Overcoming Low Germination and Low Quality of Flax Seeds (Linum usitatissimum L.) in Unfavorable Storage Using Static Magnetic Fields
Abstract
:1. Introduction
2. Materials and Methods
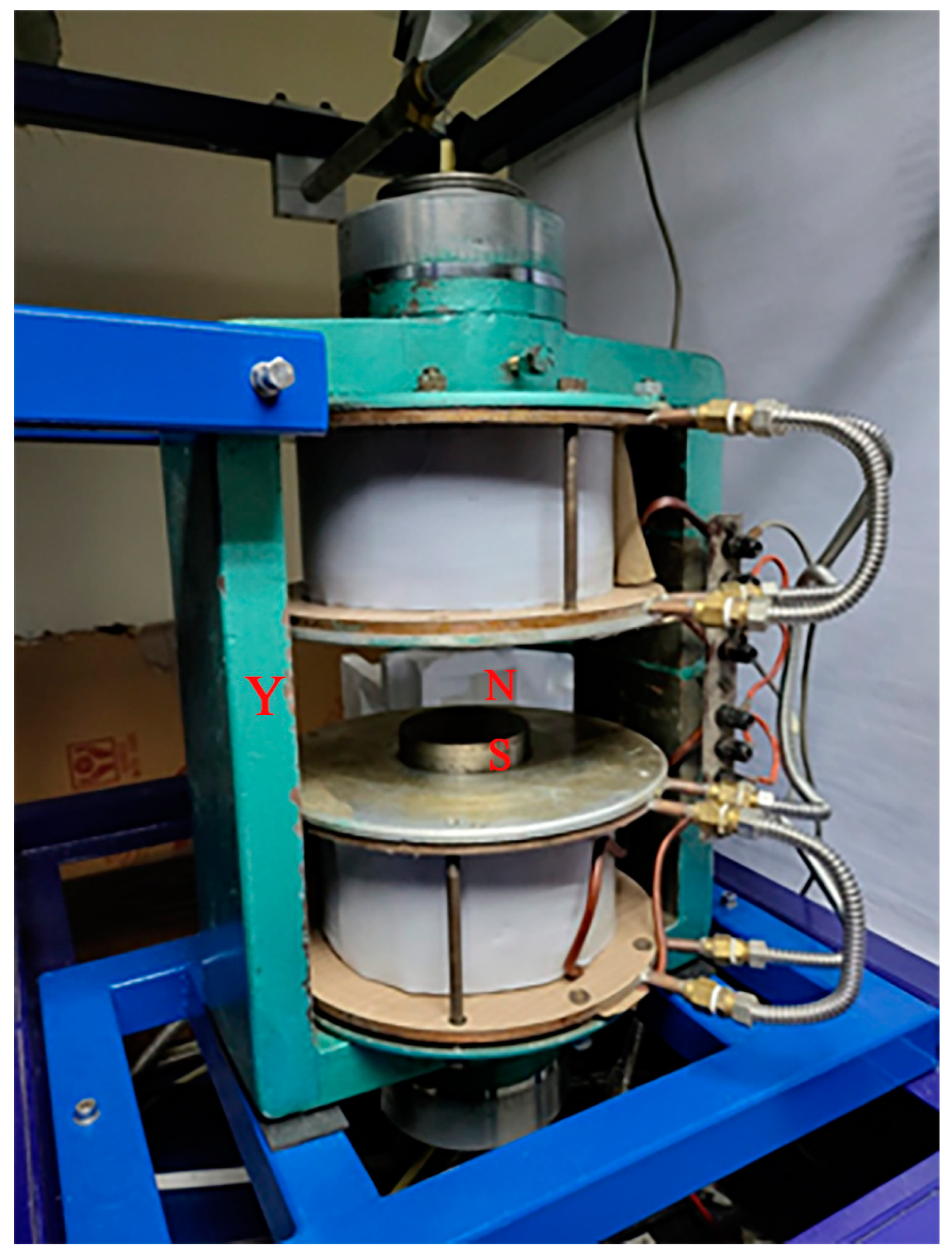
2.1. Plant Material and Magnetic Field Characteristics
2.2. Seed Germination
2.3. Seedling Growth and Vigor Indices
2.4. Seed Leachate Conductivity
2.5. Chlorophyll and Carotenoid Extractions
| Chl a = 12.47A665 − 3.62A649 Chl b = 25.06A649 − 6.5A665 C x + c = (1000A480 − 1.29C a − 53.78C b)/220 |
2.6. Enzymatic Assays
2.6.1. Total Protein Extraction
2.6.2. Spectrophotometric Determination of Catalase (CAT) and Peroxidase (POD) Activities
| Volume activity (U/mL) = (ΔA × Vq)/(0.0436 × Vs) ΔA = decrease in absorbance at 240 nm per min at 25 °C Vq = reaction volume into cuvette (in mL) 0.0436 = millimolar extinction coefficient (ɛ) of H2O2 at 240 nm (mM−1cm−1) Vs = volume (mL) of sample |
| Volume activity (U/mL) = (ΔA × Vq)/)/(2.47 × Vs) ΔA = increase in absorbance at 430 nm per min at 25 °C Vq = reaction volume into cuvette (in mL) 2.47 = extinction coefficient (ɛ) of purpurogallin at 430 nm (mM−1cm−1) Vs = volume (in mL) of sample |
2.7. Statistical Analyses
3. Results
3.1. Seed Germination
3.2. Seedling Growth
3.3. Vigor Indices
3.4. Seed Leachate Conductivity
3.5. Chlorophyll and Carotenoids Content
3.6. Catalase and Peroxidase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, A.; De Santis, A.; Maffei, M.E. Magnetoreception: An unavoidable step for plant evolution? Trends Plant Sci. 2014, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Balakhnina, T.; Bulak, P.; Nosalewicz, M.; Pietruszewski, S.; Włodarczyk, T. The influence of wheat Triticum aestivum L. seed pre-sowing treatment with magnetic fields on germination, seedling growth, and antioxidant potential under optimal soil watering and flooding. Acta Physiol. Plant. 2015, 37, 59–69. [Google Scholar] [CrossRef]
- Anand, A.; Nagarajan, S.; Verma, A.; Joshi, D.; Pathak, P.; Bhardwaj, J. Pre-treatment of seeds with magnetic field ameliorates soil wather stress in seedlings of maize (Zea mays L.). Indian J. Biochem. Biophys. 2012, 49, 63–70. [Google Scholar] [PubMed]
- Ercan, I.; Tombuloglu, H.; Alqahtani, N.; Alotaibi, B.; Bamhrez, M.; Alshumrani, R.; Ozcelik, S.; Kayed, T.S. Magnetic field effects on the magnetic properties, germination, chlorophyll fluorescence, and nutrient content of barley (Hordeum vulgare L.). Plant Physiol. Biochem. 2022, 170, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K. Alleviation of adverse effects of ambient UV stress on growth and some potential physiological attributes in soybean (Glycine max) by seed pre-treatment with a magnetic field. J. Plant Growth Regul. 2017, 36, 550–565. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of nitrate reductase-dependent nitric oxide production in magneto priming-induced salt tolerance in soybean. Physiol. Plant. 2020, 168, 422–436. [Google Scholar] [CrossRef]
- Shokrollahi, S.; Ghanati, F.; Sajedi, R.H.; Sharifi, M. Possible role of iron-containing proteins in physiological responses of soybean to magnetic field. J. Plant Physiol. 2018, 226, 163–171. [Google Scholar] [CrossRef]
- Haghighat, N.; Abdolmaleki, P.; Ghanati, F.; Behmanesh, M.; Payez, A. Modification of catalase and MAPK in Vicia faba cultivated in soil with high natural radioactivity and treated with a magnetic field. J. Plant Physiol. 2014, 171, 99–103. [Google Scholar] [CrossRef]
- Marks, N.; Szecowka, P.S. Impact of variable magnetic field stimulation on the growth of above-ground parts of potato plants. Int. Agrophys. 2010, 24, 165–170. [Google Scholar]
- Poinapen, D.; Brown, D.C.W.; Beeharry, G.K. Seed orientation and magnetic field strength have more influence on tomato seed performance than relative humidity and duration of exposure to non-uniform magnetic fields. J. Plant Physiol. 2013, 170, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and biophysical changes associated with magneto priming in germinating cucumber seeds. Plant Physiol. Bioch. 2012, 57, 67–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Hub, M.; Gaob, Z.; Chenb, X.; Huanga, D. Biological mechanisms of a novel hydro-electro hybrid priming recover potential vigor of onion seeds. Environ. Exp. Bot. 2018, 150, 260–271. [Google Scholar] [CrossRef]
- Sousa, S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Cienc. Veg. 2016, 7, 646. [Google Scholar] [CrossRef]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants, growth, antioxidant activity and phenolic compounds. Plant Physiol. Bioch. 2014, 83, 126–133. [Google Scholar] [CrossRef]
- Thomas, S.; Anand, A.; Chinnusamy, V.; Dahuja, A.; Basu, S. Magnetopriming circumvent the effect of salinity stress on germination in chickpea seeds. Acta Physiol. Plant 2013, 35, 3401–3411. [Google Scholar] [CrossRef]
- Bose, B.; Kumar, M.; Sighal, R.; Mondal, S. Impact of seed priming on the modulation of physical-chemical and molecular processes during germination, growth, and development of crops. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer Nature: Singapore, 2018; pp. 33–40. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Seed pretreatment with magnetic field alters the storage proteins and lipid profiles in harvested soybean seeds. Physiol. Mol. Biol. Plant. 2018, 24, 343–347. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Ahmad, N.; Shi, Z.; Raza, A.; Wang, X.; Li, J. Growth, physiological, biochemical and molecular changes in plants induced by magnetic fields: A review. Plant Biol. 2022, 25, 8–23. [Google Scholar] [CrossRef]
- Paul, A.L.; Ferl, R.J.; Meisel, M.W. High magnetic field induced changes of gene expression in Arabidopsis. Biomagn. Res. Technol. 2006, 4, 7. [Google Scholar] [CrossRef]
- Maffei, M.E. Plant responses to electromagnetic fields. In Biological and Medical Aspects of Electromagnetic Fields; Greenebaum, B., Barnes, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 89–109. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (SMF) application in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Zuk, M.; Richter, D.; Matuła, J.; Szopa, J. Linseed, the multipurpose plant. Ind. Crop. Prod. 2015, 75, 165–177. [Google Scholar] [CrossRef]
- Westcott, N.D.; Muir, A.D. Flaxseed lignan in disease prevention and health promotion. Phytochem. Rev. 2003, 2, 401–417. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine and modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Ramesh, M. Flax (Linum usitatissimum L.) fibre reinforced polymer composite materials: A review on preparation, properties and prospects. Prog. Mater. Sci. 2019, 102, 109–166. [Google Scholar] [CrossRef]
- Preisner, M.; Kulma, A.; Zebrowski, J.; Dymińska, L.; Hanuza, J.; Arendt, M.; Starzycki, M.; Szopa, J. Manipulating cinnamyl alcohol dehydrogenase (CAD) expression in flax affects fiber composition and properties. BMC Plant Biol. 2014, 14, 50. [Google Scholar] [CrossRef]
- Beauvais, F.; Cantat, O.; Le Gouée, P.; Brunel-Muguet, S.; Madeline, P.; Gaillard, H.; Bataille, M.P.; Sallent, A.; Preux, T.; Medjkane, M. Consequences of climate change on flax fiber in Normandy by 2100: Prospective bioclimatic simulation based on data from the ALADIN-Climate and WRF regional models. Theor. Appl. Climatol. 2022, 148, 415–426. [Google Scholar] [CrossRef]
- Austria, J.A.; Richard, M.N.; Chahine, M.N.; Edel, A.L.; Malcolmson, L.J.; Dupasquier, C.M.; Pierce, G.N. Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J. Am. Coll. Nutr. 2008, 27, 214–221. [Google Scholar] [CrossRef]
- Mundhada, S.; Muhammad Mudassir, M.; Chaudhry, A.; Erkinbaev, C.; Jitendra Paliwal, J. Development of safe storage guidelines for prairie-grown flaxseed. J. Stored Prod. Res. 2022, 97, 101965. [Google Scholar] [CrossRef]
- Thakur, M.; Tiwari, S.; Kataria, S.; Anand, A. Recent advances in seed priming strategies for enhancing planting value of vegetable seeds. Sci. Horticul. 2022, 305, 111355. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M. Magnetopriming Alleviates Adverse Effects of Abiotic Stresses in Plants. In Plant Tolerance to Environmental Stress, 1st ed.; Role of Phytoprotectants; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, T.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 427–442. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Assoc.: Zurich, Switzerland, 2011. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 2, pp. 673–684. [Google Scholar]
- Flatmark, T. Studies on the peroxidase effect of cytochrome c. III. A kinetic study of the over-all reaction. Acta Chem. Scand. 1964, 18, 2269–2279. [Google Scholar] [CrossRef]
- Wanda, M.; Waterworth, W.M.; Clifford, M.; Bray, C.M.; West, C.E. Seeds and the Art of Genome Maintenance. Front. Plant Sci. 2019, 10, 706. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. EMF radiations (1800 MHz)-inhibited early sprout growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 2016, 253, 1043–1049. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Germination characteristics of seeds of maize (Zea mays L.) exposed to magnetic fields under accelerated aging condition. J. Agric. Phys. 2009, 9, 50–58. [Google Scholar]
- Kataria, S.; Jain, M.; Rastogi, A.; Brestic, M. Static magnetic field treatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Photosynth. Res. 2021, 150, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Baghel, L.; Kataria, S.; Jain, M. Mitigation of adverse effects of salt stress on germination, growth, photosynthetic efficiency and yield in maize (Zea mays L.) through magnetopriming. Acta Agrobot. 2019, 72, 1–16. [Google Scholar] [CrossRef]
- Rathod, G.R.; Anand, A. Effect of seed magneto-priming on growth, yield and Na/K ratio in wheat (Triticum aestivum L.) under salt stress. Indian, J. Plant Physiol. 2016, 21, 15–22. [Google Scholar] [CrossRef]
- Sarraf, M.; Deamici, K.M.; Taimourya, H.; Islam, M.; Kataria, S. Effect of magnetopriming on photosynthetic performance of plants. Int. J. Mol. Sci. 2021, 22, 9353. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Niemczynowicz, A.; Kycia, R.A.; Matwijczuk, A.; Kornarzyński, K.; Stadnik, J.; Szymanek, M. Stimulation of soy seeds using environmentally friendly magnetic and electric fields. Sci. Rep. 2023, 13, 18085. [Google Scholar] [CrossRef]
- Liu, C.S.; Glahn, R.P.; Liu, R.H. Assessment of carotenoid bioavailability of whole foods using a caco-2 cell culture model coupled with an in vitro digestion. J. Agric. Food Hem. 2004, 52, 4330–4337. [Google Scholar] [CrossRef]
- Leelatanawit, R.; Sudtida, T.S.; Karoonuthaisiri, P.N.; Devahastin, S. Selection of reference genes for quantitative real-time PCR in postharvest tomatoes (Lycopersicon esculentum) treated by continuous low-voltage direct current electricity to increase secondary metabolites. Int. J. Food Sci. Technol. 2017, 52, 1942–1950. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Payez, A.; Ghanati, F.; Behmanesh, M.; Abdolmaleki, P.; Hajnorouzi, A.; Rajabbeigi, E. Increase of seed germination, growth and membrane integrity of wheat sprouts by exposure to static and a 10-KHz electromagnetic field. Electromagn. Biol. Med. 2013, 32, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins, a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Mitrov, P.P.; Kroumova, Z.; Baidanova, V.D. Auxin content of corn and tomato plants following magnetic field treatments. Fiziol No Rastenyata 1988, 14, 18–23. [Google Scholar]
- Shine, M.B.; Guruprasad, K.; Anand, A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 2012, 33, 428–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |




| SMF (mT) | Duration (min) | Germination % | Embryonic Shoot Length (cm) | Root Length (cm) | Dry Mass (g) | Vigor I | Vigor II | Anova p-Value |
|---|---|---|---|---|---|---|---|---|
| Control | control | 32 ± 3.4 e | 2.3 ± 0.2 g | 2.0 ± 0.3 f | 0.02 ± 0.002 h | 137.60 ± 13.9 l | 0.64 ± 0.08 h | ≤0.05 |
| 50 | 20 | 55 ± 5.9 d | 4.4 ± 0.4 f | 3.8 ± 0.4 ef | 0.04 ± 0.004 g | 451.00 ± 46.04 k | 2.20 ± 0.23 g | ≤0.01 |
| 40 | 60 ± 6.2 c | 4.6 ± 0.4 f | 4.1 ± 0.4 ef | 0.04 ± 0.005 g | 522.00 ± 53.27 j | 2.40 ± 0.25 g | ≤0.01 | |
| 60 | 65 ± 6.5 c | 4.9 ± 0.5 f | 4.5 ± 0.4 ef | 0.05 ± 0.005 fg | 611.00 ± 62.88 i | 3.25 ± 3.33 fg | ≤0.01 | |
| 80 | 69 ± 6.9 bc | 5.2 ± 0.5 g | 4.8 ± 0.5 e | 0.05 ± 0.005 fg | 690.00 ± 70.04 h | 3.45 ± 0.35 f | ≤0.01 | |
| 100 | 74 ± 7.2 b | 5.6 ± 0.6 ef | 5.1 ± 0.5 e | 0.05 ± 0.006 fg | 791.80 ± 79.06 g | 3.70 ± 0.38 f | ≤0.01 | |
| 120 | 78 ± 7.9 b | 5.9 ± 0.6 ef | 5.4 ± 0.6 e | 0.06 ± 0.006 f | 881.40 ± 89.05 fg | 4.68 ± 0.48 ef | ≤0.01 | |
| 100 | 20 | 60 ± 6.2 c | 6.3 ± 0.6 ef | 5.7 ± 0.6 e | 0.06 ± 0.006 f | 720.00 ± 73.55 h | 3.60 ± 0.37 f | ≤0.01 |
| 40 | 65 ± 6.7 c | 6.6 ± 0.7 ef | 6.0 ± 0.7 e | 0.06 ± 0.006 f | 819.00 ± 82.11 g | 3.90 ± 0.0.40 f | ≤0.01 | |
| 60 | 70 ± 7.1 bc | 6.9 ± 0.7 e | 6.2 ± 0.7 de | 0.07 ± 0.007 f | 917.00 ± 92.32 f | 4.90 ± 0.50 ef | ≤0.01 | |
| 80 | 74 ± 7.5 b | 7.3 ± 0.7 e | 6.6 ± 0.7 de | 0.07 ± 0.007 f | 1028.60 ± 104.03 ef | 5.18 ± 0.51 ef | ≤0.01 | |
| 100 | 79 ± 8.0 ab | 7.6 ± 0.7 e | 6.9 ± 0.7 de | 0.07 ± 0.007 f | 1145.50 ± 116.26 ef | 5.53 ± 0.56 e | ≤0.01 | |
| 120 | 83 ± 8.4 b | 7.9 ± 0.8 de | 7.2 ± 0.8 de | 0.08 ± 0.008 ef | 1253.30 ± 120.65 e | 6.00 ± 0.68 de | ≤0.01 | |
| 150 | 20 | 63 ± 6.5 c | 8.2 ± 0.8 de | 7.5 ± 0.8 d | 0.08 ± 0.008 ef | 989.10 ± 99.33 f | 5.04 ± 0.54 ef | ≤0.01 |
| 40 | 70 ± 7.2 bc | 8.5 ± 0.8 de | 7.9 ± 0.8 d | 0.08 ± 0.008 ef | 1148.00 ± 117.45 ef | 5.60 ± 0.57 e | ≤0.01 | |
| 60 | 75 ± 7.6 b | 8.7 ± 0.9 d | 8.2 ± 0.8 d | 0.08 ± 0.008 ef | 1267.50 ± 131.11 e | 6.00 ± 0.62 e | ≤0.01 | |
| 80 | 77 ± 7.9 b | 8.9 ± 0.9 d | 8.5 ± 0.9 d | 0.09 ± 0.009 e | 1339.80 ± 142.22 e | 6.93 ± 0.69 de | ≤0.01 | |
| 100 | 86 ± 8.8 b | 9.3 ± 0.9 d | 8.9 ± 0.9 d | 0.09 ± 0.01 e | 1565.20 ± 161.34 d | 7.74 ± 0.76 d | ≤0.01 | |
| 120 | 89 ± 9.0 a | 9.6 ± 1.0 d | 9.1 ± 0.9 d | 0.09 ± 0.01 e | 1664.30 ± 168.98 d | 8.01 ± 0.82 d | ≤0.01 | |
| 200 | 20 | 66 ± 6.8 c | 10.1 ± 1.1 d | 9.5 ± 0.9 cd | 0.10 ± 0.01 de | 1293.60 ± 131.54 e | 6.60 ± 0.67 e | ≤0.01 |
| 40 | 76 ± 7.7 b | 10.4 ± 1.1 d | 9.8 ± 1.0 cd | 0.10 ± 0.01 de | 1535.20 ± 155.76 d | 7.60 ± 0.77 d | ≤0.01 | |
| 60 | 79 ± 8.0 ab | 10.7 ± 1.2 d | 10.1 ± 1.0 cd | 0.10 ± 0.01 de | 1643.20 ± 172.55 d | 7.90 ± 0.79 d | ≤0.01 | |
| 80 | 85 ± 8.7 a | 11.1 ± 1.2 d | 10.5 ±1.1 c | 0.11 ± 0.01 d | 1836.00 ± 189.16 cd | 9.35 ± 0.96 c | ≤0.01 | |
| 100 | 91 ± 9.3 a | 11.6 ± 1.2 c | 10.8 ± 1.1 c | 0.11 ± 0.01 cd | 2038.40 ± 200.88 c | 10.01 ± 1.13 c | ≤0.01 | |
| 120 | 95 ± 9.5 a | 12.1 ± 1.3 c | 11.2 ± 1.1 c | 0.12 ± 0.01 cd | 2213.50 ± 229.44 c | 11.40 ± 1.15 b | ≤0.01 | |
| 250 | 20 | 70 ± 7.1 b | 12.6 ± 1.3 c | 11.5 ± 1.2 c | 0.12 ± 0.01 cd | 1687.00 ± 170.82 d | 8.40 ± 0.89 d | ≤0.01 |
| 40 | 80 ± 8.2 ab | 12.9 ± 1.3 bc | 11.8 ± 1.2 c | 0.13 ± 0.01 c | 1976.00 ± 200.03 c | 10.40 ± 1.05 c | ≤0.01 | |
| 60 | 86 ± 8.8 a | 13.1 ± 1.3 bc | 12.4 ± 1.2 b | 0.13 ± 0.01 c | 2193.00 ± 220.37 c | 11.18 ± 1.19 bc | ≤0.01 | |
| 80 | 89 ± 9.1 a | 13.5 ± 1.4 bc | 12.8 ± 1.3 b | 0.13 ± 0.01 c | 2340.70 ± 238.91 bc | 11.57 ± 1.75 b | ≤0.01 | |
| 100 | 96 ± 9.7 a | 13.8 ± 1.4 bc | 13.2 ± 1.3 b | 0.13 ± 0.01 c | 2592.00 ± 256.26 b | 12.48 ± 1.26 b | ≤0.01 | |
| 120 | 98 ± 9.9 a | 14.2 ± 1.4 bc | 13.6 ± 1.4 b | 0.14 ± 0.01 bc | 2724.40 ± 279.98 ab | 13.7 2 ± 1.39 ab | ≤0.001 | |
| 300 | 20 | 73 ± 7.2 b | 14.6 ± 1.5 b | 13.9 ± 1.4 b | 0.14 ± 0.01 bc | 2085.50 ± 210.54 c | 10.22 ± 1.04 c | ≤0.01 |
| 40 | 86 ± 8.7 a | 14.9 ± 1.5 b | 14.1 ± 1.4 b | 0.14 ± 0.01 bc | 2494.00 ± 253.12 b | 12.04 ± 1.22 b | ≤0.01 | |
| 60 | 90 ± 9.1 a | 15.3 ± 1.5 b | 14.4 ± 1.5 b | 0.15 ± 0.01 b | 2673.00 ± 272.31 b | 13.50 ± 1.37 ab | ≤0.001 | |
| 80 | 95 ± 9.5 a | 15.6 ± 1.6 b | 14.7 ± 1.5 ab | 0.15 ± 0.01 b | 2878.50 ± 290.16 ab | 14.25 ± 1.43 ab | ≤0.001 | |
| 100 | 98 ± 9.9 a | 15.8 ± 1.6 b | 15.2 ± 1.5 ab | 0.15 ± 0.01 b | 3038.00 ± 310.35 ab | 14.70 ± 1.49 ab | ≤0.001 | |
| 120 | 99 ± 10.1 a | 16.1 ± 1.6 b | 15.5 ± 1.6 ab | 0.16 ± 0.01 ab | 3128.40 ± 328.33 ab | 15.84 ± 1.59 ab | ≤0.001 | |
| 350 | 20 | 78 ± 7.9 b | 16.4 ± 1.6 b | 15.7 ± 1.6 ab | 0.16 ± 0.01 ab | 2503.80 ± 261.11 b | 12.48 ± 1.27 b | ≤0.01 |
| 40 | 89 ± 8.8 a | 16.8 ± 1.7 b | 15.9 ± 1.6 ab | 0.17 ± 0.01 ab | 2910.30 ± 300.34 ab | 15.13 ± 1.54 ab | ≤0.001 | |
| 60 | 97 ± 9.8 a | 17.1 ± 1.7 ab | 16.5 ± 1.7 ab | 0.17 ± 0.01 ab | 3259.20 ± 327.25 ab | 16.49 ± 1.68 ab | ≤0.001 | |
| 80 | 98 ± 9.7 a | 17.4 ± 1.7 ab | 16.9 ± 1.7 a | 0.17 ± 0.01 ab | 3361.40 ± 340.16 ab | 16.66 ± 1.69 ab | ≤0.001 | |
| 100 | 99 ± 9.9 a | 18.1 ± 1.8 a | 17.4 ± 1.8 a | 0.18 ± 0.01 a | 3514.50 ± 366.22 a | 17.82 ± 1.79 a | ≤0.0001 | |
| 120 | 100 a | 19.5 ± 1.9 a | 18.6 ± 1.9 a | 0.19 ± 0.01 a | 3810.00 ± 386.77 a | 19.00 ± 1.92 a | ≤0.0001 | |
| Anova p-value | ≤0.0001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
| Source of Variation | df | Seed Germination | Seedling Fresh Mass | ||
|---|---|---|---|---|---|
| F-Value | p | F-Value | p | ||
| Strength of SMF (A) | 6 | 7785 | ≤0.0001 | 777 | ≤0.0001 |
| Duration of SMF (B) | 5 | 760 | ≤0.0001 | 489 | ≤0.0001 |
| A × B | 30 | 104 | ≤0.0001 | 81 | ≤0.0001 |
| Magnetic Field (mT) | Time (min) | |||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | 120 | |
| Control | 0.577 ± 0.041 a/A | 0.577 ± 0.037 a/A | 0.577 ± 0.034 a/A | 0.577 ± 0.027 a/A | 0.577 ± 0.034 a/A | 0.577 ± 0.043 a/A |
| 50 | 0.460 ± 0.036 b/B | 0.456 ± 0.045 b/B | 0.452 ± 0.053 b/B | 0.450 ± 0.044 b/B | 0.447 ± 0.036 b/B | 0.445 ± 0.042 b/B |
| 100 | 0.435 ± 0.040 b/B | 0.430 ± 0.034 b/B | 0.425 ± 0.044 b/B | 0.421 ± 0.038 b/B | 0.418 ± 0.041 b/B | 0.413 ± 0.045 b/B |
| 150 | 0.408 ± 0.032 b/B | 0.404 ± 0.042 b/B | 0.398 ± 0.033 b/B | 0.394 ± 0.032 b/B | 0.390 ± 0.042 b/B | 0.385 ± 0.038 b/B |
| 200 | 0.385 ± 0.027 b/B | 0.381 ± 0.035 b/B | 0.377 ± 0.037 b/B | 0.372 ± 0.043 b/B | 0.369 ± 0.034 b/B | 0.363 ± 0.039 b/B |
| 250 | 0.371 ± 0.051 bc/BC | 0.368 ± 0.053 bc/BC | 0.364 ± 0.023 bc/BC | 0.360 ± 0.033 bc/BC | 0.355 ± 0.030 bc/BC | 0.350 ± 0.026 bc/BC |
| 300 | 0.344 ± 0.033 bc/BC | 0.340 ± 0.025 bc/BC | 0.335 ± 0.036 bc/BC | 0.330 ± 0.028 bc/BC | 0.326 ± 0.024 bc/BC | 0.322 ± 0.027 bc/BC |
| 350 | 0.315 ± 0.029 c/C | 0.310 ± 0.023 c/C | 0.303 ± 0.041 c/C | 0.294 ± 0.022 c/C | 0.289 ± 0.027 c/C | 0.284 ± 0.021 c/C |
| Magnetic Field Strength (mT) | Duration (min) | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Chlorophyll a/b | Carotenoids (mg/g FW) | Carotenoids/Chlorophyll a + b | ANOVA p-Value |
|---|---|---|---|---|---|---|---|
| Control | 1.40 ± 0.18 b | 1.15 ± 0.11 c | 1.21 ± 0.12 b | 0.70 ± 0.08 d | 0.26 ± 0.02 b | ≤0.01 | |
| 50 mT | 20 | 1.68 ± 0.20 b | 1.21 ± 0.13 c | 1.39 ± 0.14 a | 0.90 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 |
| 60 | 1.71 ± 0.19 b | 1.23 ± 0.12 c | 1.39 ± 0.14 a | 0.92 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 | |
| 120 | 1.74 ± 0.17 b | 1.26 ± 0.14 c | 1.38 ± 0.13 a | 0.94 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 | |
| 100 mT | 20 | 1.75 ± 0.18 b | 1.27 ± 0.13 bc | 1.38 ± 0.14 a | 0.95 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 |
| 60 | 1.77 ± 0.21 b | 1.28 ± 0.14 bc | 1.38 ± 0.14 a | 0.96 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 | |
| 120 | 1.81 ± 0.20 ab | 1.32 ± 0.14 bc | 1.37 ± 0.15 a | 0.98 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 | |
| 150 | 20 | 1.83 ± 0.22 ab | 1.33 ± 0.15 bc | 1.38 ± 0.14 a | 0.99 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 |
| 60 | 1.86 ± 0.23 ab | 1.36 ± 0.14 b | 1.36 ± 0.13 a | 1.00 ± 0.09 c | 0.31 ± 0.03 ab | ≤0.01 | |
| 120 | 1.89 ± 0.21 ab | 1.39 ± 0.15 b | 1.36 ± 0.14 a | 1.05 ± 0.09 bc | 0.32 ± 0.03 ab | ≤0.01 | |
| 200 | 20 | 1.91 ± 0.20 ab | 1.38 ± 0.14 b | 1.38 ± 0.15 a | 1.06 ± 0.09 bc | 0.32 ± 0.03 ab | ≤0.01 |
| 60 | 1.94 ± 0.21 ab | 1.41 ± 0.15 b | 1.38 ± 0.14 a | 1.08 ± 0.10 bc | 0.32 ± 0.03 ab | ≤0.01 | |
| 120 | 1.98 ± 0.19 ab | 1.44 ± 0.16 ab | 1.37 ± 0.14 a | 1.10 ± 0.11 bc | 0.32 ± 0.03 ab | ≤0.01 | |
| 250 | 20 | 2.04 ± 0.20 ab | 1.48 ± 0.15 ab | 1.38 ± 0.14 a | 1.12 ± 0.11 bc | 0.32 ± 0.03 ab | ≤0.01 |
| 60 | 2.11 ± 0.21 ab | 1.54 ± 0.17 ab | 1.37 ± 0.14 a | 1.17 ± 0.12 bc | 0.32 ± 0.03 ab | ≤0.01 | |
| 120 | 2.18 ± 0.22 ab | 1.57 ± 0.16 ab | 1.39 ± 0.13 a | 1.22 ± 0.12 bc | 0.32 ± 0.03 ab | ≤0.01 | |
| 300 | 20 | 2.22 ± 0.23 ab | 1.64 ± 0.17 ab | 1.35 ± 0.14 a | 1.30 ± 0.13 b | 0.33 ± 0.03 ab | ≤0.01 |
| 60 | 2.27 ± 0.24 ab | 1.65 ± 0.16 ab | 1.37 ± 0.14 a | 1.31 ± 0.13 b | 0.33 ± 0.03 ab | ≤0.01 | |
| 120 | 2.29 ± 0.23 ab | 1.64 ± 0.17 ab | 1.39 ± 0.14 a | 1.32 ± 0.13 b | 0.33 ± 0.03 ab | ≤0.01 | |
| 350 | 20 | 2.42 ± 0.23 a | 1.71 ± 0.18 a | 1.40 ± 0.14 a | 1.50 ± 0.14 ab | 0.36 ± 0.04 a | ≤0.001 |
| 60 | 2.66 ± 0.24 a | 1.87 ± 0.19 a | 1.42 ± 0.14 a | 1.64 ± 0.15 ab | 0.36 ± 0.04 a | ≤0.001 | |
| 120 | 2.84 ± 0.25 a | 1.97 ± 0.20 a | 1.44 ± 0.15 a | 1.84 ± 0.17 a | 0.38 ± 0.04 a | ≤0.001 | |
| Anova p-value | ≤0.001 | ≤0.001 | ≤0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćalić, D.; Ristić-Djurović, J.L.; Ćirković, S.; Milojević, J.; Belić, M.; Stanišić, M.; Zdravković-Korać, S. Overcoming Low Germination and Low Quality of Flax Seeds (Linum usitatissimum L.) in Unfavorable Storage Using Static Magnetic Fields. Agriculture 2023, 13, 2120. https://doi.org/10.3390/agriculture13112120
Ćalić D, Ristić-Djurović JL, Ćirković S, Milojević J, Belić M, Stanišić M, Zdravković-Korać S. Overcoming Low Germination and Low Quality of Flax Seeds (Linum usitatissimum L.) in Unfavorable Storage Using Static Magnetic Fields. Agriculture. 2023; 13(11):2120. https://doi.org/10.3390/agriculture13112120
Chicago/Turabian StyleĆalić, Dušica, Jasna L. Ristić-Djurović, Saša Ćirković, Jelena Milojević, Maja Belić, Mariana Stanišić, and Snežana Zdravković-Korać. 2023. "Overcoming Low Germination and Low Quality of Flax Seeds (Linum usitatissimum L.) in Unfavorable Storage Using Static Magnetic Fields" Agriculture 13, no. 11: 2120. https://doi.org/10.3390/agriculture13112120
APA StyleĆalić, D., Ristić-Djurović, J. L., Ćirković, S., Milojević, J., Belić, M., Stanišić, M., & Zdravković-Korać, S. (2023). Overcoming Low Germination and Low Quality of Flax Seeds (Linum usitatissimum L.) in Unfavorable Storage Using Static Magnetic Fields. Agriculture, 13(11), 2120. https://doi.org/10.3390/agriculture13112120







