± 0.02 d | 27.53 b | 0.217267 b | 43.83 ± 10.83 n.s. | 0.33 ± 0.05 n.s. | 78.92 ± 6.47 n.s. | 2.66 ±1.1 b | 20.1 ± 1.5 a | 0.66 ± 0.05 b | 7.33 ± 0.12 b | 3.10 ± 0.30 a |
| 110R | 19.18 ± 0.4 c | 0.15 ± 0.015 c | 17.48 c | 0.137358 d | 36.66 ± 6.90 | 0.28 ± 0.05 | 78.58 ± 3.87 | 2.22 ± 2.8 c | 14.53 ± 1.8 b | 0.55 ± 0.04 c | 8.59 ± 0.15 a | 2.50 ± 0.20 b | |
| SO4 | 22 | 21.61 ± 2.2 b | 0.21 ± 0.01 b | 29.73 a | 0.293376 a | 51.34 ± 10.83 | 0.40 ± 0.06 | 98.68 ± 3.13 n.s. | 3.62 ± 2.1 a | 19 ± 1.1 a | 0.93 ± 0.07 a | 7.47 ± 0.14 b | 3.80 ± 0.40 a |
| 110R | 25.02 ± 1.5 a | 0.24 ± 0.01 a | 16.26 d | 0.158194 c | 41.28 ± 6.90 | 0.32 ± 0.04 | 97.29 ± 2.17 | 2.62 ± 2.4 b | 15 ± 1.2 b | 0.66 ±0.04 b | 8.48 ± 0.18 a | 2.30 ± 0.30 b | |
| year | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| I = Y × T | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Year | Graft Combination | Number of Clusters per Plant | Yield per Plant Kg. plant−1 | Bunch Weight g | N° of Berries per Bunch | Bud-Break % | Real Fertility R.F. |
|---|---|---|---|---|---|---|---|
| 2021 | C/SO4 | 59.18 ± 4.37 a | 21.3 ± 1.9 a | 362 ± 25 n.s. | 51 ± 5 n.s. | 95.60 ± 2.23 n.s. | 0.77 ± 0.12 a |
| C/110R | 63.75 ± 3.3 a | 25.9 ± 2.4 a | 393 ± 22 | 56 ± 4 | 89.70 ± 2.06 | 0.87 ± 0.11 a | |
| 2022 | C/SO4 | 48.00 ± 4.19 b | 18.44 ± 1.5 b | 326.05 ± 18.64 | 54 ± 2 | 92.38 ± 3.15 | 0.66 ± 0.06 b |
| C/110R | 53.83 ± 5.90 b | 18.59 ± 1.76 b | 305.59 ± 21.64 | 59 ± 4 | 92.36 ± 2.89 | 0.73 ± 0.04 b | |
| Year | * | * | * | n.s. | n.s. | n.s. | |
| I = T × Y | n.s. | n.s | n.s. | n.s. | n.s. | n.s. | |
| Year | Graft Combination | Pn µmol CO2 m−2 s−1 | gs mol H2O m−2 s−1 | Ci ppm | E mmol H2O m−2 s−1 | Pn/E | Pn/Ci | SPAD | ΨLeaf Bar | Sap Flow cm3 cm−2 h−1 | Daily Xylem Flow L cm−2 d−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | C/SO4 | 12.21 ±0.87 b | 0.073 ±0.004 b | 118.1 ±17.80 b | 3.102 ±0.322 n.s. | 3.93 ±0.23 n.s. | 0.10 ±0.05 n.s. | 36.88 ±0.71 n.s. | −5.62 ±0.15 b | 25.74 ±1.12 b | 10.60 ±0.4 b |
| C/110R | 13.83 ±0.85 a | 0.086 ±0.008 a | 128.45 ±20.43 b | 3.008 ±0.212 | 4.59 ±0.23 | 0.10 ±0.02 | 36.14 ±0.62 | −4.56 ±0.11 a | 30.89 ±1.35 a | 12.33 ±0.15 a | |
| 2022 | C/SO4 | 10.79 ±1.13 c | 0.072 ±0.01 b | 157.21 ±33.40 a | 3.296 ±0.588 n.s. | 3.60 ±0.78 n.s. | 0.10 ±0.09 n.s. | 37.20 ±0.23 | −5.37 ±0.20 b | 20.33 ±1.24 c | 10.21 ±0.8 b |
| C/110R | 14.04 ±1.97 a | 0.090 ±0.01 a | 140.70 ±14.28 a | 3.239 ±0.708 | 5.05 ±0.28 | 0.07 ±0.01 | 36.84 ±0.38 | −4.64 ±0.10 a | 25.34 ±1.01 b | 11.74 ±0.5 a | |
| Year | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | |
| I = T × Y | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Time of Sampling | Graft Combination | Average Weight g | Polar Diameter mm | Equatorial Diameter mm | Degree of Ripening % |
|---|---|---|---|---|---|
| First week July (12 July 2021 13 July 2022) | 110R | 5.16 ± 0.21 d | 19.68 ± 0.25 d | 20.64 ± 0.30 c | 0.76 ± 0.14 f |
| SO4 | 4.91 ± 0.19 d | 19.56 ± 0.21 d | 20.04 ± 0.27 c | 1.06 ± 0.14 e | |
| Second week July 19 July 2021 20 July 2022) | 110R | 5.77 ± 0.26 c | 20.17 ± 0.27 c | 20.74 ± 0.33 c | 1.68 ± 0.14 d |
| SO4 | 5.87 ± 0.22 c | 20.33 ± 0.26 c | 20.62 ± 0.2 c | 1.98 ± 0.14 c | |
| Third ten days July (26 July 2021 26 July 2022) | 110R | 7.24 ± 0.27 a | 22.24 ± 0.25 b | 22.54 ± 0.29 b | 2.26 ± 0.10 b |
| SO4 | 6.84 ± 0.22 b | 22.20 ± 0.24 b | 22.14 ± 0.27 b | 2.22 ± 0.12 b | |
| First ten day August (2 August 2021 3 August 2022) | 110R | 7.58 ± 0.23 a | 23.05 ± 0.22 a | 22.83 ± 0.27 a | 2.36 ± 0.11 a |
| SO4 | 7.40 ± 0.24 a | 23.84 ± 0.23 a | 23.34 ± 0.28 a | 2.50 ± 0.12 a | |
| Year | n.s. | n.s. | n.s. | * | |
| I = Year × Treatment | n.s. | n.s. | n.s. | n.s. | |
| Time of Sampling | Graft Combination | TSSs (°Brix) | pH | TA (%) | TSS/TA |
|---|---|---|---|---|---|
| First week July (12 July 2021 13 July 2022) | 110R | 10.10 ± 0.31 d | 2.94 ± 0.03 d | 1.21 ± 0.05 a | 8.43 ± 0.68 c |
| SO4 | 10.40 ± 0.11 d | 2.94 ± 0.02 d | 1.28 ± 0.03 a | 8.14 ± 0.20 c | |
| Second week July (19 July 2021 20 July 2022) | 110R | 11.60 ± 0.14 c | 3.13 ± 0.02 c | 0.79 ± 0.06 b | 14.97 ± 1.17 d |
| SO4 | 11.74 ± 0.19 c | 3.10 ± 0.02 c | 0.85 ± 0.03 b | 13.91 ± 0.60 d | |
| Third ten days July (26 July 2021 26 July 2022) | 110R | 13.56 ± 0.31 b | 3.60 ± 0.33 a | 0.57 ± 0.01 d | 23.66 ± 0.71 b |
| SO4 | 13.42 ± 0.18 b | 3.28 ± 0.02 b | 0.670.02 c | 19.18 ± 0.75 c | |
| First ten days August (2 August 2021 3 August 2022) | 110R | 14.48 ± 0.30 a | 3.50 ± 0.02 a | 0.46 ± 0.01 d | 31.68 ± 0.27 a |
| SO4 | 14.74 ± 0.31 a | 3.51 ± 0.02 a | 0.53 ± 0.04 d | 28.84 ± 0.53 a | |
| Year | n.s. | n.s. | n.s. | n.s. | |
| I = T × Y | n.s. | n.s. | n.s. | n.s. | |
| Time of Sampling | Graft Combination | TPC Pulp mg Gallic acid/gr. FW | TAC Pulp µmoles trolox/gr. FW | TPC Peel mg Gallic acid/gr. FW | TAC Peel µmoles trolox/gr. FW |
|---|---|---|---|---|---|
| First week July (12 July 2021 13 July 2022) | C/110R | 0.569 ± 0.049 n.s. | 1.204 ± 0.054 n.s. | 0.994 ± 0.086 n.s. | 26.691 ± 0.494 a |
| C/SO4 | 0.650 ± 0.085 | 1.137 ± 0.207 | 1.311 ± 0.172 | 23.841 ± 0.041 b | |
| Second week July (19 July 2021 20 July 2022) | C/110R | 0.678 ± 0.036 n.s. | 1.114 ± 0.093 n.s. | 1.249 ± 0.067 n.s. | 25.859 ± 0.121 n.s. |
| C/SO4 | 0.615 ± 0.038 | 1.589 ± 0.150 | 1.172 ± 0.073 | 25.680 ± 0.013 | |
| Third ten days July (26 July 2021 26 July 2022) | C/110R | 0.590 ± 0.039 n.s. | 1.146 ± 0.068 n.s. | 1.427 ± 0.095 n.s. | 31.337 ± 0.887 a |
| C/SO4 | 0.659 ± 0.040 | 1.469 ± 0.138 | 1.239 ± 0.075 | 25.703 ± 0.529 b | |
| First ten days August (2 August 2021 3 August 2022) | C/110R | 0.592 ± 0.048 n.s. | 1.074 ± 0.052 n.s. | 1.232 ± 0.099 n.s. | 28.244 ± 0.450 n.s. |
| C/SO4 | 0.524 ± 0.010 | 1.066 ± 0.062 | 1.072 ± 0.022 | 27.407 ± 0.353 | |
| Year | n.s. | n.s. | n.s. | n.s. | |
| I = T × Y | n.s. | n.s. | n.s. | n.s. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattola, A.; Vonella, V.; Olivadese, T.; Pullia, F.M.; Zappia, R.; Gullo, G. The Evaluation of the Water Consumption and the Productive Parameters of a Table Grapevine, Cardinal Cultivar, Grafted onto Two Rootstocks. Agriculture 2023, 13, 2101. https://doi.org/10.3390/agriculture13112101
Dattola A, Vonella V, Olivadese T, Pullia FM, Zappia R, Gullo G. The Evaluation of the Water Consumption and the Productive Parameters of a Table Grapevine, Cardinal Cultivar, Grafted onto Two Rootstocks. Agriculture. 2023; 13(11):2101. https://doi.org/10.3390/agriculture13112101
Chicago/Turabian StyleDattola, Antonio, Vincenzo Vonella, Tommaso Olivadese, Francesco Maria Pullia, Rocco Zappia, and Gregorio Gullo. 2023. "The Evaluation of the Water Consumption and the Productive Parameters of a Table Grapevine, Cardinal Cultivar, Grafted onto Two Rootstocks" Agriculture 13, no. 11: 2101. https://doi.org/10.3390/agriculture13112101





