Visitation of Apis mellifera L. in Runner Bean (Phaseolus coccineus L.) and Its Exposure to Seasonal Agrochemicals in Agroecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Selection
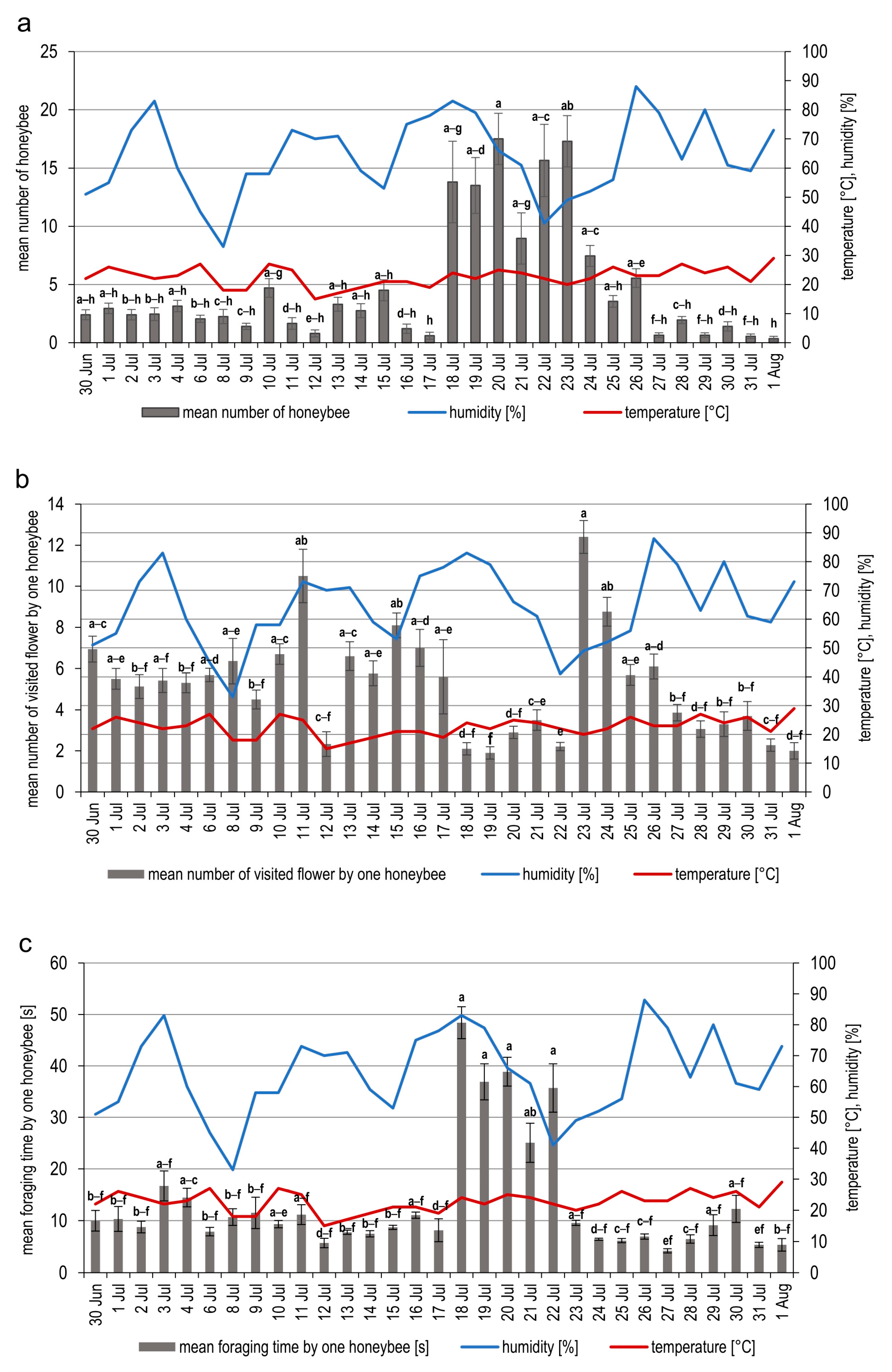
2.2. Visitation Rate
2.3. Monitoring of Honeybee Health and Behaviour including Signs of Pesticide Poisoning
2.4. Pesticide Residue Analysis
2.5. Statistical Analysis
3. Results
3.1. Visitation Rate
3.2. Comprehensive Monitoring of Honeybee Health and Behaviour including Signs of Pesticide Poisoning
3.3. Pesticide Residues in Dead Honeybee Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Ghosh, S.; Jung, C. Ecosystem services of honey bees; regulating, provisioning and cultural functions. J. Apic. 2020, 35, 119–128. [Google Scholar] [CrossRef]
- Aigner, P.A. Floral specialization without trade-offs: Optimal corolla flare in contrasting pollination environments. Ecology 2004, 85, 2560–2569. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Freitas, L. Concepts of pollinator performance: Is a simple approach necessary to achieve a standardized terminology? Braz. J. Bot. 2013, 36, 3–8. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Sáez, A.; Aizen, M.A.; Fijen, T.; Bartomeus, I. Crop pollination management needs flower-visitor monitoring and target values. J. Appl. Ecol. 2020, 57, 664–670. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/3/cb5353en/cb5353en.pdf (accessed on 28 August 2023).
- Peng, T.; Derstroff, D.; Maus, L.; Bauer, T.; Grüter, C. Forager age and foraging state, but not cumulative foraging activity, affect biogenic amine receptor gene expression in the honeybee mushroom bodies. Genes Brain Behav. 2021, 20, e12722. [Google Scholar] [CrossRef]
- Jassim, O.; Huang, Z.Y.; Robinson, G.E. Juvenile hormone profiles of worker honey bees, Apis mellifera, during normal and accelerated behavioural development. J. Insect. Physiol. 2000, 46, 243–249. [Google Scholar] [CrossRef]
- Koethe, S.; Fischbach, V.; Banysch, S.; Reinartz, L.; Hrncir, M.; Lunau, K. A comperative study of food source selection in stingless bees and honeybees: Scent marks, location, or color. Front. Plant Sci. 2020, 11, 516. [Google Scholar] [CrossRef]
- Pilati, L.; Fontana, P. Sequencing of movements of honey bee colonies between the forage sites with the microeconomic model of the migratory beekeeper. In Beekeeping-New Challenges; Ranz, R.E.R., Ed.; IntechOpen: London, UK, 2020; pp. 5–24. [Google Scholar]
- Pilati, L.; Prestamburgo, M. Sequential relationship between profitability and sustainability: The case of migratory beekeeping. Sustanability 2016, 8, 94. [Google Scholar] [CrossRef]
- Martínez-Lopez, V.; Ruiz, C.; De la Rúa, P. Migratory beekeeping and its influence on the prevalence and dispersal of pathogens to managed and wild bees. Int. J. Parasitol. Parasites Wild. 2022, 18, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Jara, L.; Ruiz, C.; Martín-Hernández, R.; Muñoz, I.; Higes, M.; Serrano, J.; De la Rúa, P. The effect of migratory beekeeping on the infestation rate of parasites in honey bee (Apis mellifera) colonies and on their genetic variability. Microorganisms 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Wilde, J. Perspektywy rozwoju polskiego pszczelarstwa w dobie zagrożeń technologicznych i zdrowotnych. Pszczelarstwo w industrialnej rzeczywistości. In Proceedings of the VII Lubelska Konferencja Pszczelarska & II Międzynarodowe Sympozjum Pszczelarskie, Pszczela Wola, Poland, 19–21 February 2016; pp. 23–39. [Google Scholar]
- Łabuda, H. Runner bean (Phaseolus coccineus L.)—Biology and use. Acta Sci. Pol. Hortorum Cultus 2010, 9, 117–132. [Google Scholar]
- Hamburdă, S.B.; Munteanu, N.; Stoleru, V.; Teliban, G.C.; Butnariu, G.; Popa, L.D. Evaluation of the possibilities of using runner bean (Phaseolus coccineus L.) in landscaping design. Lucrări Științifice Seria Horticultură 2014, 57, 87–92. [Google Scholar]
- Kołtowski, Z.; Jemioła, R.; Kołtowska, E. Kolekcja Roślin Miododajnych; Instytut Ogrodnictwa Skierniewice: Skierniewice, Poland, 2020; 35p. [Google Scholar]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef]
- Marcelino, J.; Braese, C.; Christmon, K.; Evans, J.D.; Gilligan, T.; Giray, T.; Nearman, A.; Niño, E.L.; Rose, R.; Sheppard, W.S.; et al. The movement of western honey bees (Apis mellifera L.) Among U.S. States and Territories: History, Benefits, Risks, and Mitigation Strategies. Front. Ecol. Evol. 2022, 10, 850600. [Google Scholar] [CrossRef]
- Traynor, K.S.; Tosi, S.; Rennich, K.; Steinhauer, N.; Forsgren, E.; Rose, R.; Kunkel, G.; Madella, S.; Lopez, D.; Eversole, H.; et al. Pesticides in honey bee colonies: Establishing a baseline for real world exposure over seven years in the USA. Environ. Pollut. 2021, 279, 116566. [Google Scholar] [CrossRef]
- Dixon, D.J.; Zheng, H.; Otto, C.R.V. Land conversion and pesticide use degrade forage areas for honey bees in America’s beekeeping epicenter. PLoS ONE 2021, 16, e0251043. [Google Scholar] [CrossRef]
- Łabuda, H.; Buczkowska, H.; Najda, A. Status of consumer beans production in Poland in 2004–2017 and health-promoting properties of seeds (Phaseolus ssp.). Agron. Sci. 2018, 73, 61–71. [Google Scholar] [CrossRef]
- Ministerstwo Rolnictwa i Rozwoju Wsi. Available online: https://www.gov.pl/web/rolnictwo/miod-fasolowy-odmianowy-z-nektaru-kwiatow-fasoli-tyczkowej-piekny-jas (accessed on 28 August 2023).
- Kiljanek, T.; Niewiadowska, A.; Posyniak, A. Pesticide poisoning of honeybees: A review of symptoms, incident classification, and causes of poisoning. J. Apic. Sci. 2016, 60, 5–24. [Google Scholar] [CrossRef]
- Państwowy Instytut Weterynaryjny. Available online: https://www.piwet.pulawy.pl/lims-files/wn.file/wn.file.00002605.427196883bf369bcff8701008d5b26662e64d3fb.pdf (accessed on 28 August 2023).
- Traynor, K.S.; Pettis, J.S.; Tarpy, D.R.; Mullin, C.A.; Frazier, J.L.; Frazier, M.; van Engelsdorp, D. In-hive pesticide exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci. Rep. 2016, 6, 33207. [Google Scholar] [CrossRef] [PubMed]
- Kiljanek, T.; Niewiadowska, A.; Gaweł, M.; Semeniuk, S.; Borzęcka, M.; Posyniak, A.; Pohorecka, K. Multiple pesticide residues in live and poisoned honeybees—Preliminary exposure assessment. Chemosphere 2017, 175, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Gaweł, M.; Borzęcka, M.; Posyniak, A. Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry—Honeybee poisoning incidents. J. Chromatogr. A. 2016, 1435, 100–114. [Google Scholar] [CrossRef]
- Statistica StatSoft Inc. Data Analysis Software System. Version 13.1. 2016. Available online: www.ststsoft.com (accessed on 12 May 2023).
- Lewis, K.A.; Tzilivakis, J. Wild Bee Toxicity Data for Pesticide Risk Assessments. Data 2019, 4, 98. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment for the active substance imidacloprid in light of confirmatory datasubmitted. EFSA J. 2016, 14, 4607. [Google Scholar] [CrossRef]
- Kołtowski, Z. Flowering biology, nectar secretion and insect foraging of the runner bean (Phaseolus coccineus L.). J. Apic. Sci. 2004, 48, 53–60. [Google Scholar]
- Cué-Hernández, K.A.; Gil-Muñoz, A.; Aguirre-Jaimes, A.; López, P.A.; Taboada-Gaytán, O.R. Floral visitors in the crop Phaseolus coccineus (Fabaceae) on the Altiplano of Puebla, Mexico: Importance of agricultural management and flower color. Acta Bot. Mex. 2022, 129, e2054. [Google Scholar] [CrossRef]
- Kendal, D.A.; Smith, B.D. The pollinating efficiency of honey bee and Bumblebee visits to flowers of runner bean Phaseolus coccineus. J. Appl. Ecol. 1976, 13, 749–752. [Google Scholar] [CrossRef]
- Free, J.B. The behaviour of bees visiting runner beans (Phaseolus multiflorus). J. Appl. Ecol. 1968, 5, 631–638. [Google Scholar] [CrossRef]
- Pando, J.B.; Fohouo, F.N.T.; Tamesse, J.L. Foraging and pollination behaviour of Xylocopa calens Lepeletier (Hymenoptera: Apidae) on Phaseolus coccineus L. (Fabaceae) flowers at Yaounde (Cameroon). Entomol. Res. 2011, 41, 185–193. [Google Scholar] [CrossRef]
- Tchuenguem Fohouo, F.; Femeni Tope, S.; Brückner, D. Foraging and pollination behaviour of Xylocopa olivacea (Hymenoptera: Apidae) on Phaseolus coccineus (Fabaceae) flowers at Ngaoundéré (Cameroon). Int. J. Trop. Insect Sci. 2014, 34, 127–137. [Google Scholar] [CrossRef]
- Totland, O.; Matthews, I. Determinants of pollinator activity and flower preference in the early spring blooming Crocus vernus. Acta Oecol. 1998, 19, 155–165. [Google Scholar] [CrossRef]
- Jacquemart, A.-L.; Gillet, C.; Cawoy, V. Floral visitors and the importance of honey bee on buckwheat (Fagopyrum esculentum Moench) in central Belgium. J. Hort. Sci. Biotechnol. 2007, 82, 104–108. [Google Scholar] [CrossRef]
- Djongwangwe, D.; Fohouo, F.N.T.; Messi, J.; Brückner, D. Foraging and pollination activities of Apis mellifera adansonii Latrielle (Apidae) on Syzygium guineense var. guineense (Myrtaceae) flowers at Ngaoundéré (Cameroon). J. Anim. Plant Sci. 2011, 10, 1325–1333. [Google Scholar]
- Makino, T.T.; Sakai, S. Experience changes pollinator responses to floral display size: From size-based to reward-based foraging. Funct. Ecol. 2007, 21, 854–863. [Google Scholar] [CrossRef]
- Polatto, L.P.; Chaud-Netto, J.; Alves-Junior, V.V. Influence of abiotic factors and floral resource availability on daily foraging activity of bees. J. Insect Behav. 2014, 27, 593–612. [Google Scholar] [CrossRef]
- Stanley, J.; Sah, K.; Subbanna, A.R.N.S. Hoe efficient is the Asian honey bee, Apis cerana in pollinating mustard, Brassica campestris var. toria? Pollination behaviour, pollinator efficiency, pollinator requirements and impact of pollination. J. Apicult. Res. 2017, 56, 439–451. [Google Scholar] [CrossRef]
- Su, W.; Ma, W.; Zhang, Q.; Hu, X.; Ding, G.; Jiang, Y.; Huang, J. Honey bee foraging decisions influenced by pear volatiles. Agriculture 2022, 12, 1074. [Google Scholar] [CrossRef]
- Alves, L.H.S.; Cassino, P.C.R.; Prezoto, F. Effect of abiotic factors on foraging activity of Apis mellifera Linnaeus, 1758 in inflorescences of Vernonia polyanthes Less (Asteraceae). Acta Sci. Anim. Sci. 2015, 37, 405–409. [Google Scholar] [CrossRef]
- Ribeiro, M.F.; da Silva, E.M.S.; Lima Júnior, I.O.; Kiill, L.H.P. Honey bees (Apis mellifera) visiting flowers of yellow melon (Cucumis melo) using different number of hives. Ciênc. Rural Santa Maria 2015, 45, 1768–1773. [Google Scholar] [CrossRef]
- Khan, K.A.; Bashir, M.A.; Mahmood, R.; Qadir, Z.A.; Rafiq, K.; Khan, M.H.; Saleh, M.; Hashmi, M.S.; Gulshan, A.B.; Ahmad, Z.; et al. Foraging behaviour of western honey bee (Apis mellifera) in different time intervals on Brassica campestris L. Fresen. Environ. Bull. 2021, 30, 2607–2612. [Google Scholar]
- Sanderson, C.E.; Wells, H. The flower fidelity of the honeybee. Uludag. Bee. J. 2005, 5, 145–151. [Google Scholar]
- Hennessy, G.; Harris, C.; Pirot, L.; Lefter, A.; Goulson, D.; Ratnieks, F.L.W. Wind slows play: Increasing wind speed reduces flower visiting rate in honey bees. Anim. Behav. 2021, 178, 87–93. [Google Scholar] [CrossRef]
- Manetas, Y.; Petropouloum, Y. Nectar amount, pollinator visit duration and pollination success in the Mediterranean shrub Cistus creticus. Ann. Bot. 2000, 86, 815–820. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Al-Ghamdi, A.A.; Mohamed, A.A. Tolerance of two honey bee races to various temperature and relative humidity gradients. Environ. Exp. Bot. 2012, 10, 133–138. [Google Scholar]
- Abou-Shaara, H.F.; Owayss, A.A.; Ibrahim, Y.Y.; Basuny, N.K. A review of impacts of temperature and relative humidity on various activities of honey bees. Insect. Soc. 2017, 64, 455–463. [Google Scholar] [CrossRef]
- Clarke, D.; Robert, D. Predictive modelling of honey bee foraging activity using local weather conditions. Apidologie 2018, 49, 386–396. [Google Scholar] [CrossRef]
- Blažyte-Čereškiene, L.; Vaitkevičiene, G.; Venskutonyte, S.; Būda, V. Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Zemdirb.-Agric. 2010, 97, 61–70. [Google Scholar]
- Begna, T.; Ulziibayar, D.; Noor-ul-Ane, M.; Shin, J.H.; Jung, C. Offering pollen as reward enhances foraging activity of honey bee, Apis mellifera on strawberry greenhouse during winter season. J. Apic. 2020, 35, 111–118. [Google Scholar] [CrossRef]
- Odoux, J.F.; Aupinel, P.; Gateff, S.; Requier, F.; Henry, M.; Bretagnolle, V. ECOBEE: A tool for long-term honey bee colony monitoring at the landscape scale in West European intensive agroecosystems. J. Apicult. Res. 2014, 53, 57–66. [Google Scholar] [CrossRef]
- Semkiw, P. Sektor Pszczelarski w Polsce w 2021 roku; Instytut Ogrodnictwa Zakład Pszczelnictwa w Puławach: Puławy, Polska, 2021; 15p, Available online: http://www.inhort.pl/wp-content/uploads/2022/07/Sektor-pszczelarski-w-Polsce-w-2021-roku.pdf (accessed on 10 September 2023).
- Golian, J.; Kwiatkowska, J.; Komorowski, K.; Ptaszek, M.; Jarecka-Boncela, A.; Rybczyński, D.; Soika, G.; Stepkowska, A. Program Ochrony Fasoli. 2021. Available online: https://www.inhort.pl/files/sor/programy_ochrony/Program_ochrony_fasoli.pdf (accessed on 18 September 2023).
- Dirilgen, T.; Herbertsson, L.; O’Reilly, A.D.; Mahon, N.; Stanley, D.A. Moving past neonicotinoids and honeybees: A systematic review of existing research on other insecticides and bees. Environ. Res. 2023, 235, 116612. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, E.E.W.; Chen-Wishart, Z.P.; Gill, R.J.; Leadbeater, E. Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 2016, 6, 38957. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.E.; Bonmatin, J.M.; Moineau, I.; Gaimon, C.; Brun, S.; Vermandere, J.P. A method to quantify and analyze the foraging activity of honey bees: Relevance to the sublethal effects induced by systemic insecticides. Arch. Environ. Contam. Toxicol. 2004, 47, 387–395. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Polo, R.O.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Guarna, M.M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Yang, E.C.; Chuang, C.; Chen, Y.L.; Chang, L.H. Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the Honey Bee (Hymenoptera: Apidae). J. Econ. Entomol. 2008, 101, 1743–1748. [Google Scholar] [CrossRef]
- Karahan, A.; Çakmak, I.; Hranitz, J.M.; Karaca, I.; Wells, H. Sublethal imidacloprid effects on honey bee flower choices when foraging. Ecotoxicology 2015, 24, 2017–2025. [Google Scholar] [CrossRef]
- Meikle, W.G.; Adamczyk, J.J.; Weiss, M.; Gregorc, A.; Johnson, D.R.; Stewart, S.D.; Zawislak, J.; Carrol, M.J.; Lorenz, G.M. Sublethal Effects of Imidacloprid on Honey Bee Colony Growth and Activity at Three Sites in the U.S. PLoS ONE 2016, 11, e0168603. [Google Scholar] [CrossRef]
- Bednarek, A.; Nicewicz, Ł. Efekty narażenia pszczół na działanie nikotynoidów. Pasieka 2018, 5. Available online: https://pasieka24.pl/index.php/pl-pl/pasieka-czasopismo-dla-pszczelarzy/160-pasieka-5-2018/1852-efekty-narazenia-pszczol-na-dzialanie-neonikotynoidow (accessed on 18 June 2023).
- EC European Commission. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. Eur. Union 2013, 139, 12–26. Available online: https://eur-lex.europa.eu/eli/reg_impl/2013/485/oj (accessed on 25 August 2023).
- Kadalikova, K.; Vaclavikova, M.; Halesova, T.; Kamler, M.; Markovic, M.; Erban, T. The investigation of honey bee pesticide poisoning incidents in Czechia. Chemosphere 2021, 263, 128056. [Google Scholar] [CrossRef]
- Pashte, V.V.; Patil, C.S. Toxicity and poisoning symptoms of selected insecticides to honey bees (Apis mellifera mellifera L.). Arch. Biol. Sci. 2018, 70, 5–12. [Google Scholar] [CrossRef]
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y.; Yu, L.; Cao, H. Evaluation of highly detectable pesticides sprayed in Brassica napus L.: Degradation behavior and risk assessment for honeybees. Molecules 2018, 23, 2482. [Google Scholar] [CrossRef]
- Thompson, H.M.; Fryday, S.L.; Harkin, S.; Milner, S. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie 2014, 45, 545–553. [Google Scholar] [CrossRef]
- David, A.; Botías, C.; Abdul-Sada, A.; Nicholls, E.; Rotheray, E.L.; Hill, E.M.; Goulson, D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016, 88, 169–178. [Google Scholar] [CrossRef] [PubMed]
- EC European Commission. Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 amending Implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. Off. J. Eur. Union L 2011, 153/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0540 (accessed on 20 August 2023).
- EFSA (European Food Safety Authority); Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, 7215. [Google Scholar] [CrossRef]
- Baude, F.J.; Pease, H.L.; Holt, R.F. Fate of benomyl on field soil and turf. J. Agric. Food Chem. 1974, 22, 413–418. [Google Scholar] [CrossRef]
- Miszczyk, M.; Płonka, M.; Stobiecki, T.; Kronenbach-Dylong, D.; Waleczek, K.; Weber, R. Official control of plant protection products in Poland: Detection of illegal products. Environ. Sci. Pollut. R. 2018, 25, 31906–31916. [Google Scholar] [CrossRef]
- Giesy, J.P.; Solomon, K.R.; Cutler, G.C.; Giddings, J.M.; Mackay, D.; Moore, D.R.; Purdy, J.; Williams, W.M. Ecological risk assessment of the uses of the organophosphorus insecticide chlorpyrifos in the United States. In Reviews of Environmental Contamination and Toxicology; Giesy, J., Solomon, K., Eds.; Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States; Springer: New York, NY, USA, 2014; Volume 231, pp. 1–12. [Google Scholar]
- Porrini, C.; Caprio, E.; Tesoriero, D.; Di Prosco, G. Using honey bee as bioindicator of chemicals in Campanian agroecosystems (South Italy). Bull. Insectol. 2014, 6, 137–146. [Google Scholar]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance azoxystrobin. EFSA J. 2010, 8, 15421542. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance difenoconazole. EFSA J. 2011, 9, 1967. [Google Scholar] [CrossRef]
- Serra, R.S.; Martínez, L.C.; Cossolin, J.F.S.; Santos de Resende, M.T.C.; Carneiro, L.S.; Fiaz, M.; Serrão, J.E. The fungicide azoxystrobin causes histopathological and cytotoxic changes in the midgut of the honey bee Apis mellifera (Hymenoptera: Apidae). Ecotoxicology 2023, 32, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, A.; Sharma, R.; Thakur, A.; Tuteja, S.; Shyamli; Singh, R. Effect of fungicidal contamination on survival, morphology, and cellular immunity of Apis mellifera (Hymenoptera: Apidae). Front. Physiol. 2023, 14, 1099806. [Google Scholar] [CrossRef] [PubMed]
- Almasri, H.; Tavares, D.A.; Tchamitchian, S.; Pélissier, M.; Sené, D.; Cousin, M.; Brunet, J.L.; Belzunces, L.P. Toxicological status changes the susceptibility of the honey bee Apis mellifera to a single fungicidal spray application. Environ. Sci. Pollut. R. 2021, 28, 42807–42820. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; van Engelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Sheppard, W.S. Honey bee exposure to pesticides: A four-year nationwide study. Insects 2019, 10, 13. [Google Scholar] [CrossRef]
- Friedle, C.; Wallner, K.; Rosenkranz, P.; Martens, D.; Vetter, W. Pesticide residues in daily bee pollen samples (April–July) from an intensive agricultural region in Southern Germany. Environ. Sci. Pollut. R. 2021, 28, 22789–22803. [Google Scholar] [CrossRef]
- He, Q.; Yang, Q.; Liu, Q.; Hu, Z.; Gao, Q.; Dong, Y.; Xiao, J.; Yu, L.; Cao, H. The effects of beta-cypermethrin, chlorbenzuron, chlorothalonil, and pendimethalin on Apis mellifera ligustica and Apis cerana cerana larvae reared in vitro. Pest. Manag. Sci. 2022, 78, 1407–1416. [Google Scholar] [CrossRef]
- Wilczyńska, A.; Przybyłowski, P. Residues of Organochlorine Pesticides in Polish Honeys. Apicata 2007, 42, 16–24. [Google Scholar]
- Wang, J.; Kliks, M.M.; Jun, S.; Li, Q.X. Residues of organochlorine pesticides in honeys from different geographic regions. Food Res. Int. 2010, 43, 2329–2334. [Google Scholar] [CrossRef]
- Perugini, M.; Tulini, S.M.R.; Zezza, D.; Fenucci, S.; Conte, A.; Amorena, M. Occurrence of agrochemical residues in beeswax samples collected in Italy during 2013–2015. Sci. Total Environ. 2018, 625, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Blasco, C.; Fernaändez, M.; Pena, A.; Lino, C.; Silveira, M.; Font, G.; Pico, A. Assessment of pesticide residues in honey samples from Portugal and Spain. J. Agric. Food Chem. 2003, 51, 8132–8138. [Google Scholar] [CrossRef] [PubMed]
- McArt, S.H.; Fersch, A.A.; Milano, N.J.; Truitt, L.L.; Böröczky, K. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 2017, 7, 46554. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]

| Site | Site Name | Coordinates |
|---|---|---|
| 1 | Korczmin Osada | 50°25′41.3″ N, 23°52′15.5″ E |
| 2 | Wasylów | 50°32′32.1″ N, 23°52′20.4″ E |
| 3 | Żerniki | 50°28′14.2″ N, 23°43′40.7″ E |
| 4 | Dutrów | 50°32′30.5″ N, 23°50′33.2″ E |
| 5 | Machnówek 1 | 50°24′59.2″ N, 23°54′39.8″ E |
| 6 | Machnówek 2 | 50°25′59.8″ N, 23°54′20.0″ E |
| 7 | Łasków | 50°37′45.4″ N, 23°56′23.8″ E |
| Response Variable | Kruskal—Wallis Test | |||
|---|---|---|---|---|
| H | n | df | p | |
| NB | 98.2419 | 620 | 3 | p < 0.001 |
| NVF | 94.0695 | 620 | 3 | p < 0.001 |
| FT | 81.5352 | 620 | 3 | p < 0.001 |
| Trait | T | NB | NVF | FT |
|---|---|---|---|---|
| H | −0.184 * | −0.054 | −0.295 * | −0.011 |
| T | 0.114 * | 0.089 * | 0.149 * | |
| NB | 0.339 * | 0.781 * | ||
| NVF | 0.205 * |
| Site | Date | Average Mortality (Individuals/Hive) | Behaviour | Flight Activity * | The Colony Fitness | Signs of Poisoning/ Visible Abnormalities |
|---|---|---|---|---|---|---|
| 1 | 28.06 | ≤5 | very calm | ++ | very good | not visible |
| 06.07 | ≤5 | very calm | +++ | very good | not visible | |
| 13.07 | ≤5 | very calm | +++ | very good | not visible | |
| 19.07 | 9 | very calm | ++ | very good | not visible | |
| 26.07 | 7 | very calm | ++ | very good | not visible | |
| 2 | 28.06 | 8 | very calm | ++ | very good | not visible |
| 06.07 | ≤5 | very calm | +++ | very good | not visible | |
| 13.07 | 10 | very calm | +++ | very good | not visible | |
| 19.07 | ≤5 | very calm | ++ | very good | not visible | |
| 26.07 | ≤5 | very calm | ++ | very good | not visible | |
| 3 | 28.06 | 15 | very calm | ++ | very good | not visible |
| 06.07 | ≤5 | very calm | +++ | very good | not visible | |
| 13.07 | ≤5 | very calm | +++ | very good | not visible | |
| 19.07 | ≤5 | very calm | ++ | very good | not visible | |
| 26.07 | ≤5 | very calm | ++ | very good | not visible | |
| 4 | 28.06 | 10 | very calm | ++ | very good | not visible |
| 06.07 | 44 | aggressive | + | poor | present/extended proboscis, expanded wings | |
| 13.07 | 15 | very aggressive | + | poor | present/young bees remove the larvae from the hive | |
| 19.07 | 9 | very calm | ++ | moderate | not visible | |
| 26.07 | 7 | very calm | ++ | moderate | not visible | |
| 5 | 28.06 | ≤5 | very calm | ++ | good | not visible |
| 06.07 | ≤5 | very calm | +++ | very good | not visible | |
| 13.07 | ≤5 | very calm | +++ | very good | not visible | |
| 19.07 | ≤5 | very calm | ++ | very good | not visible | |
| 26.07 | ≤5 | very calm | ++ | very good | not visible | |
| 6 | 28.06 | 7 | very calm | ++ | good | not visible |
| 06.07 | ≤5 | very calm | +++ | very good | not visible | |
| 13.07 | 8 | very calm | +++ | very good | not visible | |
| 19.07 | 10 | very calm | ++ | very good | not visible | |
| 26.07 | ≤5 | very calm | ++ | very good | not visible | |
| 7 | 28.06 | ≤5 | very calm | +++ | very good | not visible |
| 06.07 | ≥50 | aggressive | + | poor | present/no flying bees, dead bees with outstretched tongues, large losses of bees in the hives, uncoordinated and uncontrolled movements | |
| 13.07 | 44 | calm | + | poor | present/large losses of bees in the hives | |
| 19.07 | 10 | very calm | + | poor | not visible | |
| 26.07 | ≤5 | very calm | ++ | moderate | not visible |
| Pesticide | Toxicity Endpoints | Concentration in Dead Bees ± Expanded Uncertainty (mg/kg) | HQ | |||||
|---|---|---|---|---|---|---|---|---|
| Name | Type, Systemicity | Group | Oral Acute LD50 (µg/Bee) | Oral Chronic 10-day LDD50 (µg/Bee/Day) | Site 4 | Site 7 | Acute Oral | Chronic Oral |
| Azoxystrobin | fungicide, systemic | strobilurin | >25 [31] * | – | – | 0.0030 ± 0.0015 | 0 | - |
| Carbendazim | fungicide, systemic | benzimidazole | >100 [31] * | – | 0.0020 ± 0.0010 | 0.0010 ± 0.0005 | 0 | - |
| Chlorpyryfos | insecticide, non-systemic | organophosphates | 0.15 [31] * | 0.002 [31] * | – | 0.0020 ± 0.0010 | 13 | 1000 |
| Difenoconazole | fungicide, systemic | conazole | >177 [31] * | – | – | 0.0010 ± 0.0005 | 0 | - |
| Imidacloprid | insecticide, systemic | neonicotinoids | 0.0037 [31,32] * | >0.00282 [32] * | 0.0020 ± 0.0010 | – | 541 | 709 |
| p,p’-DDT | insecticide, non-systemic | organochlorine | 5.1 [31] * | – | – | 0.0020 ± 0.0010 | 0 | - |
| Pendimethalin | herbicide, systemic | dinitroanilines | >101.2 [31] * | >96.5 [31] * | 0.0020 ± 0.0010 | 0.0020 ± 0.0010 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, I.; Lisecka, M.; Kmieć, K.; Golan, K.; Górska-Drabik, E.; Kiljanek, T.; Zimowska, B.; Skwaryło-Bednarz, B. Visitation of Apis mellifera L. in Runner Bean (Phaseolus coccineus L.) and Its Exposure to Seasonal Agrochemicals in Agroecosystems. Agriculture 2023, 13, 2138. https://doi.org/10.3390/agriculture13112138
Kot I, Lisecka M, Kmieć K, Golan K, Górska-Drabik E, Kiljanek T, Zimowska B, Skwaryło-Bednarz B. Visitation of Apis mellifera L. in Runner Bean (Phaseolus coccineus L.) and Its Exposure to Seasonal Agrochemicals in Agroecosystems. Agriculture. 2023; 13(11):2138. https://doi.org/10.3390/agriculture13112138
Chicago/Turabian StyleKot, Izabela, Magdalena Lisecka, Katarzyna Kmieć, Katarzyna Golan, Edyta Górska-Drabik, Tomasz Kiljanek, Beata Zimowska, and Barbara Skwaryło-Bednarz. 2023. "Visitation of Apis mellifera L. in Runner Bean (Phaseolus coccineus L.) and Its Exposure to Seasonal Agrochemicals in Agroecosystems" Agriculture 13, no. 11: 2138. https://doi.org/10.3390/agriculture13112138
APA StyleKot, I., Lisecka, M., Kmieć, K., Golan, K., Górska-Drabik, E., Kiljanek, T., Zimowska, B., & Skwaryło-Bednarz, B. (2023). Visitation of Apis mellifera L. in Runner Bean (Phaseolus coccineus L.) and Its Exposure to Seasonal Agrochemicals in Agroecosystems. Agriculture, 13(11), 2138. https://doi.org/10.3390/agriculture13112138








