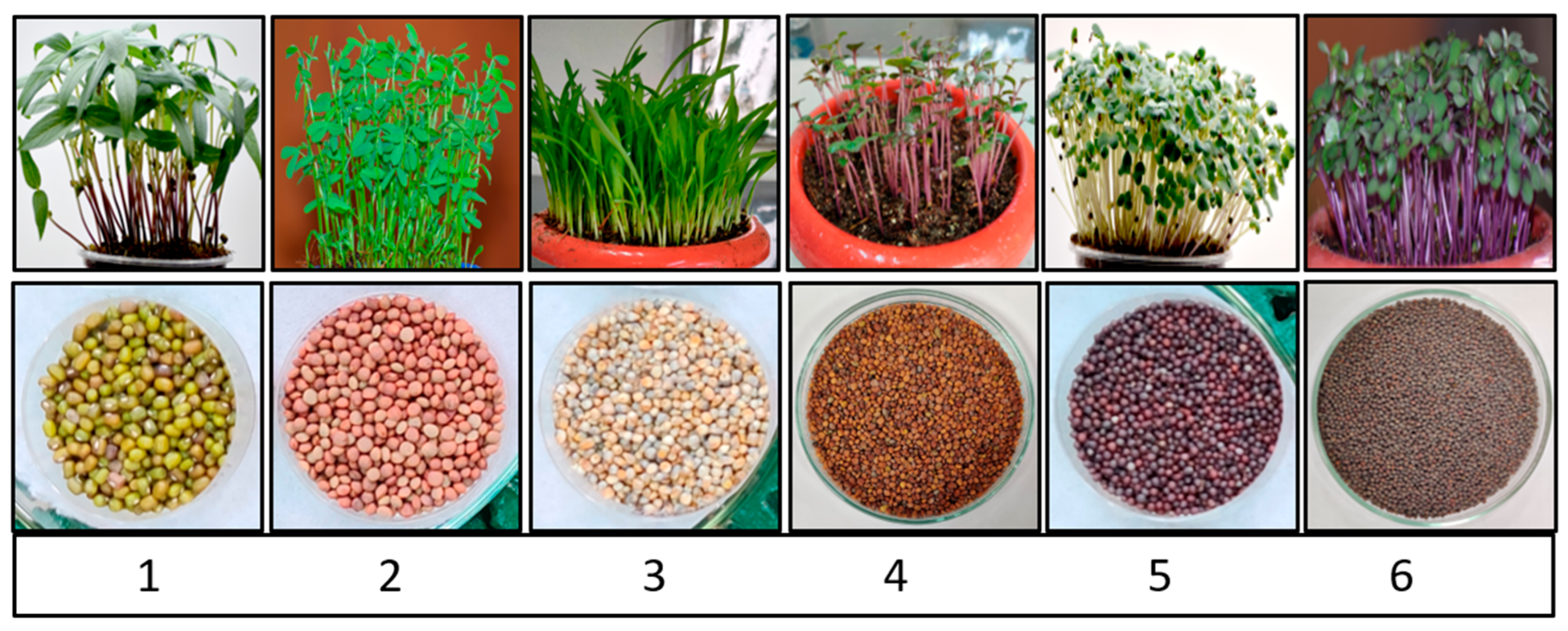
3.1. Optimization of Growth Conditions for Microgreen Production
Microgreens, unlike sprouts (the early stage of microgreens), require regular watering and a growing medium. Under controlled conditions, the experimental setup for optimizing six microgreens (mungbean, lentil, pearl millet, red radish, mustard, and red cabbage) was standardized (
Figure 1). To begin, the germination percentage of each microgreens seed was calculated at various temperatures (20–30 °C), and the ideal temperature for their maximum germination percentage was determined. At 28 °C, mungbean, lentil, and pearl millet germinated at maximum rates of 95, 90, and 85%, respectively. While red radish germinated at a maximum of 75% at 26 °C, mustard and red cabbage germinated at a maximum of 90 and 75% at 24 °C, respectively (
Table 4). Germination was majorly affected by the temperature; extreme high/low temperatures decreased the seed germination percentage significantly. Seedlings require only water and light for microgreens cultivation after they have sprouted [
28]. The plants were exposed to warm yellow light and watered every day until the first set of real leaves emerged. Indoor grow lamps are frequently used instead of natural lighting by growers. Customization is possible with LED lighting to optimize spectral composition to meet plant photoreceptors’ productivity, plant shape, and nutritional content [
29]. LED lighting systems provide many advantages in terms of efficiency and are less harmful to the environment than other types of light [
30].
After standardizing the optimum temperature for each microgreens species, the best day to harvest them was determined based on their morphological characteristics. When their cotyledonary leaf gets fully extended, they are ready to harvest. On the 7th day, mung bean and lentil were harvested, red radish, mustard, and red cabbage on the 8th day, and pearl millet on the 10th day. Each microgreen’s height was measured at the time of harvesting. Mungbean and lentils reached heights of 9 and 8 cm on the seventh day, respectively. While red radish, mustard, and red cabbage reached their maximum heights of 8, 9, and 8 cm, respectively, on the 8th day. On the 10th day of harvest, pearl millet reached its maximum height of 14 cm (
Table 5). Xiao et al. [
31] recommended harvesting microgreens from the Brassica family on day 7. Harvesting microgreens depends upon the height and leaf area. Green radish microgreens reached their maximum height of roughly 6 cm on the 8th day, whereas lettuce, mustard, and sesame reached their maximum height within 14 days. The intensity of sole-source light-emitting diodes have a significant impact on the growth, height and appearance quality of microgreens [
32]. Mostly, microgreen’s true leaves fully developed in 8–10 days, with few exceptions like finger millet and red amaranth showed comparatively slow growth, and true leaves emerged on days 13 and 14 in finger millet and red amaranth, respectively [
33].
3.3. Antioxidant Potential of Microgreens
The antioxidant potential in terms of radical scavenging activity (DPPH), total phenolics, anthocyanin, ascorbic acid, and phytic acid contents of each microgreen was estimated in the freshly harvested microgreens samples (
Table 7). In the DPPH assay, free radicals containing DPPH react with sample antioxidants, and by donating a hydrogen atom, the antioxidants reduce the DPPH [
33]. Each microgreens sample was evaluated for free radical scavenging activity, and the values ranged from 72 to 87%. Among all six microgreens, pearl millet showed the lowest 72%, whereas red cabbage showed the highest 87% free radical activity at the time of harvesting. Ghoora et al. [
11] also reported high values of DPPH activity in a few of the microgreens.
Phenolic compounds, the largest group of secondary metabolites, are primarily found in almost all fruits and vegetables and are the products of the phenylpropanoid biosynthesis pathway. Plant phenolic compounds include proanthocyanidins, flavonoids, lignans and lignin, cinnamic acid, benzoic acid, stilbenes, coumarins, etc. [
34]. The current study observed a significant variation in the total phenolic content in all six selected microgreens with a range of 52.62 to 1136.10 μg GAE/100 g FW. The highest total phenolics content (TPC) was found in pearl millet microgreens, and the lowest was found in the mustard microgreens at the time of harvesting. It should be highlighted as well that pearl millet and red cabbage recorded the highest DPPH activity and mustard as the lowest DPPH activity. A high positive association between TPC and DPPH scavenging activity has also been shown in another research [
11]. Agarwal et al. [
32] also reported a higher total phenolics content of radish, fenugreek, and roselle microgreens than the broccoli, red cabbage, radish, and purple radish microgreens. Different intrinsic and extrinsic factors, including species, growth circumstances, harvest maturity, and postharvest circumstances, may contribute to the variation in total phenolics content among different microgreens [
35,
36]. The phenolic compounds have antioxidant properties and are beneficial to human health in a number of ways. Its strong antioxidant efficacy comes from its capacity to scavenge free radicals, give electrons to oxidizing species, and indirectly reduce the buildup of reactive oxygen species. (ROS) [
34].
Another crucial phytochemical component in plants, i.e., anthocyanin pigments, are responsible for the attractive orange, red, blue, or purple coloration of several plant tissues [
37]. Red cabbage and red radish exhibit purplish-red and radish hypocotyls, respectively, owing to the abundance of anthocyanins. The total anthocyanin content was estimated in all six selected microgreens, and observed the highest content in red radish (186 mg/100 g FW). The accumulation of these color pigments not only contributes to the attractiveness of the microgreens but also has biological activities, making them an important component of human health [
11,
38,
39].
The most valuable antioxidant for living beings, including plants, is ascorbic acid (vitamin C). During various physical or physiological stresses, the free ascorbic acid oxidizes into dehydroascorbic acid and protects from oxidative damage to plants as well as human beings [
39]. The present study determined the amount of ascorbic acid in each microgreen on the day of harvest, as shown in
Table 6. The ascorbic acid content ranged from 28.48 mg/100 g FW (lowest; red radish) to 140.22 mg/100 g FW (highest; red cabbage). Mustard microgreens showed 60.68 mg/100 g, the second-highest amount of ascorbic acid in the brassica family. Xiao et al. [
31] also evaluated ascorbic acid content in 25 commercial plant micro-sprouts (20.4–131.6 mg/100 g FW), and rice grass (0.243 mg/g FW) and wheatgrass (0.487 mg/g FW) found similar to this research. The dietary fiber content also has prominent health benefits, and their content ranges from 2.26 to 6.48%. The highest amount of dietary fiber was recorded in pearl millet. Mlinarić et al. [
40] also reported the antioxidant potential and dietary fiber content of radish microgreens and showed higher as compared to the mature counterpart. In addition to the antioxidant potential of the microgreens, we have also estimated the growth inhibitory factor, phytic acid, in all the six selected microgreens. Phytic acid is known as a major anti-nutritional factor in several plants. As expected, phytic acid contents were reduced in the microgreens as compared to the content in their seeds. In the selected microgreens, pearl millet microgreens showed the highest (0.304 g/100 g FW), and red radish showed the lowest (0.156 g/100 g FW) phytic acid content.
Antioxidant enzymes are major components of the living system and determine the antioxidant potential of the cell. Four major antioxidant enzymes viz catalase, peroxidase, ascorbate peroxidase, and superoxide dismutase activities were estimated in all the microgreens. Increased activity indicates a lesser accumulation of reactive oxygen species (ROS). Plants have developed an antioxidant defense system, which includes antioxidant enzymes like; catalase, peroxidase, ascorbate peroxidase, superoxide dismutase, etc., to combat oxidative damage in severely unfavorable environments [
41]. A heme-containing enzyme called catalase has the ability to directly dismutate H
2O
2 into H
2O and O
2. Its rate of turnover is among the highest among all enzymes. Red cabbage had the lowest activity levels, while pearl millet had the highest. During the investigation, peroxidase—another significant enzyme involved in H
2O
2-scavenging—was also examined. Moreover, it is an enzyme that uses heme to catalyze the single electron oxidation of a number of substrates at the expense of H
2O
2. In plants, peroxidase is engaged in a number of processes. Red cabbage had the lowest activity levels, and pearl millet had the highest levels. Ascorbate peroxidase utilizes ascorbate as the electron donor and scavenges ROS (H
2O
2) through the ascorbate-GSH cycle. Compared to other scavenging enzymes, it has a higher affinity for H
2O
2. Its activity was highest in red cabbage and lowest in pearl millet microgreens. Superoxide dismutase, the most effective intracellular enzymatic antioxidant, removes O
-2 by catalyzing its dismutation and produces H
2O
2. Its activity was also highest in red cabbage and lowest in mungbean. Wang et al. [
41] also reported the increased activities of antioxidant enzymes in microgreens. Several conditions, like seed germination, biotic and abiotic stresses may cause the occurrence of oxidative stresses in plants by increasing the production and accumulation of ROS. The production and accumulation of H
2O
2 in plants can be controlled or reduced by the plant’s antioxidants and antioxidant enzymes system. Whereas under severe stresses, ROS are overproduced and cause damage to cellular machinery [
41]. Hydrogen peroxide accumulation was determined in all the microgreens samples and the highest accumulation was in mungbean, whereas the lowest accumulation was in red radish (
Table 8).
3.4. Sensory Evaluation of Microgreens
On sensory evaluation, firstly, the visual quality and olfactive results were recorded (
Table 9). Both visual quality and olfactive depend upon the different enzymatic and metabolic activities that occurred in the selected microgreens. Mustard microgreen was the first to undergo deterioration, and its visual quality showed some changes after four days. However, the other microgreens retained their visual quality after four days. After six days, the olfactive properties of pearl millet, mustard, and red radish were still optimal, while all other sensory scores pointed out the presence of visual and olfactive defects. However, the sensory quality was still satisfactory. After ten days, all the selected microgreens samples differed substantially. Red cabbage had the lowest overall score for both visual and olfactory quality, whereas pearl millet received the highest overall score for both.
Among all, mungbean and lentil microgreens showed only moderately objectionable defects and were still above the marketability threshold. Broccoli microgreens were used for a sensory shelf-life extension of up to 21 days by other authors using some salts preharvest treatments [
42,
43,
44]. According to Kou et al. [
45], broccoli microgreens washed with chlorine continued to taste good even after 11 days, whereas post-harvest treated microgreens showed a shorter sensory shelf-life. According to Chandra et al. [
46], chlorine-treated fresh-cut Tah Tasai cabbage microgreens had off-odors that were tolerable for seven days. Some authors also observed the increased shelf-life of microgreens treated with chemicals other than chlorine. Xiao et al. [
32] reported the sensory acceptability of radish microgreens from 8 days to 16 days by using different conditions with different rates of respiration. In a sensory evaluation test of 12 microgreen species in young adult participants, Caracciolo et al. [
29] found that the total acceptability was significantly determined by sensory qualities (e.g., aroma, bitterness, astringency, grassy, heat, and sourness).