Can Nanomaterials Improve the Soil Microbiome and Crop Productivity?
Abstract
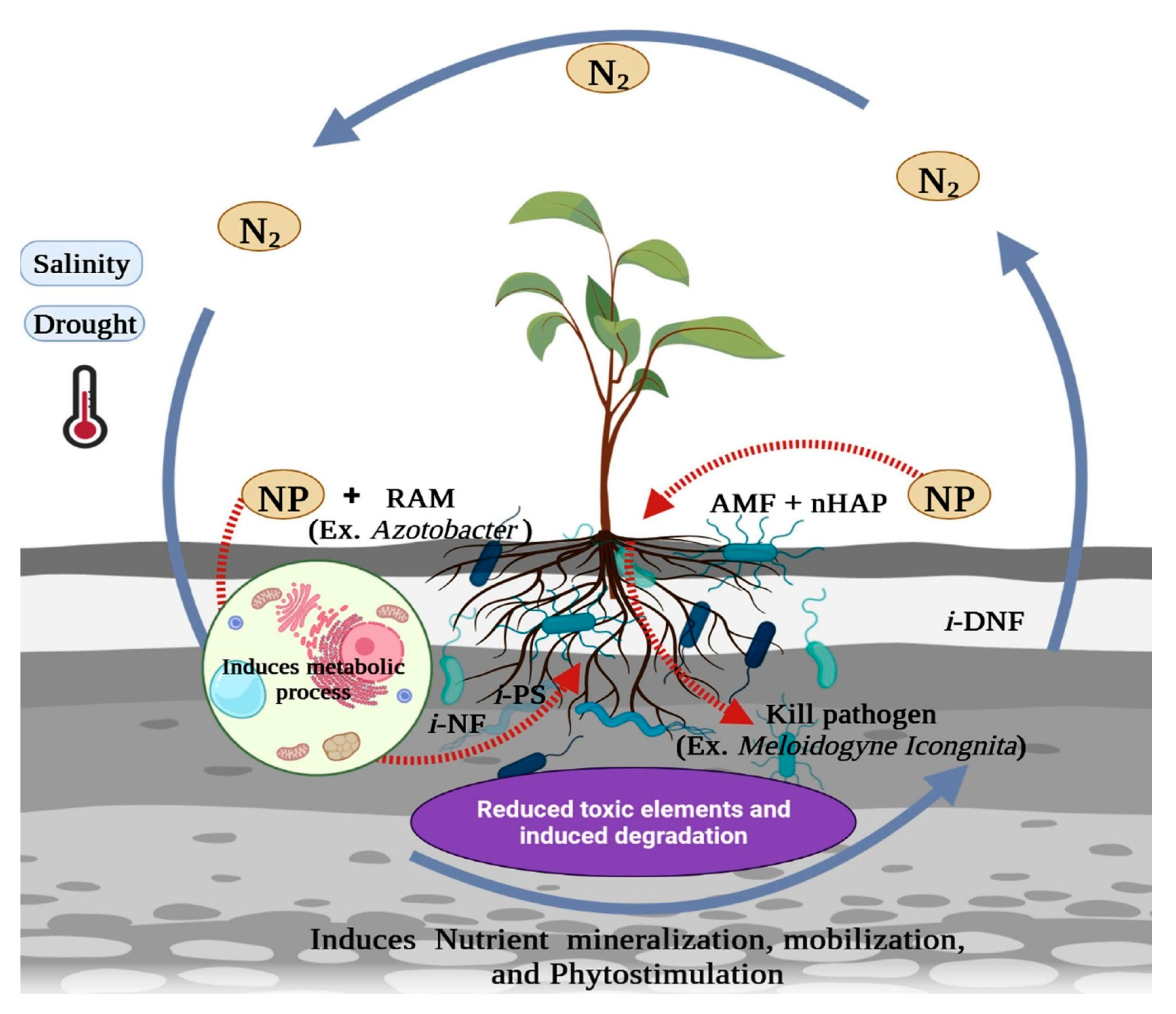
:1. Introduction
2. Nanoparticles in Restoration of Soils
2.1. Manipulation in Soil Microbiome via Nanotechnology for Soil Health Improvements
2.2. Nanotechnology in Reducing Soil Stress for Plant Growth
2.2.1. Salinity Stress
2.2.2. Drought Stress
2.2.3. Availability of Nutrients
3. Future Aspects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strer, M.; Svoboda, N.; Herrmann, A. Abundance of adverse environmental conditions during critical stages of crop production in Northern Germany. Environ. Sci. Eur. 2018, 30, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the Restoration of Polluted Soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.K.; Satya, P. Research advances in major cereal crops for adaptation to abiotic stresses. GM Crop. Food 2014, 5, 259–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [Green Version]
- Rajput, V.D.; Singh, A.; Singh, V.K.; Minkina, T.M.; Sushkova, S. Impact of nanoparticles on soil resource. In Nanomaterials for Soil Remediation; Amrane, A., Mohan, D., Nguyen, T.A., Assadi, A.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 65–85. [Google Scholar]
- Kalwani, M.; Chakdar, H.; Srivastava, A.; Pabbi, S.; Shukla, P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere 2022, 287, 132107. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered Nanomaterials in Soil: Their Impact on Soil Microbiome and Plant Health. Plants 2022, 11, 109. [Google Scholar] [CrossRef]
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.H.; Mohamed, S.M.S.; Osman, M.M. Engineered staphylococcus aureus via immobilization on magnetic Fe3O4-phthalate nanoparticles for biosorption of divalent ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3810–3824. [Google Scholar] [CrossRef]
- Baragaño, D.; Forján, R.; Welte, L.; Gallego, J.L.R. Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 2020, 10, 1896. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, S.; Zhong, Q.; Wang, G.; Xu, X.; Li, T.; Wang, L.; Jia, Y.; Li, Y. Feasibility of nanoscale zero-valent iron to enhance the removal efficiencies of heavy metals from polluted soils by organic acids. Ecotoxicol. Environ. Saf. 2018, 162, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Z.; Kang, Y.; Tsang, E.P. Immobilization and phytotoxicity of chromium in contaminated soil remediated by CMC-stabilized nZVI. J. Hazard. Mater. 2014, 275, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; El-shahawy, A.; Ibrahim, M.; El-Halim, A.E.; Talha, N.; Sedky, A.; Alfwuaires, M.; Alabbad, H.; Almeri, N.; Mahmoud, E. Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agriculture 2022, 12, 2096. [Google Scholar] [CrossRef]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of carbon nanoparticles to improve soil fertility, crop growth and nutrient uptake by corn (Zea mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Tian, D.-D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.-R. Recent Trends in Nano-Fertilizers for Sustainable Agriculture under Climate Change for Global Food Security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Chaudhary, P.; Khati, P.; Chaudhary, A.; Maithani, D.; Kumar, G.; Sharma, A. Cultivable and metagenomic approach to study the combined impact of nanogypsum and Pseudomonas taiwanensis on maize plant health and its rhizospheric microbiome. PLoS ONE 2021, 16, e0250574. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Handa, N.; Kaur, H.; Ohri, P.; Bhardwaj, R.; Yousaf, B.; Rinklebe, J.; Ahmad, P. Enthralling the impact of engineered nanoparticles on soil microbiome: A concentric approach towards environmental risks and cogitation. Ecotoxicol. Environ. Saf. 2021, 222, 112459. [Google Scholar] [CrossRef]
- Tian, H.; Kah, M.; Kariman, K. Are Nanoparticles a Threat to Mycorrhizal and Rhizobial Symbioses? A Critical Review. Front. Microbiol. 2019, 10, 1660. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Deng, J.; Shao, L.; Jiang, S.; Xiao, T.; Sun, W.; Xiao, E. The rhizosphere microbiome improves the adaptive capabilities of plants under high soil cadmium conditions. Front. Plant Sci. 2022, 13, 914103. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Z.; Liu, Y.; Wang, F.; Liu, Y.; Zhao, J.; Li, Y.; Gao, Y.; Zhu, N. Roles of plant-associated microorganisms in regulating the fate of Hg in croplands: A perspective on potential pathways in maintaining sustainable agriculture. Sci. Total Environ. 2022, 834, 155204. [Google Scholar] [CrossRef]
- Jatav, H.S.; Sharma, L.D.; Sadhukhan, R.; Singh, S.K.; Singh, S.; Rajput, V.D.; Parihar, M.; Jatav, S.S.; Jinger, D.; Kumar, S.; et al. An Overview of Micronutrients: Prospects and Implication in Crop Production. In Plant Micronutrients: Deficiency and Toxicity Management; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–30. [Google Scholar]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Chung, H.; Kim, M.J.; Ko, K.; Kim, J.H.; Kwon, H.-a.; Hong, I.; Park, N.; Lee, S.-W.; Kim, W. Effects of graphene oxides on soil enzyme activity and microbial biomass. Sci. Total Environ. 2015, 514, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Mazher, J.; Azmat, A.; Nosheen, A.; Naz, R.; Hassan, M.N.; Noureldeen, A.; Ahmad, P. Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol. Environ. Saf. 2021, 218, 112262. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Verma, K.K.; et al. Nano-enabled products: Challenges and opportunities for sustainable agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef]
- Alamri, S.; Nafady, N.A.; El-Sagheer, A.M.; El-Aal, M.A.; Mostafa, Y.S.; Hashem, M.; Hassan, E.A. Current Utility of Arbuscular Mycorrhizal Fungi and Hydroxyapatite Nanoparticles in Suppression of Tomato Root-Knot Nematode. Agronomy 2022, 12, 671. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Chauhan, P.K. Optimization of eco-friendly amendments as sustainable asset for salt-tolerant plant growth-promoting bacteria mediated maize (Zea mays L.) plant growth, Na uptake reduction and saline soil restoration. Environ. Res. 2022, 211, 113081. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, P.K.; Upadhyay, S.K.; Singh, R.K.; Dwivedi, P.; Wang, J.; Jain, D.; Jiang, M. Mechanistic Insights and Potential Use of Siderophores Producing Microbes in Rhizosphere for Mitigation of Stress in Plants Grown in Degraded Land. Front. Microbiol. 2022, 13, 898979. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [Green Version]
- Lucke, M.; Correa, M.G.; Levy, A. The Role of Secretion Systems, Effectors, and Secondary Metabolites of Beneficial Rhizobacteria in Interactions With Plants and Microbes. Front. Plant Sci. 2020, 11, 589416. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Liu, H.; Xu, Z.; Li, R.; Shen, Q.; Salles, J.F. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum. NPJ Biofilms Microbiomes 2021, 7, 33. [Google Scholar] [CrossRef]
- Hunter, P. Plant microbiomes and sustainable agriculture. EMBO Rep. 2016, 17, 1696–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Liu, S. Identification and Characterization of the Phosphate-Solubilizing Bacterium Pantoea sp. S32 in Reclamation Soil in Shanxi, China. Front. Microbiol. 2019, 10, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Behera, M.; Kumar, S. Nano-bioremediation: An Innovative Remediation Technology for Treatment and Management of Contaminated Sites. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 165–182. [Google Scholar]
- Layet, C.; Auffan, M.; Santaella, C.; Chevassus-Rosset, C.; Montes, M.; Ortet, P.; Barakat, M.; Collin, B.; Legros, S.; Bravin, M.N.; et al. Evidence that Soil Properties and Organic Coating Drive the Phytoavailability of Cerium Oxide Nanoparticles. Environ. Sci. Technol. 2017, 51, 9756–9764. [Google Scholar] [CrossRef]
- Bray, N.; Kao-Kniffin, J.; Frey, S.D.; Fahey, T.; Wickings, K. Soil Macroinvertebrate Presence Alters Microbial Community Composition and Activity in the Rhizosphere. Front. Microbiol. 2019, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef] [Green Version]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J. Plant Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.-K.; Arshad, M. Growth and Metabolic Responses of Rice (Oryza sativa L.) Cultivated in Phosphorus-Deficient Soil Amended with TiO2 Nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Bioactivity and Biomodification of Ag, ZnO, and CuO Nanoparticles with Relevance to Plant Performance in Agriculture. Ind. Biotechnol. 2012, 8, 344–357. [Google Scholar] [CrossRef]
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. BioMetals 2010, 23, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Barton, L.E.; Quicksall, A.N.; Maurice, P.A. Siderophore-Mediated Dissolution of Hematite (α-Fe2O3): Effects of Nanoparticle Size. Geomicrobiol. J. 2012, 29, 314–322. [Google Scholar] [CrossRef]
- Avellan, A.; Auffan, M.; Masion, A.; Levard, C.; Bertrand, M.; Rose, J.; Santaella, C.; Achouak, W. Remote Biodegradation of Ge–Imogolite Nanotubes Controlled by the Iron Homeostasis of Pseudomonas brassicacearum. Environ. Sci. Technol. 2016, 50, 7791–7798. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Merten, D.; Svatos, A.; Büchel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Brandel, J.; Humbert, N.; Elhabiri, M.; Schalk, I.J.; Mislin, G.L.; Albrecht-Gary, A.M. Pyochelin, a siderophore of Pseudomonas aeruginosa: Physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans. 2012, 41, 2820–2834. [Google Scholar] [CrossRef]
- Neubauer, U.; Nowack, B.; Furrer, G.; Schulin, R. Heavy Metal Sorption on Clay Minerals Affected by the Siderophore Desferrioxamine B. Environ. Sci. Technol. 2000, 34, 2749–2755. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A Paradigm Shift in the Application of Microorganisms for Sustainable Agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef]
- Panke-Buisse, K.; Poole, A.C.; Goodrich, J.K.; Ley, R.E.; Kao-Kniffin, J. Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J. 2015, 9, 980–989. [Google Scholar] [CrossRef] [Green Version]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Santos, L.F.; Olivares, F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021, 26, 100198. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, P.M.; Johnson, M.T.J. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA 2018, 115, E1157–E1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, F.; Kost, E.; Nowack, B. Engineered nanomaterials in water and soils: A risk quantification based on probabilistic exposure and effect modeling. Environ. Toxicol. Chem. 2013, 32, 1278–1287. [Google Scholar] [CrossRef]
- Moll, J.; Klingenfuss, F.; Widmer, F.; Gogos, A.; Bucheli, T.D.; Hartmann, M.; van der Heijden, M.G.A. Effects of titanium dioxide nanoparticles on soil microbial communities and wheat biomass. Soil Biol. Biochem. 2017, 111, 85–93. [Google Scholar] [CrossRef]
- Grün, A.-L.; Manz, W.; Kohl, Y.L.; Meier, F.; Straskraba, S.; Jost, C.; Drexel, R.; Emmerling, C. Impact of silver nanoparticles (AgNP) on soil microbial community depending on functionalization, concentration, exposure time, and soil texture. Environ. Sci. Eur. 2019, 31, 15. [Google Scholar] [CrossRef] [Green Version]
- Johansen, A.; Pedersen, A.L.; Jensen, K.A.; Karlson, U.; Hansen, B.M.; Scott-Fordsmand, J.J.; Winding, A. Effects of C60 fullerene nanoparticles on soil bacteria and protozoans. Environ. Toxicol. Chem. 2008, 27, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Grün, A.-L.; Straskraba, S.; Schulz, S.; Schloter, M.; Emmerling, C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on microbial biomass, enzyme activity, and functional genes involved in the nitrogen cycle of loamy soil. J. Environ. Sci. 2018, 69, 12–22. [Google Scholar] [CrossRef]
- Liu, J.; Williams, P.C.; Geisler-Lee, J.; Goodson, B.M.; Fakharifar, M.; Peiravi, M.; Chen, D.; Lightfoot, D.A.; Gemeinhardt, M.E. Impact of wastewater effluent containing aged nanoparticles and other components on biological activities of the soil microbiome, Arabidopsis plants, and earthworms. Environ. Res. 2018, 164, 197–203. [Google Scholar] [CrossRef]
- Kibbey, T.C.G.; Strevett, K.A. The effect of nanoparticles on soil and rhizosphere bacteria and plant growth in lettuce seedlings. Chemosphere 2019, 221, 703–707. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Doolette, C.L.; Gupta, V.V.; Lu, Y.; Payne, J.L.; Batstone, D.J.; Kirby, J.K.; Navarro, D.A.; McLaughlin, M.J. Quantifying the Sensitivity of Soil Microbial Communities to Silver Sulfide Nanoparticles Using Metagenome Sequencing. PLoS ONE 2016, 11, e0161979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berhe, A.A.; Barnes, R.T.; Six, J.; Marín-Spiotta, E. Role of Soil Erosion in Biogeochemical Cycling of Essential Elements: Carbon, Nitrogen, and Phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- Eivazi, F.; Afrasiabi, Z.; Jose, E. Effects of Silver Nanoparticles on the Activities of Soil Enzymes Involved in Carbon and Nutrient Cycling. Pedosphere 2018, 28, 209–214. [Google Scholar] [CrossRef]
- Zhao, S.; Su, X.; Wang, Y.; Yang, X.; Bi, M.; He, Q.; Chen, Y. Copper oxide nanoparticles inhibited denitrifying enzymes and electron transport system activities to influence soil denitrification and N2O emission. Chemosphere 2020, 245, 125394. [Google Scholar] [CrossRef]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [Green Version]
- Judy, J.D.; McNear, D.H., Jr.; Chen, C.; Lewis, R.W.; Tsyusko, O.V.; Bertsch, P.M.; Rao, W.; Stegemeier, J.; Lowry, G.V.; McGrath, S.P.; et al. Nanomaterials in Biosolids Inhibit Nodulation, Shift Microbial Community Composition, and Result in Increased Metal Uptake Relative to Bulk/Dissolved Metals. Environ. Sci. Technol. 2015, 49, 8751–8758. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Impact of exopolysaccharides on the stability of silver nanoparticles in water. Water Res. 2011, 45, 5184–5190. [Google Scholar] [CrossRef]
- Lin, D.; Drew Story, S.; Walker, S.L.; Huang, Q.; Cai, P. Influence of extracellular polymeric substances on the aggregation kinetics of TiO2 nanoparticles. Water Res. 2016, 104, 381–388. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.F.; Tan, Z.Q.; Bekana, D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017, 230, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahkami, A.H.; Allen White, R.; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, M.D.A. Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE 2019, 14, e0213040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Chau, N.H.; Van, N.T. Metal-Based Nanoparticles Enhance Drought Tolerance in Soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V.V. High-Temperature Stress Alleviation by Selenium Nanoparticle Treatment in Grain Sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Wang, X.; Saleem, M.H.; Hafeez, A.; Afridi, M.S.; Khan, S.; Alatawi, A.; Ullah, I.; Amaral Júnior, A.T.d.; Ali, S.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.; El-Monem, A.M.A.; Abd El-Razek, U.A.; Hafez, E.M. Interactive Impacts of Beneficial Microbes and Si-Zn Nanocomposite on Growth and Productivity of Soybean Subjected to Water Deficit under Salt-Affected Soil Conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Biochem. 2017, 118, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.K.; Upadhyay, S.K.; Tripathi, M.; Singh, R.; Krishna, D.; Singh, S.K.; Dwivedi, P. Understanding the salinity stress on plant and developing sustainable management strategies mediated salt-tolerant plant growth-promoting rhizobacteria and CRISPR/Cas9. Biotechnol. Genet. Eng. Rev. 2022, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Fatemi, H.; Rizwan, M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021, 225, 112769. [Google Scholar] [CrossRef]
- Karami, A.; Sepehri, A. Beneficial role of MWCNTs and SNP on growth, physiological and photosynthesis performance of barley under NaCl stress. J. Soil Sci. Plant Nutr. 2018, 18, 752–771. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Basahi, M. Seed germination with titanium dioxide nanoparticles enhances water supply, reserve mobilization, oxidative stress and antioxidant enzyme activities in pea. Saudi J. Biol. Sci. 2021, 28, 6500–6507. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Su, M.; Liu, C.; Qu, C.; Zheng, L.; Chen, L.; Huang, H.; Liu, X.; Wu, X.; Hong, F. Nano-anatase relieves the inhibition of electron transport caused by linolenic acid in chloroplasts of spinach. Biol. Trace Elem. Res. 2008, 122, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Alabdallah, N.M.; Hasan, M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Kaur, R.; Kaur, R. An insight into drought stress and signal transduction of abscisic acid. Plant Sci. Today 2018, 5, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Hojjat, S.S. Effects of TiO2 Nanoparticles on Germination and Growth Characteristics of Grass Pea (Lathyrus sativus L.) Seed under Drought Stress. Nanotechnol. Russ. 2020, 15, 204–211. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Exogenous Nitric Oxide Improves the Protective Effects of TiO2 Nanoparticles on Growth, Antioxidant System, and Photosynthetic Performance of Wheat Seedlings Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P.; Tohidi Moghadam, H.R.; Zahedi, H. Influence of Bulk and Nanoparticles Titanium Foliar Application on some Agronomic Traits, Seed Gluten and Starch Contents of Wheat Subjected to Water Deficit Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Ray, R.; Mandal, N.; Chakrabarti, K. An analysis of transcripts and enzyme profiles in drought stressed jute (Corchorus capsularis) and rice (Oryza sativa) seedlings treated with CaCl2, hydroxyapatite nano-particle and β-amino butyric acid. Plant Growth Regul. 2016, 79, 401–412. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite Nanoparticles Acts as Nanozymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Song, F.; Guo, J.; Zhu, X.; Liu, S.; Liu, F.; Li, X. Nano-ZnO-Induced Drought Tolerance Is Associated with Melatonin Synthesis and Metabolism in Maize. Int. J. Mol. Sci. 2020, 21, 782. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Debnath, A.; Guru, A.; Singh, B.N.; Upadhyay, S.K.; Dwivedi, P. Mechanistic and recent updates in nano-bioremediation for developing green technology to alleviate agricultural contaminants. Int. J. Environ. Sci. Technol. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Application of Nanofertilizer and Nanopesticides for Improvements in Crop Production and Protection. In Nanoscience and Plant–Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 405–427. [Google Scholar]
- Soltys, L.; Mironyuk, I.; Tatarchuk, T.; Tsinurchyn, V. Zeolite-based Composites as Slow Release Fertilizers (Review). Phys. Chem. Solid State 2020, 21, 89–104. [Google Scholar] [CrossRef]
- Arshad, M.; Nisar, S.; Gul, I.; Nawaz, U.; Irum, S.; Ahmad, S.; Sadat, H.; Mian, I.A.; Ali, S.; Rizwan, M.; et al. Multi-element uptake and growth responses of Rice (Oryza sativa L.) to TiO2 nanoparticles applied in different textured soils. Ecotoxicol. Environ. Saf. 2021, 215, 112149. [Google Scholar] [CrossRef]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Das, P.; Barua, S.; Sarkar, S.; Karak, N.; Bhattacharyya, P.; Raza, N.; Kim, K.H.; Bhattacharya, S.S. Plant extract-mediated green silver nanoparticles: Efficacy as soil conditioner and plant growth promoter. J. Hazard. Mater. 2018, 346, 62–72. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, X.; Nie, X.; Li, Y.; Liang, T.; Ci, L. Alleviation role of functional carbon nanodots for tomato growth and soil environment under drought stress. J. Hazard. Mater. 2022, 423, 127260. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N. Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil 2022, 477, 99–115. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Wang, C.; Tabl, K.M.; Khatab, A.; Kuai, J.; Wang, J.; Wang, B.; et al. Mitigation of the salinity stress in rapeseed (Brassica napus L.) productivity by exogenous applications of bio-selenium nanoparticles during the early seedling stage. Environ. Pollut. 2022, 310, 119815. [Google Scholar] [CrossRef] [PubMed]
- Waqas Mazhar, M.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy 2022, 12, 982. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Mahawar, L.; Rajput, P.; Rajput, V.D.; Minkina, T.; Singh, R.K. Role of Engineered carbon nanoparticles (CNPs) in promoting growth and metabolism of Vigna radiata (L.) Wilczek: Insights into the biochemical and physiological responses. Plants 2021, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.B.; Yin, Q.S.; Zhang, Y.L.; Wang, B.L.; Guo, W.M.; Wang, J.W.; Xie, J. Effects of carbon nano-particles application on the growth, physiological characteristics and nutrient accumulation in tobacco plants. J. Food Agric. Environ. 2013, 11, 954–958. [Google Scholar]
- González-García, Y.; López-Vargas, E.R.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Pérez-Labrada, F.; Juárez-Maldonado, A. Seed priming with carbon nanomaterials improves the bioactive compounds of tomato plants under saline stress. Plants 2022, 11, 1984. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticles | Concentration | Plants and Application Mode | Beneficial Effects on Plants | Reference |
|---|---|---|---|---|
| ZnO-NMs | 20, 40, and 60mg L−1 [w/v] | Lupinus termis; seed priming | Treatment with ZnO-NMs improved the growth of plants under salinity stress. Increased the contents of photosynthetic pigments, organic solutes, total phenols, and ascorbic acid. Additionally, showed an increment in the activities of SOD, CAT, POD, and APX enzymes. While ZnO-NMs seed priming resulted in a decrement in MDA and Na contents in salt-stressed plants. Thus, seed priming with ZnO-NMs at 60 mg L−1 was revealed as an effective method to enhance the salt tolerance of lupine plants. | [124] |
| Si NMs | 100, 200, 300 and 400 mg kg−1 [w/w] | Cucumis sativus var (Beit Alpha); the suspension was directly added to the soil | Regardless of the amount of water given, Si NMs increased cucumber growth and production. The application of Si NMs at a rate of 200 mg kg−1 demonstrated the greatest improvement, particularly when cucumber plants received 85% of their evapotranspiration, leading to an increase in morphological parameters when compared to the control. | [101] |
| ZnO-NMs | 1, 3, and 5 mg kg−1 [w/w] | Sorghum bicolor var. 251 | ZnO-NMs (5 mg kg−1) increased grain N translocation in comparison to drought conditions and restored total N levels to normal. While grain P translocation was restricted by drought, shoot absorption of phosphorus was promoted. However, ZnO-NMs decreased overall P acquisition during stress by drought. | [125] |
| Poly(acrylic acid)-coated cerium oxide nanoparticles (PNC) | -- | Gossypium hirsutum L.; seed priming | PNC significantly improved the morphometric parameters by increasing the length, fresh weight, and dry weight of roots, modifying root anatomical structure, and increasing root vitality under salt stress compared to controls. Furthermore, treatment with PNC reduced ROS accumulation in plants. | [126] |
| Functional carbonnanodots (FCNs) | 0, 1, 3, 10, and 30 mg kg−1 [w/w] | Solanum lycopersicum; supplemented in soil | Treatments with FCNs improved plant growth, development, and production under drought stress by enhancing physiological plant functions such as photosynthesis, the antioxidant system, osmotic adjustment, etc. FCNs assist to increase root vigor and osmolytes levels, which in turn moderates the decrease in tissue water content and water usage efficiency. | [127] |
| SiO2 NMs | 150 mg kg−1 [w/w] | Triticum aestivum L. | Under drought stress, nano-silica improved the germination percentage, germination index, and germination vigor index. Increased shoot length and root length. Additionally, nano-silica boosted the concentration of photosynthetic pigments, osmolytes, relative water, membrane stability index, phenol, and flavonoids. The use of nano-silica significantly increased antioxidant activity. Compared to control, it also boosted indole acetic acid and cytokinin. Under stressful circumstances, a rise in hundred-grain weight and grains per spike due to the application of nano-silica was seen. | [128] |
| Zero-valent copper nanoparticles (n-Cuo) | -- | Zea mays; seed priming | When compared to plants under drought stress, n-Cuo treatment boosted the anthocyanin, chlorophyll, and carotenoid levels. Under drought-stress settings, applying n-Cuo to the plant enhanced overall seed production and grain yield. Thus, this study revealed seed priming with n-Cuo as a potential strategy for the development of drought-tolerant agricultural plants via the regulation of plant defensive mechanisms linked to drought tolerance. | [114] |
| ZnO-NMs | 0, 5, 10, 15, 25 and 50 ppm | Oryza sativa | Priming with ZnO-NMs at 25 ppm increased seed and straw yield under water deficit conditions. Additionally, seed priming with ZnO-NMs alleviated the oxidative stress. | [129] |
| BioSe-NMs | 0, 5, 50, 100, and 150 μmol L−1 | Brassica napus L. | The most effective concentration of bioSe-NMs was 150 μmol/L against the salinity stress in rapeseed seedlings. The applications of bioSe-NMs mainly imparted positive effects on seedlings under salinity stress via enhancing the germination, adjusting osmotic homeostasis, and switching on enzymatic and on-enzymatic defense systems. | [130] |
| Iron oxide (IO) NMs | 0, 25, 50, and 100 ppm | Linum usitatissimum L. | Seed priming with IO-NMs improved the plant’s growth and activities of antioxidative enzymes under water stress. Seed priming using IO-NMs also enhanced yield parameters such as the number of fruit branches, capsules, seeds/capsule, and total fresh and dry stem fiber production. | [131] |
| Engineered Carbon Nanoparticles (CNPs) | 25–200 μmol L−1 | Vigna radiata L. | CNPs improved growth by improving the content of total chlorophyll, protein, and plant biomass in Vigna radiata. | [132] |
| Carbon nanotubes (CNTs) | 0, 25, 75, and 125 mg pot−1 | Nicotiana tabacum | The plant height increased by 6.33%, 10.56%, and 10.00%, and the leaf area increased by 6.64%, 19.51%, and 21.58% at the maturity stage. It also improved the contents of chlorophyll and soluble proteins in leaves. | [133] |
| CNTs | 50, 250, and 500 mg L−1 | Solanum lycopersicum | CNTs as a seed primer coped with the saline stress and improved the antioxidant defense system, and in combination with GP enhanced the chlorophylls (9.1–21.7%), ascorbic acid (19.5%), glutathione (≈13%), proteins (9.9–11.9%), and phenols (14.2%) in leaves. | [134] |
| Single-walled CNTs | 50–800 μg mL−1 | Hyoscyamus niger | A reduction in oxidative damage indices and electrolyte leakage, and activate the defense system. Improved water absorption, protein biosynthesis, phenolics, and proline under drought-stress conditions. | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajput, V.D.; Kumari, A.; Upadhyay, S.K.; Minkina, T.; Mandzhieva, S.; Ranjan, A.; Sushkova, S.; Burachevskaya, M.; Rajput, P.; Konstantinova, E.; et al. Can Nanomaterials Improve the Soil Microbiome and Crop Productivity? Agriculture 2023, 13, 231. https://doi.org/10.3390/agriculture13020231
Rajput VD, Kumari A, Upadhyay SK, Minkina T, Mandzhieva S, Ranjan A, Sushkova S, Burachevskaya M, Rajput P, Konstantinova E, et al. Can Nanomaterials Improve the Soil Microbiome and Crop Productivity? Agriculture. 2023; 13(2):231. https://doi.org/10.3390/agriculture13020231
Chicago/Turabian StyleRajput, Vishnu D., Arpna Kumari, Sudhir K. Upadhyay, Tatiana Minkina, Saglara Mandzhieva, Anuj Ranjan, Svetlana Sushkova, Marina Burachevskaya, Priyadarshani Rajput, Elizaveta Konstantinova, and et al. 2023. "Can Nanomaterials Improve the Soil Microbiome and Crop Productivity?" Agriculture 13, no. 2: 231. https://doi.org/10.3390/agriculture13020231
APA StyleRajput, V. D., Kumari, A., Upadhyay, S. K., Minkina, T., Mandzhieva, S., Ranjan, A., Sushkova, S., Burachevskaya, M., Rajput, P., Konstantinova, E., Singh, J., & Verma, K. K. (2023). Can Nanomaterials Improve the Soil Microbiome and Crop Productivity? Agriculture, 13(2), 231. https://doi.org/10.3390/agriculture13020231















