Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety
Abstract
:1. Introduction
2. Soil Amendment to Reduce Cd Bioavailability
2.1. Inorganic Amendments
2.1.1. Phosphorous (P)
2.1.2. Zinc (Zn)
2.1.3. Calcium (Ca)
2.1.4. Silicon (Si)
2.1.5. Liming Materials
2.1.6. Nitrogen
2.1.7. Potassium (K)
2.1.8. Iron/Manganese (Fe/Mn)
2.2. Organic Amendments
2.2.1. Biochar
| Amendment | Pyrolysis Temperature | Doses Applied | Cd Treatment mg kg−1 | Plant Species | Effects/Results | References |
|---|---|---|---|---|---|---|
| Biochar | ||||||
| Rice hull | 500 °C | 0, 0.5, 1, 2, 5, 10% | Cd, Cu, Pb, Zn | Lettuce | No significant increase in yield, a decrease in the bioavailability of heavy metals in soil. | [173] |
| Rice straw | 500 °C | 0, 10, 20 ton/ha | 3.3, 5.9 | Lettuce | Exchangeable Cd decreased due to increased soil pH | [174] |
| Rice husk + nano-Fe3O4 particles coating | 400 °C | 0.05, 0.1, 0.2, 0.4, 0.8, 1.6% | 1.6 | Rice | BC-Fe treatments promoted iron plaque formation and increased soil CEC and reduced Cd availability by 6.81–25.0%. | [175] |
| Rice straw | 450 °C and 550 °C | 0, 3.0, 5.0% | 2.86 | Wheat | Increased soil pH, 35, 47, and 57% decrease in roots, shoots, and grains Cd content. | [161] |
| Wheat straw | 485 °C | 0, 20, 40 ton/ha | 0.9 | Rice | Increased soil pH and reduced CaCl2-extractable Cd in soil and grain Cd concentration. The effect decreased over time. | [61] |
| Wheat straw | 450 °C | 0.7–2.9% | 22.65 | Rice | Metal ions Precipitate with CO and/or PO4 Binding of Cd and Pb to the inner biochar particles, with 8.0–44.6% reduction in exchangeable Cd. | [176] |
| Willow chips | 450 °C and 600 °C | 0, 0.2, 1.0, 5% | 0, 1, 5 | Pepper | Low-temperature biochar was more efficient in immobilizing Cd in soil and higher biochar application decreased the Cd in roots. | [177] |
| Willow biomass + Zeolite | 350 °C and 500 °C | 0.50% | 2.5 | Tall fescue and cocksfoot | Higher biomass production was observed in the tested grasses. | [178] |
| Sugarcane straw | 700 °C | 0, 1.5, 3.0, 5.0% | 8.4 | Jack bean, Mucuna aterrima | Metal bioavailability in the soil and plant uptake by roots was reduced. | [179] |
| Olive mill waste | 450 °C | 0, 5, 10, 15% | 7.1 | Common bean | Increase in shoot length and dry weights of leaves and roots was observed. Cd in leaves was below the detection limit at the highest rate of biochar applied. | [180] |
| Pigeon pea stalk | 300 °C | 0, 0.25, 0.5% | 0, 5, 10 | Spinach | Increased soil pH and organic matter contents, DTPA extractable Cd was decreased in the soil and decreased Cd concentration in leaf and roots was observed. | [181] |
| Cotton sticks | 450 °C | 0, 3, 5% | 0, 25, 50, 75, 100 | Spinach | Decreased the shoot and root Cd concentration and increased the biomass and chlorophyll contents and gas exchange parameters. | [182] |
| Hickory nutshell and Maize straw | 0, 15, 30 ton/ha | 0.7, 2.04 | Rice | Reduce Cd accumulation in rice grains by immobilizing soil Cd. | [183] | |
| Bamboo chips | 350 °C | 1.00% | 3, 20 | Rice | Reduced Cd contents in rice plants in highly contaminated soil, supported metal-resistant and growth-promoting bacteria in the rhizosphere. | [184] |
| Coconut shell and GSA-4 (compositing organic manure with lime and sepiolite) | 1% | 0.83 | Rice and Wheat | Cadmium fractionation showed a significant decrease in the extractable fractions. | [125] | |
| peanut shell and wheat straw | 300–350 | 5% | 0.507 | Rice | led to significantly higher pH, soil organic carbon (SOC), and cation exchange capacity (CEC) in paddy soil, while the content of MgCl2-extractable Cd and Pb was lower | [185] |
| wheat chaff | 750 | 0.5, 5% | 0, 10, or 50 | Juncus Subsecundus | pH increased and CaCl2-extractable Cd decreased significantly. Biochar immobilized soil Cd but did notimprove the growth of the emergent wetland plant species atthe early growth stage | [186] |
| Sewage sludge, soybean straw, rice straw, and peanut shell | 0, 2, 5% | 0.81 | Turnip | Fresh biomass was the highest with lower biochar (2%) compared to the control and higher biochar (5%) treatment. The highest reduction in metal uptake was recorded with peanut shell biochar. | [187] | |
| Compost | ||||||
| Agriculturalpostharvest wastecompost | 6.25, 12.5% | 25 | Sorghum and barnyard grass | Compost decreased the solubility and mobilization of Cd (especially in dry soil). | [188] | |
| Bamboo biochar, rice, and wheat straw | 750 °C | 2% biochar or 1% straw | 2 | Maize and ryegrass | Increase in soil pH and organic carbon. The Cd concentration in shoots of maize was reduced by 50.9%, 69.5%, and 66.9% with biochar, rice straw, and wheat straw, respectively. | [177] |
| composted sewage sludge and green waste compost | 5, 10,15% | 813 | Ryegrass | Compost immobilized Cu and Cd in contaminated soils. | [189] | |
| Manure | ||||||
| chicken manure | 0, 5.5, 11, 16.5, 22 ton/ha | 0.41 | Rice | Converted Cd to more immobilized fractions by decreasing the exchangeable Cd fraction and increasing the carbonate-, oxide-, and organic matter-bound fractions. | [190] | |
| Farmyard manure | 20–30 kg/ha | 0.35 | Wheat | The release of organic ligands immobilizes soil Zn and Cd | [191] | |
| Pig manure | 1.3, 4 g/kg | 6.79 | Rice | Increased the grain yield by 0.3–15.3 fold, and effectively decreased the Cu and Cd concentrations in grain. | [192] | |
| Swine manure | 30 g/kg | 2.91 | Sunflower | Swine manure and salicylic acid reduced the Cd/Zn ratio in the sunflower. | [193] |
2.2.2. Compost
2.2.3. Animal Waste/Manure
3. Irrigation Management to Reduce Cd Uptake
4. Effect of the Cropping Pattern on the Cd Contamination of Crop
4.1. Intercropping
4.2. Crop Rotation
5. Effect of Microorganisms
5.1. Bacteria
5.2. Fungi
5.3. Algae
6. Novel Sustainable Strategies for Mitigating Cd Toxicity
6.1. Nanoremediation
6.2. Phytoremediation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, T.; Zhu, J.-M.; Gao, T.; Xiong, Y.; Zhu, Z.; Ning, Z.; Liu, C. Redistribution and isotope fractionation of endogenous Cd in soil profiles with geogenic Cd enrichment. Sci. Total Environ. 2022, 852, 158447. [Google Scholar] [CrossRef]
- Wang, F.; Peng, L.; Zhou, X.; Zeng, Q.; Luo, S. Typical sources of Cd to paddy fields in different contaminated areas and their impacts on Cd accumulation in topsoil and rice in Changzhutan, China. Environ. Res. 2021, 193, 110523. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Lin, C. Response of toxic metal distributions and sources to anthropogenic activities and pedogenic processes in the Albic Luvisol profile of northeastern China. Environ. Adv. 2021, 6, 100142. [Google Scholar] [CrossRef]
- Yin, X.; Wei, R.; Chen, H.; Zhu, C.; Liu, Y.; Wen, H.; Guo, Q.; Ma, J. Cadmium isotope constraints on heavy metal sources in a riverine system impacted by multiple anthropogenic activities. Sci. Total Environ. 2021, 750, 141233. [Google Scholar] [CrossRef]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuan, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef] [PubMed]
- Romero-Estévez, D.; Yánez-Jácome, G.S.; Navarrete, H. Non-essential metal contamination in Ecuadorian agricultural production: A critical review. J. Food Compos. Anal. 2023, 115, 104932. [Google Scholar] [CrossRef]
- Han, Y.; Gu, X. Enrichment, contamination, ecological and health risks of toxic metals in agricultural soils of an industrial city, northwestern China. J. Trace Elem. Miner. 2023, 3, 100043. [Google Scholar] [CrossRef]
- Mazumder, P.; Khwairakpam, M.; Kalamdhad, A.S. Assessment of multi-metal contaminant in agricultural soil amended with organic wastes, speciation and translocation–an approach towards sustainable crop production. Total Environ. Res. 2023, 5, 100025. [Google Scholar] [CrossRef]
- Tariq, F. Heavy metals concentration in vegetables irrigated with municipal wastewater and their human daily intake in Erbil city. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100475. [Google Scholar] [CrossRef]
- Marini, M.; Angouria-Tsorochidou, E.; Caro, D.; Thomsen, M. Daily intake of heavy metals and minerals in food–a case study of four Danish dietary profiles. J. Clean. Prod. 2021, 280, 124279. [Google Scholar] [CrossRef]
- Huo, J.; Huang, Z.; Li, R.; Song, Y.; Lan, Z.; Ma, S.; Wu, Y.; Chen, J.; Zhang, L. Dietary cadmium exposure assessment in rural areas of Southwest China. PLoS ONE 2018, 13, e0201454. [Google Scholar] [CrossRef] [Green Version]
- Nishijo, M.; Nakagawa, H.; Suwazono, Y.; Nogawa, K.; Kido, T. Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: A nested case–control analysis of a follow-up study in Japan. BMJ Open 2017, 7, e015694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melila, M.; Rajaram, R.; Ganeshkumar, A.; Kpemissi, M.; Pakoussi, T.; Agbere, S.; Lazar, I.M.; Lazar, G.; Paray, B.A.; Gulnaz, A. Assessment of renal and hepatic dysfunction by co-exposure to toxic metals (Cd, Pb) and fluoride in people living nearby an industrial zone. J. Trace Elem. Med. Biol. 2022, 69, 126890. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.-Y.; Li, M.-Y.; Qin, Y.-S.; Bao, X.-C.; Wang, C.-C.; Cui, D.-L.; Xiang, P.; Ma, L.Q. Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: Oxidative stress, cell cycle arrest and apoptosis. Sci. Total Environ. 2021, 756, 143951. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Cai, Y.; Liu, B.; Cai, K.; Lv, W.; Tian, J.; Wang, W. Abatement of Cd in rice grain and toxic risks to human health by the split application of silicon at transplanting and jointing period. J. Environ. Manag. 2022, 302, 114039. [Google Scholar] [CrossRef] [PubMed]
- Chaudri, A.; Zhao, F.; McGrath, S.; Crosland, A. The cadmium content of British wheat grain. J. Environ. Qual. 1995, 24, 850–855. [Google Scholar] [CrossRef]
- Gawalko, E.J.; Garrett, R.G.; Nowicki, T.W. Trace elements in western Canadian hard red spring wheat (Triticum aestivum L.): Levels and quality assurance. J. AOAC Int. 2001, 84, 1953–1963. [Google Scholar] [CrossRef] [Green Version]
- Wiersma, D.; Van Goor, B.J.; Van der Veen, N.G. Cadmium, lead, mercury and arsenic concentrations in crops and corresponding soils in the Netherlands. J. Agric. Food Chem. 1986, 34, 1067–1074. [Google Scholar] [CrossRef]
- Wolnik, K.A.; Fricke, F.L.; Capar, S.G.; Braude, G.L.; Meyer, M.W.; Satzger, R.D.; Bonnin, E. Elements in major raw agricultural crops in the United States. 1. Cadmium and lead in lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J. Agric. Food Chem. 1983, 31, 1240–1244. [Google Scholar] [CrossRef]
- Sriprachote, A.; Kanyawongha, P.; Ochiai, K.; Matoh, T. Current situation of cadmium-polluted paddy soil, rice and soybean in the Mae Sot District, Tak Province, Thailand. Soil Sci. Plant Nutr. 2012, 58, 349–359. [Google Scholar] [CrossRef]
- Song, W.-e.; Chen, S. The toxicity thresholds (ECx) of cadmium to rice cultivars as determined by root elongation tests in soils and its predicted models. Sci. Agric. Sin. 2014, 47, 3434–3443. [Google Scholar]
- US Food and Drug Administration. Total Diet Study Statistics on Element Results Market Baskets 2006-1 through 2008-4; US Food and Drug Administration: College Park, MD, USA, 2010.
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Parveen, Z.; Khuhro, M.; Rafiq, N. Market basket survey for lead, cadmium, copper, chromium, nickel, and zinc in fruits and vegetables. Bull. Environ. Contam. Toxicol. 2003, 71, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Al-Qahtani, K.M. Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012, 38, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-G.; Lee, Y.; Kim, C.S.; Han, S.B. Codex Alimentarius commission on ensuring food safety and promoting fair trade: Harmonization of standards between Korea and codex. Food Sci. Biotechnol. 2021, 30, 1151–1170. [Google Scholar] [CrossRef]
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M. Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam Kohat. J. Pharm. Sci. Res. 2015, 7, 89. [Google Scholar]
- Kulsum, P.G.P.S.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2022, 220, 115098. [Google Scholar] [CrossRef]
- ur Rehman, Z.; Junaid, M.F.; Ijaz, N.; Khalid, U.; Ijaz, Z. Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci. Total Environ. 2023, 864, 161468. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar]
- Wang, X.-L.; Wang, M.-H.; Quan, S.-X.; Yan, B.; Xiao, X.-M. Influence of thermal treatment on fixation rate and leaching behavior of heavy metals in soils from a typical e-waste processing site. J. Environ. Chem. Eng. 2016, 4, 82–88. [Google Scholar] [CrossRef]
- Siebers, N.; Siangliw, M.; Tongcumpou, C. Cadmium uptake and subcellular distribution in rice plants as affected by phosphorus: Soil and hydroponic experiments. J. Soil Sci. Plant Nutr. 2013, 13, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.J.; Azeem, M.T.; Jan, M.T.; Perveen, S. Effect of amendments on chemical immobilization of heavy metals in sugar mill contaminated soils. Soil Environ. 2012, 31, 55–66. [Google Scholar]
- Chen, S.; Xu, M.; Ma, Y.; Yang, J. Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotoxicol. Environ. Saf. 2007, 67, 278–285. [Google Scholar] [CrossRef]
- Fei, C.; Jing, D.; Wang, F.; Wu, F.; Zhang, G.; Li, G.; Chen, Z.; Chen, J.; Kang, W. Identification of barley genotypes with low grain Cd accumulation and its interaction with four microelements. Chemosphere 2007, 67, 2082–2088. [Google Scholar]
- Zhou, C.; Yuan, H.; Ning, C.; Li, S.; Xia, Z.; Zhu, M.; Ma, Q.; Yu, W. Evaluation of Different Types and Amounts of Amendments on Soil Cd Immobilization and its Uptake to Wheat. Environ. Manag. 2020, 65, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ali, Q.; Javid, R.; Asghar, H.N.; Ahmad, I.; Iqbal, M.Z.; Khaliq, A. Organic and Inorganic Amendments Immobilized Cadmium and Improved Maize Growth and Yield in Cd-Contaminated Soil. Int. J. Agric. Biol. 2019, 22, 1497–1506. [Google Scholar]
- McGowen, S.; Basta, N.; Brown, G. Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J. Environ. Qual. 2001, 30, 493–500. [Google Scholar] [CrossRef]
- Chizzola, R.; Mitteregger, U.S. Cadmium and zinc interactions in trace element accumulation in chamomile. J. Plant Nutr. 2005, 28, 1383–1396. [Google Scholar] [CrossRef]
- Ueno, D.; Zhao, F.-l.; Ma, J.F. Interactions between Cd and Zn in relation to their hyperaccumulation in Thlaspi caerulescens. Soil Sci. Plant Nutr. 2004, 50, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, G.; Javed, W.; Hussain, A.; Qadir, M.; Aslam, M. Soil-applied zinc and copper suppress cadmium uptake and improve the performance of cereals and legumes. Int. J. Phytoremediation 2017, 19, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Z.; Li, H.; Smith, S.; Smith, F. Effect of zinc–cadmium interactions on the uptake of zinc and cadmium by winter wheat (Triticum aestivum) grown in pot culture. Bull. Environ. Contam. Toxicol. 2003, 71, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Erdem, H.; Tosun, Y.K.; Ozturk, M. Effect of cadmium-zinc interactions on growth and Cd-Zn concentration in durum and bread wheats. Fresenius Environ. Bull 2012, 21, 1046–1051. [Google Scholar]
- Chen, H.; Shu, F.; Yang, S.; Li, Y.; Wang, S. Competitive Inhibitory Effect of Calcium Polypeptides on Cd Enrichment of Brassia campestris L. Int. J. Environ. Res. Public Health 2019, 16, 4472. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.-S.P. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar]
- Bolan, N.S.; Adriano, D.C.; Duraisamy, P.; Mani, A.; Arulmozhiselvan, K. Immobilization and phytoavailability of cadmium in variable charge soils. I. Effect of phosphate addition. Plant Soil 2003, 250, 83–94. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, H.; Lu, J.; Chen, Q.; Li, W.; Wu, L.; Tang, J.; Ma, L. The immobilization of Soil cadmium by the combined Amendment of Bacteria and Hydroxyapatite. Sci. Rep. 2020, 10, 2189. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Li, Z.; Wu, J.; Shen, Y.; Li, Y.; Zou, B.; Tang, Y.; Zhuang, P. Influences of calcium silicate on chemical forms and subcellular distribution of cadmium in Amaranthus hypochondriacus L. Sci. Rep. 2017, 7, 40583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Q.; Fang, J.; Huang, F.; Cai, K. Silicon amendment reduces soil Cd availability and Cd uptake of two Pennisetum species. Int. J. Environ. Res. Public Health 2019, 16, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Wong, J.W.; Wei, L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef]
- Naeem, A.; Ghafoor, A.; Farooq, M. Suppression of cadmium concentration in wheat grains by silicon is related to its application rate and cadmium accumulating abilities of cultivars. J. Sci. Food Agric. 2015, 95, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Mench, M.; Vangronsveld, J.; Didier, V.; Clijsters, H. Evaluation of metal mobility, plant availability and immobilization by chemical agents in a limed-silty soil. Environ. Pollut. 1994, 86, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Murata, Y.; Nakamura, T.; Sakai, Y.; Osaki, M. Effect of zero-valent iron application on cadmium uptake in rice plants grown in cadmium-contaminated soils. J. Plant Nutr. 2009, 32, 1164–1172. [Google Scholar] [CrossRef]
- Chlopecka, A.; Adriano, D. Influence of zeolite, apatite and Fe-oxide on Cd and Pb uptake by crops. Sci. Total Environ. 1997, 207, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.F.; ur Rehman, M.Z.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Ahmad, M.; Usman, A.R.; Lee, S.S.; Jeon, W.-T.; Oh, S.-E.; Yang, J.E.; Ok, Y.S. Effects of natural and calcined poultry waste on Cd, Pb and As mobility in contaminated soil. Environ. Earth Sci. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Rehman, M.Z.-u.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef]
- Chen, D.; Guo, H.; Li, R.; Li, L.; Pan, G.; Chang, A.; Joseph, S. Low uptake affinity cultivars with biochar to tackle Cd-tainted rice—A field study over four rice seasons in Hunan, China. Sci. Total Environ. 2016, 541, 1489–1498. [Google Scholar] [CrossRef]
- Ashrafi, M.; Mohamad, S.; Yusoff, I.; Shahul Hamid, F. Immobilization of Pb, Cd, and Zn in a contaminated soil using eggshell and banana stem amendments: Metal leachability and a sequential extraction study. Environ. Sci. Pollut. Res. 2015, 22, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Matusik, J.; Bajda, T.; Manecki, M. Immobilization of aqueous cadmium by addition of phosphates. J. Hazard. Mater. 2008, 152, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.O.; Owens, V.N.; Kim, Y.G.; Lee, S.M.; Park, H.C.; Kim, K.K.; Son, H.J.; Suh, J.M.; Kim, P.J. Soil pH effect on phosphate induced cadmium precipitation in arable soil. Bull. Environ. Contam. Toxicol. 2014, 93, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar]
- He, M.; Shi, H.; Zhao, X.; Yu, Y.; Qu, B. Immobilization of Pb and Cd in contaminated soil using nano-crystallite hydroxyapatite. Procedia Environ. Sci. 2013, 18, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Ruangcharus, C.; Kim, S.U.; Hong, C.O. Mechanism of cadmium immobilization in phosphate-amended arable soils. Appl. Biol. Chem. 2020, 63, 36. [Google Scholar] [CrossRef]
- Jiang, H.M.; Yang, J.C.; Zhang, J.F. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ. Pollut. 2007, 147, 750–756. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Shams, M.S.; Khalifa, M.R.; Mohamed, A.; Rinklebe, J. Various soil amendments and environmental wastes affect the (im) mobilization and phytoavailability of potentially toxic elements in a sewage effluent irrigated sandy soil. Ecotoxicol. Environ. Saf. 2017, 142, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Rinklebe, J. Phytoextraction of potentially toxic elements by Indian mustard, rapeseed, and sunflower from a contaminated riparian soil. Environ. Geochem. Health 2015, 37, 953–967. [Google Scholar] [CrossRef]
- Zare, A.; Khoshgoftarmanesh, A.; Malakouti, M.; Bahrami, H.; Chaney, R. Root uptake and shoot accumulation of cadmium by lettuce at various Cd: Zn ratios in nutrient solution. Ecotoxicol. Environ. Saf. 2018, 148, 441–446. [Google Scholar] [CrossRef]
- Küpper, H.; Kochian, L.V. Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol. 2010, 185, 114–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Palusińska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maślińska, K.; Antosiewicz, D.M. Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Biol. 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Cojocaru, P.; Gusiatin, Z.M.; Cretescu, I. Phytoextraction of Cd and Zn as single or mixed pollutants from soil by rape (Brassica napus). Environ. Sci. Pollut. Res. 2016, 23, 10693–10701. [Google Scholar] [CrossRef]
- Green, C.E.; Chaney, R.L.; Bouwkamp, J. Interactions Between Cadmium Uptake and Phytotoxic Levels of Zinc in Hard Red Spring Wheat. J. Plant Nutr. 2003, 26, 417–430. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Clarke, J.M.; Kochian, L.V. Zinc effects on cadmium accumulation and partitioning in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol. 2005, 167, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Köleli, N.; Eker, S.; Cakmak, I. Effect of zinc fertilization on cadmium toxicity in durum and bread wheat grown in zinc-deficient soil. Environ. Pollut 2004, 131, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Qaswar, M.; Hussain, S.; Rengel, Z. Zinc fertilisation increases grain zinc and reduces grain lead and cadmium concentrations more in zinc-biofortified than standard wheat cultivar. Sci. Total Environ. 2017, 606, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, C.; Luo, Z.-c.; Zhu, H.-h.; Wang, S.; Zhu, Q.-h.; Huang, D.-y.; Zhang, Y.-z.; Xiong, J.; He, Y.-b. Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions. Environ. Sci. Pollut. Res. 2018, 25, 29287–29294. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Zhou, Z.; Zhang, T.; Wang, X. A tillering application of zinc fertilizer based on basal stabilization reduces Cd accumulation in rice (Oryza sativa L.). Ecotoxicol Environ. Saf 2019, 167, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, G.P. Genotypic Differences in Effect of Cd on Growth and Mineral Concentrations in Barley Seedlings. Bull. Environ. Contam. Toxicol. 2002, 69, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, G.; Yu, J. Interaction of Cadmium and Four Microelements for Uptake and Translocation in Different Barley Genotypes. Commun. Soil Sci. Plant Anal. 2003, 34, 2003–2020. [Google Scholar] [CrossRef]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Bioresour. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Khurana, M.; Kansal, B. Influence of zinc supply on the phytotoxicity of cadmium in maize (Zea mays L.) grown on cadmium-contaminated soil. Acta Agron. Hung. 2012, 60, 37–46. [Google Scholar] [CrossRef]
- Sikka, R.; Nayyar, V. Cadmium Accumulation and Its Effects on Uptake of Micronutrients in Indian Mustard [Brassica juncea (L.) Czern.] Grown in a Loamy Sand Soil Artificially Contaminated with Cadmium. Commun. Soil Sci. Plan 2012, 43, 672–688. [Google Scholar] [CrossRef]
- McKenna, I.M.; Chaney, R.L.; Williams, F.M. The effects of cadmium and zinc interactions on the accumulation and tissue distribution of zinc and cadmium in lettuce and spinach. Environ. Pollut. 1993, 79, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.; Li, Y.; Schneiter, A.; Green, C.; Miller, J.; Hopkins, D. Progress in developing technologies to produce low Cd concentration sunflower kernels. In Proceedings of the 15th Sunflower Research Workshop, Fargo, ND, USA, 14–15 January 1993; pp. 14–15. [Google Scholar]
- Cherif, J.; Mediouni, C.; Ben Ammar, W.; Jemal, F. Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solanum lycopersicum). J Environ. Sci. 2011, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Herath, D.; Weerasinghe, A.; Bandara, D.; Wijayawardhana, D. Synergistic Effect of Zinc and Cadmium for Uptake, Accumulation and Growth Responses in Rice (Oryza sativa) Varieties. Int. J. Chem. Environ. Biol. Sci. 2016, 4, 69–73. [Google Scholar]
- Nan, Z.; Li, J.; Zhang, J.; Cheng, G. Cadmium and zinc interactions and their transfer in soil-crop system under actual field conditions—ScienceDirect. Sci. Total Environ. 2002, 285, 187–195. [Google Scholar] [CrossRef]
- Grant, C.; Buckley, W.; Bailey, L.; Selles, F. Cadmium accumulation in crops. Can. J. Plant Sci. 1998, 78, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Green, C.E.; Chaney, R.L.; Bouwkamp, J. Increased Zinc Supply Does Not Inhibit Cadmium Accumulation by Rice (Oryza sativa L.). J. Plant Nutr. 2016, 40, 869–877. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.; Cui, H.; Fan, X.; Zhou, D.; Zhou, J. Effects of zinc application on cadmium (Cd) accumulation and plant growth through modulation of the antioxidant system and translocation of Cd in low-and high-Cd wheat cultivars. Environ. Pollut. 2020, 265, 115045. [Google Scholar] [CrossRef]
- Wang, C.X.; Mo, Z.; Wang, H.; Wang, Z.J.; Cao, Z.H. The transportation, time-dependent distribution of heavy metals in paddy crops. Chemosphere 2003, 50, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Wei, D.; Chen, S.; Li, J.; Ma, Y. Field Evidence of Cadmium Phytoavailability Decreased Effectively by Rape Straw and/or Red Mud with Zinc Sulphate in a Cd-Contaminated Calcareous Soil. PLoS ONE 2014, 9, e109967. [Google Scholar] [CrossRef] [PubMed]
- Bozena, G.; Zakrzewska, D.; Szymczycha, B. Sorption of Cr, Pb, Cu, Zn, Cd, Ni, and Co to nano-TiO2 in seawater. Water Sci. Technol. 2018, 77, 145–158. [Google Scholar] [CrossRef]
- Chaney, R.L. How Does Contamination of Rice Soils with Cd and Zn Cause High Incidence of Human Cd Disease in Subsistence Rice Farmers. Curr. Pollut. Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Hui, Y.; Junli, W.; Wei, F.; Jiangang, Y.; Zhongyi, Y. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar]
- Chaney, R.L.; Ryan, J.A.; Reeves, P.G. Cadmium in Soils and its Transfer to Plants and the Human Food Chain. Cell 2013, 301, 395–4852. [Google Scholar]
- Chen, X.; Ouyang, Y.; Fan, Y.; Qiu, B.; Zhang, G.; Zeng, F. The pathway of transmembrane cadmium influx via calcium-permeable channels and its spatial characteristics along rice root. J. Exp. Bot. 2018, 69, 5279–5291. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, N.; Tomioka, R.; Takenaka, C. Effects of calcium on cadmium uptake and transport in the tree species Gamblea innovans. Soil Sci. Plant Nutr. 2011, 57, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotechnol. 2005, 22, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Kurtyka, R.; MbBkowski, E.; Kita, A.; Karcz, W. Effect of Calcium and Cadmium on Growth and Accumulation of Cadmium, Calcium, Potassium and Sodium in Maize Seedlings. Pol. J. Environ. Stud. 2008, 17, 51–56. [Google Scholar]
- Godbold, D.L. Cadmium uptake in Norway spruce (Picea abies (L.) Karst.) seedlings. Tree Physiol. 1991, 9, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-Y.; Yang, Y.-Y.; Lee, Y. Pb and Cd uptake in rice roots. Physiol. Plant. 2002, 116, 368–372. [Google Scholar] [CrossRef]
- Jiang, X.J.; Luo, Y.M.; Liu, Q.; Liu, S.L.; Zhao, Q.G. Effects of Cadmium on Nutrient Uptake and Translocation by Indian Mustard. Environ. Geochem. Health 2004, 26, 319–324. [Google Scholar] [CrossRef]
- Gong, X.; Liu, Y.; Huang, D.; Zeng, G.; Liu, S.; Tang, H.; Zhou, L.; Hu, X.; Zhou, Y.; Tan, X. Effects of exogenous calcium and spermidine on cadmium stress moderation and metal accumulation in Boehmeria nivea (L.) Gaudich. Environ. Sci. Pollut. Res. 2016, 23, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Juang, K. Alleviation effects of calcium and potassium on cadmium rhizotoxicity and absorption by soybean and wheat roots. J. Plant Nutr. Soil Sci. 2015, 178, 748–754. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, F.; Liu, S.; Du, Y.; Li, F.; Du, R.; Wen, D.; Zhao, J. Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. sp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2016, 131, 173–180. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.-D.; Davidian, J.-C.; Pokrovsky, O.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. 2016, 23, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Głazowska, S.; Baldwin, L.; Mravec, J.; Bukh, C.; Hansen, T.H.; Jensen, M.M.; Fangel, J.U.; Willats, W.G.T.; Glasius, M.; Felby, C.; et al. The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnol. Biofuels 2018, 11, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Zhang, C.; Wang, H.; Zhang, F. Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 2005, 272, 53–60. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q.; Liu, C.; Wu, L. Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul. 2010, 61, 45–52. [Google Scholar] [CrossRef]
- Lukačová, Z.; Švubová, R.; Kohanová, J.; Lux, A. Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul. 2013, 70, 89–103. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, J.; He, C.; Li, X.; Zhang, W.; Xu, F.; Lin, Y.; Wang, L. Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol 2013, 200, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cai, H.; He, C.; Zhang, W.; Wang, L. A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 2015, 206, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.M.T.; Chacón-Madrid, K.; Galazzi, R.M.; Campos, B.K.; Arruda, S.C.C.; Azevedo, R.A.; Arruda, M.A.Z. Evaluation of silicon influence on the mitigation of cadmium-stress in the development of Arabidopsis thaliana through total metal content, proteomic and enzymatic approaches. J. Trace Elem. Med. Biol. 2017, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.A.; Prity, S.A.; Das, U.; Akther, M.S.; Sajib, S.A.; Reza, M.A.; Kabir, A.H. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020, 22, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 2016, 211, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.F.; Fujii-Kashino, M.; Yamaji, N.; Fukuoka, S.; Shen, R.F.; Ma, J.F. Isolation and characterization of a rice line with high Cd accumulation for potential use in phytoremediation. Plant Soil 2017, 410, 357–368. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Yaseen, M.; Hussain, B.; Zehra, A.; Aziz, M.Z.; He, Z.-l.; Yang, X. Comparative efficacy of organic and inorganic amendments for cadmium and lead immobilization in contaminated soil under rice-wheat cropping system. Chemosphere 2019, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Guo, Z.; Peng, C.; Xiao, X.; He, Y. Factors influencing the effectiveness of liming on cadmium reduction in rice: A meta-analysis and decision tree analysis. Sci. Total Environ. 2021, 779, 146477. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.-l.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhou, Z.; Yi, Y.; Chen, G. Transcriptome analysis reveals the roles of stem nodes in cadmium transport to rice grain. BMC Genom. 2020, 21, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chaney, R.L.; Green, C.E.; Ajwa, H.A.; Smith, R.F. Zinc fertilization plus liming to reduce cadmium uptake by Romaine lettuce on Cd-mineralized Lockwood soil. In Proceedings of the International Plant Nutrition Colloquium XVI; University of California: Oakland, CA, USA, 2009. [Google Scholar]
- Krebs, R.; Gupta, S.K.; Furrer, G.; Schulin, R. Solubility and Plant Uptake of Metals with and without Liming of Sludge-Amended Soils. J. Environ. Qual. 1998, 27, 18–23. [Google Scholar] [CrossRef]
- Hong, C.O.; Lee, D.K.; Chung, D.Y.; Kim, P.J. Liming effects on cadmium stabilization in upland soil affected by gold mining activity. Arch. Environ. Contam. Toxicol. 2007, 52, 496–502. [Google Scholar] [CrossRef]
- Smith, S. Effect of soil pH on availability to crops of metals in sewage sludge-treated soils. II. Cadmium uptake by crops and implications for human dietary intake. Environ. Pollut. 1994, 86, 5–13. [Google Scholar] [CrossRef]
- Cavallaro, N.; McBride, M. Activities of Cu2+ and Cd2+ in soil solutions as affected by pH. Soil Sci. Soc. Am. J. 1980, 44, 729–732. [Google Scholar] [CrossRef]
- Elliott, H.; Liberati, M.; Huang, C. Competitive Adsorption of Heavy Metals by Soils; 0047-2425; Wiley Online Library: Hoboken, NJ, USA, 1986. [Google Scholar]
- Knox, A.; Seaman, J.; Mench, M.; Vangronsveld, J. Remediation of metal-and radionuclides-contaminated soils by in situ stabilization techniques. In Environmental Restoration of Metals-Contaminated Soils; CRC Press: Boca Raton, FL, USA, 2000; pp. 21–60. [Google Scholar]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar]
- He, Q.; Singh, B. Crop uptake of cadmium from phosphorus fertilizers: I. Yield and cadmium content. Water Air Soil Pollut. 1994, 74, 251–265. [Google Scholar] [CrossRef]
- Zaccheo, P.; Crippa, L.; Pasta, V.D.M. Ammonium nutrition as a strategy for cadmium mobilisation in the rhizosphere of sunflower. Plant Soil 2006, 283, 43–56. [Google Scholar] [CrossRef]
- Giansoldati, V.; Tassi, E.; Morelli, E.; Gabellieri, E.; Pedron, F.; Barbafieri, M. Nitrogen fertilizer improves boron phytoextraction by Brassica juncea grown in contaminated sediments and alleviates plant stress. Chemosphere 2012, 87, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Panković, D.; Plesničar, M.; Arsenijević-Maksimović, I.; Petrović, N.; Sakač, Z.; Kastori, R. Effects of nitrogen nutrition on photosynthesis in Cd-treated sunflower plants. Ann. Bot. 2000, 86, 841–847. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Wang, X.; Shi, W.; Yan, W.; Cao, Z. Plant physiological responses to the interactions between heavy metal and nutrients. Soil Environ. Sci. 2002, 11, 392–396. [Google Scholar]
- Hassan, M.J.; Wang, F.; Ali, S.; Zhang, G. Toxic Effect of Cadmium on Rice as Affected by Nitrogen Fertilizer Form. Plant Soil 2005, 277, 359–365. [Google Scholar] [CrossRef]
- Maqbool, A.; Ali, S.; Rizwan, M.; Arif, M.S.; Yasmeen, T.; Riaz, M.; Hussain, A.; Noreen, S.; Abdel-Daim, M.M.; Alkahtani, S. N-Fertilizer (Urea) Enhances the Phytoextraction of Cadmium through Solanum nigrum L. Int. J. Environ. Res. Public Health 2020, 17, 3850. [Google Scholar] [CrossRef]
- Xie, H.L.; Jiang, R.F.; Zhang, F.S.; McGrath, S.P.; Zhao, F.J. Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator Thlaspi caerulescens. Plant Soil 2009, 318, 205–215. [Google Scholar] [CrossRef]
- Zhu, E.; Liu, D.; Li, J.G.; Li, T.Q.; Yang, X.E.; He, Z.L.; Stoffella, P.J. Effect of Nitrogen Fertilizer on Growth and Cadmium Accumulation in Sedum alfredii Hance. J. Plant Nutr. 2010, 34, 115–126. [Google Scholar] [CrossRef]
- Qin, D.; Chen, M.-X.; Rong, Z.; Chao, Z.-Y.; Zhu, Z.-W.; Shao, G.-S.; Wang, G.-M. Cd toxicity and accumulation in rice plants vary with soil nitrogen status and their genotypic difference can be partly attributed to nitrogen uptake capacity. Rice Sci. 2009, 16, 283–291. [Google Scholar]
- Umar, S.; Diva, I.; Anjum, N.; Iqbal, M. Potassium nutrition reduces cadmium accumulation and oxidative burst in mustard (Brassica campestris L.). Electron. Int. Fertil. Corresp. 2008, 16, 6–9. [Google Scholar]
- Sun, Y.; Li, Z.; Guo, B.; Chu, G.; Wei, C.; Liang, Y. Arsenic mitigates cadmium toxicity in rice seedlings. Environ. Exp. Bot. 2008, 64, 264–270. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhu, Y.G.; Li, H.Y.; Smith, S.E.; Smith, F.A. Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum L.). Environ. Int. 2004, 29, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [PubMed]
- Kumpiene, J. Trace Element Immobilization in Soil Using Amendments; Chapter 15; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zhang, C.; Ge, Y.; Yao, H.; Chen, X.; Hu, M. Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: A review. Front. Environ. Sci. Eng. 2012, 6, 509–517. [Google Scholar] [CrossRef]
- Ran, L.; Altschul, E.B.; Hedin, R.S.; Nakles, D.V.; Dzombak, D.A. Sequestration Enhancement of Metals in Soils by Addition of Iron Oxides Recovered from Coal Mine Drainage Sites. J. Soil Contam. 2014, 23, 374–388. [Google Scholar]
- Liu, H.; Zhang, J.; Christie, P.; Zhang, F. Influence of iron plaque on uptake and accumulation of Cd by rice (Oryza sativa L.) seedlings grown in soil. Sci. Total Environ. 2008, 394, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, G.; Chen, M.; Wang, D.; Xu, C.; Mou, R.; Cao, Z.; Zhang, X. Using iron fertilizer to control Cd accumulation in rice plants: A new promising technology. Sci. China Ser. C Life Sci. 2008, 51, 245–253. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ. Pollut. 2007, 147, 366–373. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M.; Ransom, M.D. In Situ Stabilization of Soil Lead Using Phosphorus and Manganese Oxide. Environ. Sci. Technol. 2000, 34, 4614–4619. [Google Scholar] [CrossRef]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; D’Souza, T. Plant uptake of cadmium, zinc, and manganese from four contrasting soils amended with Cd-enriched sewage sludge. J. Environ. Sci. Health Part A 2002, 37, 1337–1346. [Google Scholar] [CrossRef]
- Chang, R.; Sohi, S.P.; Jing, F.; Liu, Y.; Chen, J. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollut. 2019, 254, 113123. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Farooq Qayyum, M.; Abbas, F.; Hannan, F.; Rinklebe, J.; Sik Ok, Y. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Mohammad Hojjati, S.; Kammann, C.; Ghorbani, M.; Biparva, P. The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth. Appl. Sci. 2020, 10, 3410. [Google Scholar] [CrossRef]
- Shaheen, S.M.; El-Naggar, A.; Wang, J.; Hassan, N.E.E.; Niazi, N.K.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Chapter 14—Biochar as an (Im)mobilizing Agent for the Potentially Toxic Elements in Contaminated Soils. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 255–274. [Google Scholar]
- Hu, Y.; Zhang, P.; Yang, M.; Liu, Y.; Zhang, X.; Feng, S.; Guo, D.; Dang, X. Biochar is an effective amendment to remediate Cd-contaminated soils—A meta-analysis. J. Soils Sediments 2020, 20, 3884–3895. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Wang, S.-W.; Wang, C.-Q.; Zhang, Z.-Y.; Zhang, J.-Q.; Meng, M.; Li, M.; Uchimiya, M.; Yuan, X.Y. Simultaneous Immobilization of Soil Cd(II) and As(V) by Fe-Modified Biochar. Int. J. Environ. Res. Public Health 2020, 17, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Wang, B.; Shi, Z.; Li, L.; Li, Y.; Mao, Z.; Liao, L.; Zhang, H.; Wu, Y. Immobilization of heavy metals (Cd, Zn, and Pb) in different contaminated soils with swine manure biochar. Environ. Pollut. Bioavailab. 2021, 33, 55–65. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C.; et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Klasson, K.T.; Wartelle, L.H.; Lima, I.M. Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere 2011, 82, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Jin, F.; Wang, F.; McMillan, O.; Al-Tabbaa, A. Sorption of lead by Salisbury biochar produced from British broadleaf hardwood. Bioresour. Technol. 2015, 193, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Fahmi, A.H.; Samsuri, A.W.; Jol, H.; Singh, D. Bioavailability and leaching of Cd and Pb from contaminated soil amended with different sizes of biochar. R Soc. Open Sci. 2018, 5, 181328. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Kim, K.-R.; Kim, H.-J.; Yoon, J.-H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.-H. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Li, Z.-G.; Liu, X.-D.; Wang, B.-c.; Zhou, G.-L.; Huang, X.-X.; Lin, C.-F.; Wang, A.-h.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhou, H.; Gu, J.-F.; Huang, F.; Yang, W.-J.; Wang, S.-L.; Yuan, T.-Y.; Liao, B.-H. Effects of nano-Fe3O4-modified biochar on iron plaque formation and Cd accumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113970. [Google Scholar] [CrossRef]
- Cui, L.; Pan, G.; Li, L.; Bian, R.; Liu, X.; Yan, J.; Quan, G.; Ding, C.; Chen, T.; Liu, Y. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.-X.; Ye, X.-Z.; Xiao, W.-D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef]
- Puga, A.; Abreu, C.; Melo, L.; Paz-Ferreiro, J.; Beesley, L. Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw biochar. Environ. Sci. Pollut. Res. 2015, 22, 17606–17614. [Google Scholar] [CrossRef] [PubMed]
- Hmid, A.; Al Chami, Z.; Sillen, W.; De Vocht, A.; Vangronsveld, J. Olive mill waste biochar: A promising soil amendment for metal immobilization in contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Coumar, M.V.; Parihar, R.; Dwivedi, A.; Saha, J.; Rajendiran, S.; Dotaniya, M.; Kundu, S. Impact of pigeon pea biochar on cadmium mobility in soil and transfer rate to leafy vegetable spinach. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shan, S.; Chen, Y.; Wang, F.; Yang, D.; Ren, J.; Lu, H.; Ping, L.; Chai, Y. Biochar reduces cadmium accumulation in rice grains in a tungsten mining area-field experiment: Effects of biochar type and dosage, rice variety, and pollution level. Environ. Geochem. Health 2019, 41, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wei, S.; Jia, P.; Liu, T.; Hou, D.; Xie, R.; Lin, Z.; Ge, J.; Qiao, Y.; Chang, X. Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Sci. Total Environ. 2019, 676, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Seshadri, B.; Sarkar, B.; Wang, H.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.; Bolan, N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Solaiman, Z.M.; Meney, K.; Murphy, D.V.; Rengel, Z. Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J. Soils Sediments 2013, 13, 140–151. [Google Scholar] [CrossRef]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Balbaa, A.A.; Khatab, A.M.; Rinklebe, J. Compost and sulfur affect the mobilization and phyto-availability of Cd and Ni to sorghum and barnyard grass in a spiked fluvial soil. Environ. Geochem. Health 2017, 39, 1305–1324. [Google Scholar] [CrossRef] [PubMed]
- Gadepalle, V.P.; Ouki, S.K.; Hutchings, T. Remediation of copper and cadmium in contaminated soils using compost with inorganic amendments. Water Air Soil Pollut. 2009, 196, 355–368. [Google Scholar] [CrossRef]
- Huang, Q.; Wan, Y.; Luo, Z.; Qiao, Y.; Su, D.; Li, H. The effects of chicken manure on the immobilization and bioavailability of cadmium in the soil-rice system. Arch. Agron. Soil Sci. 2020, 66, 1753–1764. [Google Scholar] [CrossRef]
- Grüter, R.; Meister, A.; Schulin, R.; Tandy, S. Green manure effects on zinc and cadmium accumulation in wheat grains (Triticum aestivum L.) on high and low zinc soils. Plant Soil 2018, 422, 437–453. [Google Scholar] [CrossRef]
- Ping, L.; Xingxiang, W.; Zhang, T.; Dongmei, Z.; Yuanqiu, H. Effects of several amendments on rice growth and uptake of copper and cadmium from a contaminated soil. J. Environ. Sci. 2008, 20, 449–455. [Google Scholar]
- Xiu-Zhen, H.; Dong-Mei, Z.; Dan-Dan, L.; Jiang, P. Growth, cadmium and zinc accumulation of ornamental sunflower (Helianthus annuus L.) in contaminated soil with different amendments. Pedosphere 2012, 22, 631–639. [Google Scholar]
- Białobrzewski, I.; Mikš-Krajnik, M.; Dach, J.; Markowski, M.; Czekała, W.; Głuchowska, K. Model of the sewage sludge-straw composting process integrating different heat generation capacities of mesophilic and thermophilic microorganisms. Waste Manag. 2015, 43, 72–83. [Google Scholar] [CrossRef]
- de la Fuente, C.; Clemente, R.; Martínez-Alcalá, I.; Tortosa, G.; Bernal, M.P. Impact of fresh and composted solid olive husk and their water-soluble fractions on soil heavy metal fractionation; microbial biomass and plant uptake. J. Hazard. Mater. 2011, 186, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Udovic, M.; McBride, M.B. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. J. Hazard. Mater. 2012, 205, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Clemente, R.; Escolar, Á.; Bernal, M.P. Heavy metals fractionation and organic matter mineralisation in contaminated calcareous soil amended with organic materials. Bioresour. Technol. 2006, 97, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- van Herwijnen, R.; Hutchings, T.R.; Al-Tabbaa, A.; Moffat, A.J.; Johns, M.L.; Ouki, S.K. Remediation of metal contaminated soil with mineral-amended composts. Environ. Pollut. 2007, 150, 347–354. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef] [PubMed]
- Milojković, J.; Pezo, L.; Stojanović, M.; Mihajlović, M.; Lopičić, Z.; Petrović, J.; Stanojević, M.; Kragović, M. Selected heavy metal biosorption by compost of Myriophyllum spicatum—A chemometric approach. Ecol. Eng. 2016, 93, 112–119. [Google Scholar] [CrossRef]
- Karaca, A. Effect of organic wastes on the extractability of cadmium, copper, nickel, and zinc in soil. Geoderma 2004, 122, 297–303. [Google Scholar] [CrossRef]
- Hanafi, M.M.; Salwa, H. Cadmium and zinc in acid tropical soils: Il influence of humic acid addition on soil properties and their adsorption. Commun. Soil Sci. Plant Anal. 1998, 29, 1933–1947. [Google Scholar] [CrossRef]
- Karer, J.; Wawra, A.; Zehetner, F.; Dunst, G.; Wagner, M.; Pavel, P.-B.; Puschenreiter, M.; Friesl-Hanl, W.; Soja, G. Effects of biochars and compost mixtures and inorganic additives on immobilisation of heavy metals in contaminated soils. Water Air Soil Pollut. 2015, 226, 342. [Google Scholar] [CrossRef]
- Bolan, N.; Adriano, D.; Mani, S.; Khan, A. Adsorption, complexation, and phytoavailability of copper as influenced by organic manure. Environ. Toxicol. Chem. Int. J. 2003, 22, 450–456. [Google Scholar] [CrossRef]
- Li, M.; Mohamed, I.; Raleve, D.; Chen, W.; Huang, Q. RETRACTED ARTICLE: Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environ. Geochem. Health 2016, 38, 1193–1201. [Google Scholar] [CrossRef]
- Houben, D.; Pircar, J.; Sonnet, P. Heavy metal immobilization by cost-effective amendments in a contaminated soil: Effects on metal leaching and phytoavailability. J. Geochem. Explor. 2012, 123, 87–94. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Mao, P.; Li, Y.; Li, Y.; McBride, M.B.; Wu, J.; Zhuang, P. Heavy metal availability, bioaccessibility, and leachability in contaminated soil: Effects of pig manure and earthworms. Environ. Sci. Pollut. Res. 2019, 26, 20030–20039. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Bingham, F.; Page, A.; Mahler, R.; Ganje, T. Cadmium availability to rice in sludge-amended soil under “flood” and “nonflood” culture. Soil Sci. Soc. Am. J. 1976, 40, 715–719. [Google Scholar] [CrossRef]
- Qingqing, H.; Yiyun, L.; Xu, Q.; Lijie, Z.; Xuefeng, L.; Yingming, X. Selenite mitigates cadmium-induced oxidative stress and affects Cd uptake in rice seedlings under different water management systems. Ecotoxicol. Environ. Saf. 2019, 168, 486–494. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Camara, A.Y.; Wang, Q.; Li, H. Water management impacts on the solubility of Cd, Pb, As, and Cr and their uptake by rice in two contaminated paddy soils. Chemosphere 2019, 228, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ouyang, Y.; Wu, L.; Shen, L.; Luo, Y.; Christie, P. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar. J. Environ. Sci. 2015, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhou, H.; Gu, J.; Jia, R.; Li, H.; Wang, Q.; Zeng, M.; Liao, B. Cadmium accumulation and bioavailability in paddy soil under different water regimes for different growth stages of rice (Oryza sativa L.). Plant Soil 2019, 440, 327–339. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Mei, X.-Q.; Yang, Y.; Tam, N.F.-Y.; Wang, Y.-W.; Li, L. Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res. 2014, 50, 147–159. [Google Scholar] [CrossRef]
- Mei, X.; Li, Q.; Wang, H.; Fang, H.; Chen, H.; Chen, X.; Yang, Y.; Rizwan, M.; Ye, Z. Effects of cultivars, water regimes, and growth stages on cadmium accumulation in rice with different radial oxygen loss. Plant Soil 2020, 453, 529–543. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, M.; Wong, M.H.; Ye, Z. Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 2014, 375, 137–148. [Google Scholar] [CrossRef]
- Xiao, Q.; Wong, M.H.; Huang, L.; Ye, Z. Effects of cultivars and water management on cadmium accumulation in water spinach (Ipomoea aquatica Forsk.). Plant Soil 2015, 391, 33–49. [Google Scholar] [CrossRef]
- Jackson, M.; Armstrong, W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999, 1, 274–287. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y. Immobilization remediation of Cd-polluted soil with different water condition. J. Environ. Manag. 2017, 193, 607–612. [Google Scholar] [CrossRef]
- Cattani, I.; Romani, M.; Boccelli, R. Effect of cultivation practices on cadmium concentration in rice grain. Agron. Sustain. Dev. 2008, 28, 265–271. [Google Scholar] [CrossRef]
- Luo, W.; Yang, S.; Khan, M.A.; Ma, J.; Xu, W.; Li, Y.; Xiang, Z.; Jin, G.; Jia, J.; Zhong, B. Mitigation of Cd accumulation in rice with water management and calcium-magnesium phosphate fertilizer in field environment. Environ. Geochem. Health 2020, 42, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Valente, M.; Langasco, I.; Barracu, F.; Orlandoni, A.M.; Sanna, G. Sprinkler irrigation is effective in reducing cadmium concentration in rice (Oryza sativa L.) grain: A new twist on an old tale? Sci. Total Environ. 2018, 628, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of Water Management on Cadmium and Arsenic Accumulation and Dimethylarsinic Acid Concentrations in Japanese Rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Hu, P.; Li, Z.; Yuan, C.; Ouyang, Y.; Zhou, L.; Huang, J.; Huang, Y.; Luo, Y.; Christie, P.; Wu, L. Effect of water management on cadmium and arsenic accumulation by rice (Oryza sativa L.) with different metal accumulation capacities. J. Soils Sediments 2013, 13, 916–924. [Google Scholar] [CrossRef]
- Smolders, E.; McLaughlin, M.J. Chloride increases cadmium uptake in Swiss chard in a resin-buffered nutrient solution. Soil Sci. Soc. Am. J. 1996, 60, 1443–1447. [Google Scholar] [CrossRef]
- Salimi, M.; Amin, M.M.; Ebrahimi, A.; Ghazifard, A.; Najafi, P. Influence of electrical conductivity on the phytoremediation of contaminated soils to Cd 2+ and Zn2+. Int. J. Environ. Health Eng. 2012, 1, 11. [Google Scholar]
- Khoshgoftar, A.; Shariatmadari, H.; Karimian, N.; Kalbasi, M.; Van der Zee, S.; Parker, D. Salinity and zinc application effects on phytoavailability of cadmium and zinc. Soil Sci. Soc. Am. J. 2004, 68, 1885–1889. [Google Scholar] [CrossRef]
- Hattori, H.; Kuniyasu, K.; Chiba, K.; Chino, M. Effect of chloride application and low soil pH on cadmium uptake from soil by plants. Soil Sci. Plant Nutr. 2006, 52, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lin, L.; Liao, M.a.; Wang, J.; Tang, Y.; Sun, G.; Liang, D.; Xia, H.; Deng, Q.; Wang, X. Effects of intercropping with floricultural accumulator plants on cadmium accumulation in grapevine. Environ. Sci. Pollut. Res. 2019, 26, 24474–24481. [Google Scholar] [CrossRef]
- Zu, Y.; Qin, L.; Zhan, F.; Wu, J.; Li, Y.; Chen, J.; Wang, J.; Hu, W. Intercropping of Sonchus asper and Vicia faba affects plant cadmium accumulation and root responses. Pedosphere 2020, 30, 457–465. [Google Scholar] [CrossRef]
- Whiting, S.N.; Leake, J.R.; McGrath, S.P.; Baker, A.J. Assessment of Zn mobilization in the rhizosphere of Thlaspi caerulescens by bioassay with non-accumulator plants and soil extraction. Plant Soil 2001, 237, 147–156. [Google Scholar] [CrossRef]
- Yi, T.; Juan, H.; Xuena, Y.; Yongdong, X.; Lijin, L.; Guochao, S.; Huanxiu, L.; Mingan, L.; LIANG, D.; Hui, X. Intercropping with Solanum nigrum and Solanum photeinocarpum from two ecoclimatic regions promotes growth and reduces cadmium uptake of eggplant seedlings. Pedosphere 2017, 27, 638–644. [Google Scholar]
- Yongzhen, D.; Shirong, T.; Zhian, L.; Murray, M. Effects of low molecule weight organic acids on Cd solubility in paddy and red soils in South China. Res. J. Chem. Environ. 2008, 12, 7–16. [Google Scholar]
- Hei, L.; Wu, Q.-T.; Long, X.-X.; Hu, Y.-M. Effect of co-planting of Sedum alfredii and Zea mays on Zn-contaminated sewage sludge. Huan Jing Ke Xue = Huanjing Kexue 2007, 28, 852–858. [Google Scholar] [PubMed]
- Li, N.Y.; Li, Z.A.; Zhuang, P.; Zou, B.; McBride, M. Cadmium Uptake From Soil by Maize with Intercrops. Water Air Soil Pollut. 2009, 199, 45–56. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Tang, J.; Hu, L.; Chen, X. Plant coexistence can enhance phytoextraction of cadmium by tobacco (Nicotiana tabacum L.) in contaminated soil. J. Environ. Sci. 2011, 23, 453–460. [Google Scholar] [CrossRef]
- Azeez, J.O.; Hassan, O.A.; Adesodun, J.K.; Arowolo, T.A. Soil metal sorption characteristics and its influence on the comparative effectiveness of EDTA and legume intercrop on the phytoremediative abilities of Maize (Zea mays), Mucuna (Mucuna pruriens), Okra (Abelmoschus esculentus), and Kenaf (Hibiscus cannabinus). Soil Sediment Contam. Int. J. 2013, 22, 930–957. [Google Scholar]
- Yang, Y.; Zhou, X.; Tie, B.; Peng, L.; Li, H.; Wang, K.; Zeng, Q. Comparison of three types of oil crop rotation systems for effective use and remediation of heavy metal contaminated agricultural soil. Chemosphere 2017, 188, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ae, N.; Ishikawa, S.; Ibaraki, T.; Ito, M. Phytoextraction by a high-Cd-accumulating rice: Reduction of Cd content of soybean seeds. Environ. Sci. Technol. 2008, 42, 6167–6172. [Google Scholar] [CrossRef]
- Murakami, M.; Nakagawa, F.; Ae, N.; Ito, M.; Arao, T. Phytoextraction by rice capable of accumulating Cd at high levels: Reduction of Cd content of rice grain. Environ. Sci. Technol. 2009, 43, 5878–5883. [Google Scholar] [CrossRef]
- Wu, F.-L.; Lin, D.-Y.; Su, D.-C. The effect of planting oilseed rape and compost application on heavy metal forms in soil and Cd and Pb uptake in rice. Agric. Sci. China 2011, 10, 267–274. [Google Scholar] [CrossRef]
- Su, D.; Lu, X.; Wong, J. Could cocropping or successive cropping with Cd accumulator oilseed rape reduce Cd uptake of sensitive Chinese Cabbage? Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 224–228. [Google Scholar] [CrossRef]
- Tang, L.; Luo, W.; Chen, W.; He, Z.; Gurajala, H.K.; Hamid, Y.; Deng, M.; Yang, X. Field crops (Ipomoea aquatica Forsk. and Brassica chinensis L.) for phytoremediation of cadmium and nitrate co-contaminated soils via rotation with Sedum alfredii Hance. Environ. Sci. Pollut. Res. 2017, 24, 19293–19305. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, Q.; Liang, C.; Yang, X. Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii. Environ. Sci. Pollut. Res. 2014, 21, 5899–5908. [Google Scholar] [CrossRef]
- Li, W.C.; Wong, M.H. Interaction of Cd/Zn hyperaccumulating plant (Sedum alfredii) and rhizosphere bacteria on metal uptake and removal of phenanthrene. J. Hazard. Mater. 2012, 209, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Liu, J.; Wu, J.; Dai, G.; Wei, D.; Shu, Y. Assessing biochar application to immobilize Cd and Pb in a contaminated soil: A field experiment under a cucumber–sweet potato–rape rotation. Environ. Geochem. Health 2020, 42, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Guo, Z.; Shi, L.; Feng, W.; Xiao, X.; Peng, C.; Xue, Q. Effects of mixed amendments on the phytoavailability of Cd in contaminated paddy soil under a rice-rape rotation system. Environ. Sci. Pollut. Res. 2019, 26, 14128–14136. [Google Scholar] [CrossRef]
- Guo, L.; Wu, G.; Li, Y.; Li, C.; Liu, W.; Meng, J.; Liu, H.; Yu, X.; Jiang, G. Effects of cattle manure compost combined with chemical fertilizer on topsoil organic matter, bulk density and earthworm activity in a wheat–maize rotation system in Eastern China. Soil Tillage Res. 2016, 156, 140–147. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Song, J.; Jiang, W.; Liu, Y.; Fan, W. Bioremediation of cadmium-and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere 2018, 197, 33–41. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, L.M.; Rezende, H.C.; Coelho, L.M.; de Sousa, P.; Melo, D.; Coelho, N. Bioremediation of polluted waters using microorganisms. Adv. Bioremediation Wastewater Polluted Soil 2015, 10, 60770. [Google Scholar]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yuan, M.; Tan, S.; Yang, X.; Lan, Z.; Jiang, Q.; Ye, Z.; Jing, Y. Enhancement of arbuscular mycorrhizal fungus (Glomus versiforme) on the growth and Cd uptake by Cd-hyperaccumulator Solanum nigrum. Appl. Soil Ecol. 2015, 89, 44–49. [Google Scholar] [CrossRef]
- Ali, A.; Bilal, S.; Khan, A.L.; Mabood, F.; Al-Harrasi, A.; Lee, I.-J. Endophytic Aureobasidium pullulans BSS6 assisted developments in phytoremediation potentials of Cucumis sativus under Cd and Pb stress. J. Plant Interact. 2019, 14, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Nayuki, K.; Kuga, Y.; Zhang, X.; Wu, S.; Ohtomo, R. Uptake and Intraradical Immobilization of Cadmium by Arbuscular Mycorrhizal Fungi as Revealed by a Stable Isotope Tracer and Synchrotron Radiation μX-Ray Fluorescence Analysis. Microbes Environ. 2018, 33, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Hu, Z.-H.; Yan, T.-X.; Lu, R.-R.; Peng, C.-L.; Li, S.-S.; Jing, Y.-X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Degola, F.; Fattorini, L.; Bona, E.; Sprimuto, C.T.; Argese, E.; Berta, G.; di Toppi, L.S. The symbiosis between Nicotiana tabacum and the endomycorrhizal fungus Funneliformis mosseae increases the plant glutathione level and decreases leaf cadmium and root arsenic contents. Plant Physiol. Biochem. 2015, 92, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhipeng, W.; Weidong, W.; Shenglu, Z.; Shaohua, W. Mycorrhizal inoculation affects Pb and Cd accumulation and translocation in Pakchoi (Brassica chinensis L.). Pedosphere 2016, 26, 13–26. [Google Scholar]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Fungal endophyte Penicillium janthinellum LK5 can reduce cadmium toxicity in Solanum lycopersicum (Sitiens and Rhe). Biol. Fertil. Soils 2014, 50, 75–85. [Google Scholar] [CrossRef]
- Khan, A.R.; Ullah, I.; Waqas, M.; Park, G.-S.; Khan, A.L.; Hong, S.-J.; Ullah, R.; Jung, B.K.; Park, C.E.; Ur-Rehman, S. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol. Environ. Saf. 2017, 136, 180–188. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.; Marques, A.P.; Franco, A.R.; Rangel, A.O.; Castro, P.M. Phytomanagement of Cd-contaminated soils using maize (Zea mays L.) assisted by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2014, 21, 9742–9753. [Google Scholar] [CrossRef]
- Saluja, B.; Sharma, V. Cadmium resistance mechanism in acidophilic and alkalophilic bacterial isolates and their application in bioremediation of metal-contaminated soil. Soil Sediment Contam. Int. J. 2014, 23, 1–17. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.-D.; He, L.-Y.; Wang, Q.; Sheng, X.-F. Cd immobilization and reduced tissue Cd accumulation of rice (Oryza sativa wuyun-23) in the presence of heavy metal-resistant bacteria. Ecotoxicol. Environ. Saf. 2017, 138, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Abd_Allah, E.F.; Hashem, A.; Ahmad, P. Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 2019, 217, 463–474. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.M.; Juwarkar, A.A. In vivo studies to elucidate the role of extracellular polymeric substances from Azotobacter in immobilization of heavy metals. Environ. Sci. Technol. 2009, 43, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jia, Y.; Li, X.; Jiang, W.; Lu, L. Phytoavailability and geospeciation of cadmium in contaminated soil remediated by Rhodobacter sphaeroides. Chemosphere 2012, 88, 751–756. [Google Scholar] [CrossRef]
- Chen, L.; He, L.-y.; Wang, Q.; Sheng, X.-f. Synergistic effects of plant growth-promoting Neorhizobium huautlense T1-17 and immobilizers on the growth and heavy metal accumulation of edible tissues of hot pepper. J. Hazard. Mater. 2016, 312, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Mou, R.; Cao, Z.; Xu, P.; Wu, X. Characterization of cadmium-resistant bacteria and their potential for reducing accumulation of cadmium in rice grains. Sci. Total Environ. 2016, 569, 97–104. [Google Scholar] [CrossRef]
- Suksabye, P.; Pimthong, A.; Dhurakit, P.; Mekvichitsaeng, P.; Thiravetyan, P. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 962–973. [Google Scholar] [CrossRef]
- Li, X.; Peng, W.; Jia, Y.; Lu, L.; Fan, W. Bioremediation of lead contaminated soil with Rhodobacter sphaeroides. Chemosphere 2016, 156, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fan, W. Bioremediation of Heavy Metal–Contaminated Soils by Sulfate-Reducing Bacteria. Ann. N. Y. Acad. Sci. 2008, 1140, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, J.; Yuan, Z.; Wang, T.; Zhang, Y.; Wang, F. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation. Environ. Sci. Pollut. Res. 2017, 24, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Deng, Y.; Zhang, S.; Jiang, H. Bioremediation of cadmium-contaminated paddy soil using an autotrophic and heterotrophic mixture. RSC Adv. 2020, 10, 26090–26101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, W.D.; Yang, L.Y.; An, J.; Zhang, X.X.; Hong, H.; Xu, L.Z.; Wang, Y.P. Microcystis bloom containing microcystin-LR induces type 2 diabetes mellitus. Toxicol. Lett. 2018, 294, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.-M.; Wang, Q.; Liu, C. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit. Rev. Environ. Sci. Technol. 2012, 42, 741–775. [Google Scholar] [CrossRef]
- Júnior, L.B.; Macedo, G.; Duarte, M.; Silva, E.; Lobato, A.K.C.L. Biosorption of cadmium using the fungus Aspergillus niger. Braz. J. Chem. Eng. 2003, 20, 229–239. [Google Scholar] [CrossRef]
- Manguilimotan, L.C.; Bitacura, J.G. Biosorption of cadmium by filamentous fungi isolated from coastal water and sediments. J. Toxicol. 2018, 2018, 7170510. [Google Scholar] [CrossRef]
- Abhijit, M.; Ellairaja, S.; Chinnaiah, A.; Sivasamy, V.V. Efficient Removal of Cadmium Using Edible Fungus and Its Quantitative Fluorimetric Estimation Using (Z)-2-(4H-1,2,4-Triazol-4-yl)iminomethylphenol. ACS Omega 2018, 3, 6243–6250. [Google Scholar]
- Abeed, A.H.A.; Mahdy, R.E.; Alshehri, D.; Hammami, I.; Eissa, M.A.; Abdel Latef, A.A.H.; Mahmoud, G.A. Induction of resilience strategies against biochemical deteriorations prompted by severe cadmium stress in sunflower plant when Trichoderma and bacterial inoculation were used as biofertilizers. Front Plant Sci. 2022, 13, 1004173. [Google Scholar] [CrossRef] [PubMed]
- Oshiquiri, L.H.; Dos Santos, K.R.A.; Ferreira Junior, S.A.; Steindorff, A.S.; Barbosa Filho, J.R.; Mota, T.M.; Ulhoa, C.J.; Georg, R.C. Trichoderma harzianum transcriptome in response to cadmium exposure. Fungal Genet. Biol. 2020, 134, 103281. [Google Scholar] [CrossRef]
- Shahabivand, S.; Maivan, H.Z.; Goltapeh, E.M.; Sharifi, M.; Aliloo, A.A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Biochem. 2012, 60, 53–58. [Google Scholar] [CrossRef]
- Luo, N.; Li, X.; Chen, A.Y.; Zhang, L.J.; Zhao, H.M.; Xiang, L.; Cai, Q.Y.; Mo, C.H.; Wong, M.H.; Li, H. Does arbuscular mycorrhizal fungus affect cadmium uptake and chemical forms in rice at different growth stages? Sci. Total Environ. 2017, 599, 1564–1572. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R. Use of fungi in mitigating cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–426. [Google Scholar]
- Chen, B.; Liu, Y.; Shen, H.; Li, X.; Christie, P. Uptake of cadmium from an experimentally contaminated calcareous soil by arbuscular mycorrhizal maize (Zea mays L.). Mycorrhiza 2004, 14, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhang, S.; Chen, B.-D.; Wu, N.; Shan, X.-Q.; Christy, P. Uptake of Atrazine and Cadmium from Soil by Maize (Zea mays L.) in Association with the Arbuscular Mycorrhizal Fungus Glomus etunicatum. J. Agric. Food Chem. 2006, 54, 9377–9382. [Google Scholar] [CrossRef] [Green Version]
- Bauddh, K.; Korstad, J. Phycoremediation: Use of algae to sequester heavy metals. Hydrobiology 2022, 1, 288–303. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2021, 29, 41909–41922. [Google Scholar] [CrossRef]
- Saini, S.; Dhania, G. Cadmium as an environmental pollutant: Ecotoxicological effects, health hazards, and bioremediation approaches for its detoxification from contaminated sites. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Springer: Singapore, 2020; pp. 357–387. [Google Scholar]
- Lodeiro, P.; Herrero, R.; de Vicente, M.S. The use of protonated Sargassum muticum as biosorbent for cadmium removal in a fixed-bed column. J. Hazard. Mater. 2006, 137, 244–253. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Alabdullatif, J.A.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabbad, A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015, 22, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rama, M.; Alonso, J.A.; López, C.H.; Vaamonde, E.T. Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour. Technol. 2002, 84, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Shi, D.; Dai, J.; Ru, B. Expression of the mouse metallothionein-I gene conferring cadmium resistance in a transgenic cyanobacterium. FEMS Microbiol. Lett. 1998, 158, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S.; May, M.J.; Howden, R.; Rolls, B. The glutathione-deficient, cadmium-sensitive mutant, cad2–1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 1998, 16, 73–78. [Google Scholar] [CrossRef]
- Yoshida, N.; Ikeda, R.; Okuno, T. Identification and characterization of heavy metal-resistant unicellular alga isolated from soil and its potential for phytoremediation. Bioresour. Technol. 2006, 97, 1843–1849. [Google Scholar] [CrossRef]
- Hu, R.; Wang, H.; Liu, Q.; Lin, L.; Liao, M.A.; Deng, H.; Wang, Z.; Liang, D.; Wang, X.; Xia, H. An algal biostimulant promotes growth and decreases cadmium uptake in accumulator plant Nasturtium officinale. Int. J. Environ. Anal. Chem. 2022, 102, 4403–4411. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.; Sohn, J. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhao, M.; Yu, Z.; Rong, H.; Zhang, C. Utilization of nanomaterials for in-situ remediation of heavy metal(loid) contaminated sediments: A review. Sci. Total Environ. 2019, 662, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Forján, R.; Álvarez, N.; Gallego, J.; González, A. Zero valent iron nanoparticles and organic fertilizer assisted phytoremediation in a mining soil: Arsenic and mercury accumulation and effects on the antioxidative system of Medicago sativa L. J. Hazard. Mater. 2022, 433, 128748. [Google Scholar] [CrossRef]
- Bakshi, M.; Abhilash, P. Nanotechnology for soil remediation: Revitalizing the tarnished resource. In Nano-Materials as Photocatalysts for Degradation of Environmental Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–370. [Google Scholar]
- Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Asghar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H.M. In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air—A review. Chemosphere 2022, 289, 133252. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Rizwan, M.; Noureen, S.; Ali, S.; Anwar, S.; Rehman, M.Z.U.; Qayyum, M.F.; Hussain, A. Influence of biochar amendment and foliar application of iron oxide nanoparticles on growth, photosynthesis, and cadmium accumulation in rice biomass. J. Soils Sediments 2019, 19, 3749–3759. [Google Scholar] [CrossRef]
- Li, P.; Wen, J.; Chen, P.; Guo, P.; Ke, Y.; Wang, M.; Liu, M.; Tran, L.P.; Li, J.; Du, H. MYB Superfamily in Brassica napus: Evidence for Hormone-Mediated Expression Profiles, Large Expansion, and Functions in Root Hair Development. Biomolecules 2020, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Peng, Z.; Zeng, G.; Xu, P.; Cheng, M.; Wang, R.; Wan, J. Remediation of contaminated soils by biotechnology with nanomaterials: Bio-behavior, applications, and perspectives. Crit. Rev. Biotechnol. 2018, 38, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ye, J.; Fang, H.; Zhang, S.; Xu, C. Effects of copper oxide nanoparticles on paddy soil properties and components. Nanomaterials 2018, 8, 839. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of phytoremediation potential of five Cd (hyper) accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef]
- Iqbal, N.; Hayat, M.T.; Zeb, B.S.; Abbas, Z.; Ahmed, T. Phytoremediation of Cd-contaminated soil and water. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 531–543. [Google Scholar]
- Luo, J.-S.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van Der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Zhou, Q.; Wang, X.; Zhang, K.; Guo, G.; Ma, L.Q. A newly-discovered Cd-hyperaccumulator Solatium nigrum L. Chin. Sci. Bull. 2005, 50, 33–38. [Google Scholar] [CrossRef]
- Sheng, X.-F.; Xia, J.-J. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 2006, 64, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Q.; Zhang, Z.; Hua, T.; Cai, Z. Evaluation of cadmium phytoremediation potential in Chinese cabbage cultivars. J. Agric. Food Chem. 2011, 59, 8324–8330. [Google Scholar] [CrossRef]
- Saad-Allah, K.M.; Elhaak, M. Hyperaccumulation activity and metabolic responses of Solanum nigrum in two differentially polluted growth habitats. J. Saudi Soc. Agric. Sci. 2017, 16, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Khaokaew, S.; Landrot, G. A field-scale study of cadmium phytoremediation in a contaminated agricultural soil at Mae Sot District, Tak Province, Thailand: (1) Determination of Cd-hyperaccumulating plants. Chemosphere 2015, 138, 883–887. [Google Scholar] [CrossRef]
- Palutoglu, M.; Akgul, B.; Suyarko, V.; Yakovenko, M.; Kryuchenko, N.; Sasmaz, A. Phytoremediation of cadmium by native plants grown on mining soil. Bull. Environ. Contam. Toxicol. 2018, 100, 293–297. [Google Scholar] [CrossRef] [PubMed]

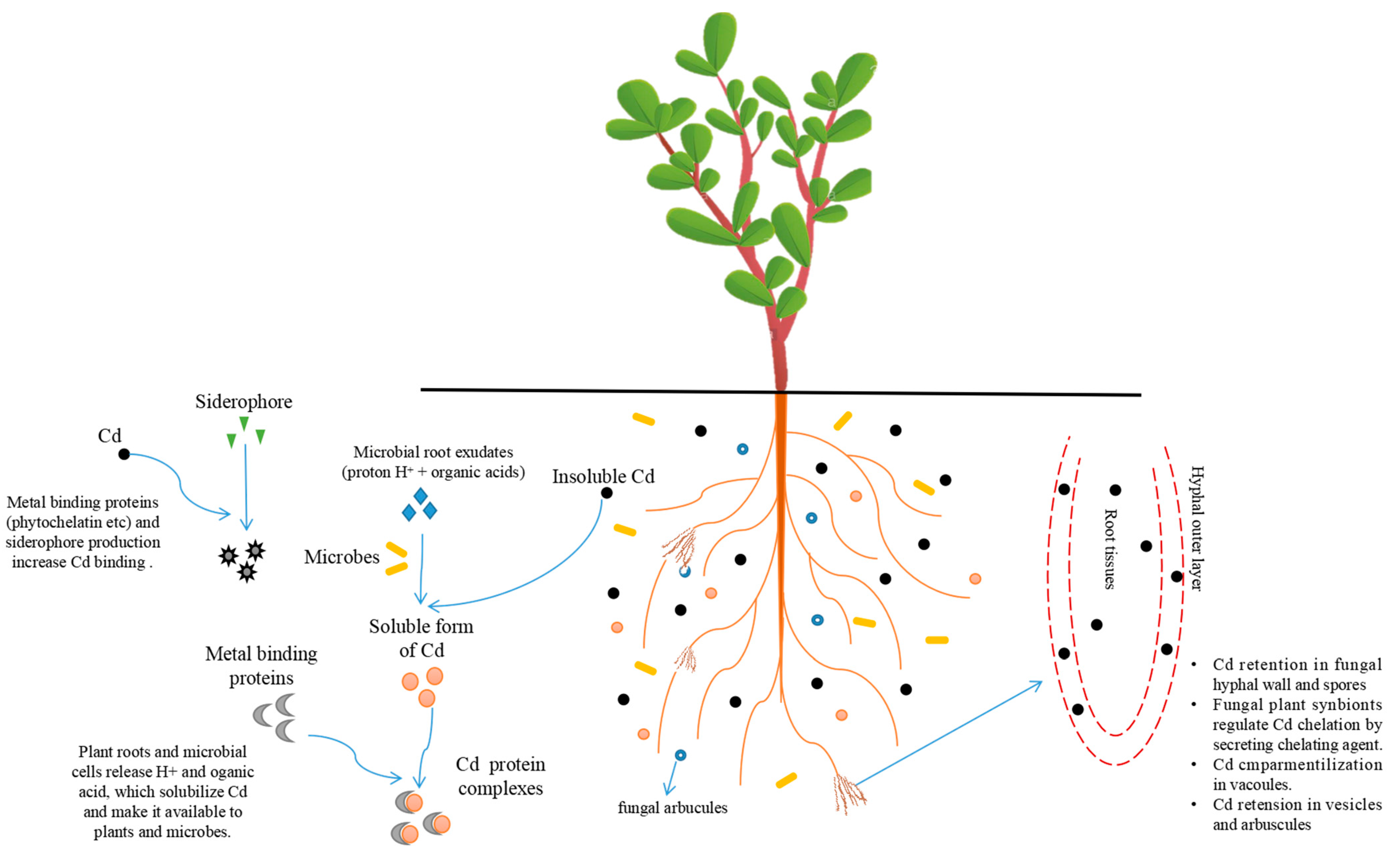
| Amendment Type | Applied Concentration (mg kg−1) | Cd Treatment (mg kg−1) | Plant | Soil Type | Results/Observation | References |
|---|---|---|---|---|---|---|
| Phosphorous Fertilizer | 50, 200, 1000 | 82 | Rice | Sandy loam | Increased soil pH and converted Cd to a less mobile form. | [34] |
| Diammonium phosphate (DAP) | 230 | 0.19 | - | silt loam | Acted as a stabilizing agent to reduce Cd uptake. | [35] |
| Phosphate Rock (PR) | 2500 | 0.6, 1.5 | Brassica campestris | Red soil | Immobilized Cd via formation or co-precipitation of insoluble metal phosphates in the soils. | [36,37] |
| Super Phosphate (SP), | 5000 | 0.20, 0.15, 0.02, 0.04, 0.06 | Wheat | Surface agriculture soil | SP efficiently immobilized the soil Cd but caused potential soil acidification risk. | [38] |
| Phosphate Rock (PR) + Mud compost (CP) | 10,000, 20,000 +20,000 | 0, 10, 30 | Maize | Sandy loam | The combined application of PR + CP improved the growth of maize and reduced soil Cd bioavailability. | [39] |
| Diammonium Phosphate (DAP) | 60, 920, 2300 | 1090 | - | Sandy loam | Application of 2300 mg kg−1 was the most effective for immobilizing Cd, Pb, and Zn from the contaminated soil. | [40] |
| Zinc (Zn) | 0, 100, 200 | 0, 1.5, 3 | Chamomile | Mixture of sandy + humus garden soil | The addition of Zn to the soils led to a suppressed Cd accumulation into the above-ground plant parts. | [41] |
| ZnS04 | 0, 80.7, 322 | 104 | Thlaspi caerulescens | - | Cd competed with Zn uptake while Zn did not compete with Cd uptake. | [42] |
| Zinc Sulfate (ZnSO4·7H2O) | 60 | 0, 1, 2, 5 | Chickpeas, mung beans, wheat, and maize | Sandy loam | Soil-applied Zn antagonized Cd to cope with its toxicity, thus favoring plant growth. | [43] |
| Zinc Oxide Nanoparticles (ZnO NPs) | 0, 25, 50,75, 100 | 7.38 | Wheat | Sandy loam | The Cd concentrations were reduced in the grains (16–78%) with the soil application of ZnO NPs as compared to the control. | [44] |
| Zinc (Zn) | 0, 2, 10, 100, 1000 | 0, 15, 30, 50 | Wheat | Loamy | Zn application decreased Cd concentration in plants. | [45] |
| Zinc (Zn) | 0, 2.5, 10 | 0, 5 | Wheat | Clay loam | Zn treatment alleviated Cd toxicity by decreasing Cd concentrations in wheat. | [46] |
| Calcium polypeptide | 0, 210, 420, 840, 1260, 1680 | 2.0, 5.0 | Brassica campestris | Red loam | Competitive inhibition effect of calcium on Cd enrichment in plants. | [47] |
| Calcium dichloride (CaCl2) | 200.4 | 200, 300 | Brassica juncea | Peat, Perlite and Sand (1:1:1, v/v/v) | Decreased Cd content and Improved growth and biomass yield of Brassica plants. | [48] |
| Ca(OH)2 | 5.62–23.1 | 0–10 | Brassica juncea | Egmont and Tokomaru soil | Transformed Cd to fewer mobile fractions and reduced phytoavailability. | [49] |
| Hydroxyapatite (HAP) + Cupriavidus sp. strain ZSK | 30,000 + 108 cells/g | 13.82 | Ramie, Dandelion, Daisy | Smelter soil | Combined application of HAP+ Cupriavidus sp. reduced Cd accumulation in ramie, dandelion, and daisy by 44.9%, 51.0%, and 38.7%, respectively. | [50] |
| Calcium Silicate (Ca2O4Si) | 0, 410, 830, 1650, 3310 | 6.1 | Amaranths | - | Free Cd ions convert into inactive Cd forms by Ca amendment in soil and are sequestered in subcellular compartments. | [51] |
| Potassium Silicate (K2SiO3) | 8 | 0, 10, 50, 100 | Pennis etumglaucum and Pennisetum glaucum | peat soil and sand | Significantly increased plant biomass and Si content, reduced Cd content, and decreased the enrichment factor in shoots and roots. | [52] |
| Sodium Metasilicate (Na2SiO3) | 400 | 20, 40 | Maize | Weathered acidic soil | Si significantly increased soil pH and decreased soil Cd availability. | [53] |
| Calcium Silicate (CaSiO3) | 50, 100, 150 | 10 | Wheat | Surface soil | Si application caused a decrease in the Cd contents of shoots and grains and the translocation from roots to shoots and grains. | [54] |
| Hydrous manganese oxides (HMO) | 1000 | 18 | Ryegrass, tobacco, and bean | Limed silty soil | The amendment application did not increase biomass production, but treatment with HMO markedly decreased the mobility of Cd, Zn, and Pb. | [55] |
| Zero-valent iron (Fe(0)) | 0, 500, 1000, 5000 | 10 | Rice | - | The Fe(0) application increased the less available Cd content, and decreased the exchangeable and Fe-Mn-oxide-bound (more available) Cd content. | [56] |
| Iron oxide (Fe2O3) | 50,000 | 0.5, 1.5, 3.0, 4.0, 8.5 | Maize, Barley | Silt loam. | Fe2O3 appears to be effective in response to plant yield, metal content in plant tissues, and bioavailable Cd. | [57] |
| gypsum | 0, 2000, 4000, 8000 | 3.02 | Wheat | Sandy clay loam | Increased pH and reduces the availability of Cd due to increased Cd precipitation and surface adsorption on the amendment. | [58] |
| CaCO3 and CaO | 0, 10,000, 30,000, 50,000 | 15.27 | Loam | Increased soil pH; formation of Cd-carbonate, phosphate, or hydroxide. | [59] | |
| Monoammonium phosphate (MAP) and gypsum | 0, 2000, 4000, 8000 | 3.15 | Rice | Sandy clay loam | MAP and gypsum increase grain yield and biomass of rice, whereas, decreased gain and straw Cd concentrations and uptake in rice. | [60] |
| Lime + peat | 0, 500, 1250 | 15.44 | Mixed clay | Liming reduced Cd available fraction in soil. | [61] | |
| Eggshell | 50,000 | 0.24 | Alkaline soil | Decreased mobility of Pb, Cd, and Zn in the soil by transforming their readily available forms to less accessible fractions. | [62] | |
| Sodium nitroprusside | 100 um/L | 150 um/L | Lycopersicon esculentum | Mixture of sand, perlite, and peat | Improve resistance mechanism by modulation of antioxidative defense system. NO boosts mineral uptake and reduced Cd accumulation. | [63] |
| Group | Species | Type | Plant/Crop | Resistance Mechanism/References | References |
|---|---|---|---|---|---|
| Fungi | Glomus versiforme | AMF | Solanum nigrum | Enhancement of soil acid phosphate activity | [255] |
| Aureobasidium pullulans | Endophytic fungi | Cucumis sativus | Regulate soil enzymatic activities to reduce Cd uptake | [256] | |
| Rhizophagus irregularis | AMF | Lotus japonicus | Enhanced intraradical immobilization of Cd | [257] | |
| Rhizophagus intraradices and Glomus versiforme | AMF | Zea mays | PC and GSH transformed Cd into the inactive form | [258] | |
| Funneliformis mosseae | Endo-mycorrhizal fungus | Nicotiana tabacum | Enhanced GSH content reduced Cd accumulation | [259] | |
| Funneliformis mosseae, Glomus versiforme, and Rhizophagus intraradices | AMF | Brassica chinensis | Altered plant–soil interaction by increased soil pH and electrical conductivity | [260] | |
| Penicillium janthinellum | Endophytic fungi | Solanum lycopersicum | Reduced electrolytes and lipid peroxidation and increased glutathione content and catalase activity | [261] | |
| Fusarium tricinctum and Alternaria alternata | Endophytic fungi | Solanum nigrum | Improve tolerance mechanism by low POD and PPO activities and high CAT activity | [262] | |
| Bacteria | Methylobacterium oryzae and Burkholderia sp. | PGPB | Lycopersicon esculentum | Reduced stressed ethylene and ACC deaminase activity | [263] |
| Ralstonia eutropha and Chryseobacterium humii | PGPR | Zea mays | Cd retention in roots by immobilization and reduced Cd translocation to shoots | [264] | |
| Pseudomonas putida | Acidophilic bacteria | Vigna radiata | Metallothioneins and ABC transporter/P-type ATPase, intracellular Cd bioaccumulation | [265] | |
| Rhodobacter sphaeroides | Purple non-sulfur bacteria | Triticum aestivum | Reduced the bioavailable Cd fractions (e.g., exchangeable and carbonate-bound phases) | [251] | |
| Bacillus megaterium and Neorhizobium huautlense | PGPB | Oryza sativa | Increased Cd immobilization in rhizosphere soil and reduced Cd uptake | [266] | |
| Pseudomonas aeruginosa and Burkholderia gladioli | PGPR | Lycopersicon esculentum | Improve resistance mechanism by modulation of antioxidative defense system | [267,268] | |
| Azotobacter sp. | Nitrogen-fixing bacteria | Triticum aestivum | Metal ion complexation either through f extracellular polymeric substance (EPS) or through cell wall lipopolysaccharides (LPS) | [269] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubeen, S.; Ni, W.; He, C.; Yang, Z. Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety. Agriculture 2023, 13, 471. https://doi.org/10.3390/agriculture13020471
Mubeen S, Ni W, He C, Yang Z. Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety. Agriculture. 2023; 13(2):471. https://doi.org/10.3390/agriculture13020471
Chicago/Turabian StyleMubeen, Samavia, Wenjuan Ni, Chuntao He, and Zhongyi Yang. 2023. "Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety" Agriculture 13, no. 2: 471. https://doi.org/10.3390/agriculture13020471
APA StyleMubeen, S., Ni, W., He, C., & Yang, Z. (2023). Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety. Agriculture, 13(2), 471. https://doi.org/10.3390/agriculture13020471






