Abstract
The present study aimed to alleviate the negative effects of the peripartum and postpartum periods on the timing of ovarian follicle development, milk composition, as well as blood and metabolic profiles due to Nigella sativa (N. sativa) supplementation. Twenty-seven pregnant Ardi goats were classified using a randomized complete design into three groups: a control group and two N. sativa groups (10.0 and 20.0 g N. sativa seeds per kg diet). Productive and reproductive performances, in addition to blood and metabolic profiles, were investigated and compared using Duncan’s multiple test. N. sativa supplementation increased dry matter intake and body weight. Ruminal pH and total bacterial counts were increased versus a decreased total protozoal count due to N. sativa inclusion. Additionally, N. sativa supplementation increased the concentration of protein, lactose, solids not fat, fat, and ash in milk. Pulse rates were the lowest (p < 0.05) in the N. sativa group and the partial pressure of oxygen was the lowest in the control group. Red and white blood cells and their related parameters (hemoglobin, hematocrit, neutrophils, and lymphocytes) showed significant increases due to N. sativa inclusion. Total protein, albumin, globulin, glucose, and minerals (calcium, phosphorus, and magnesium) values were higher (p < 0.05) in the N. sativa group. Lower concentrations of blood urea nitrogen were found in N. sativa groups compared to control one. In conclusion, N. sativa inclusion from 4 weeks prepartum to 4 weeks postpartum of Ardi goats modified productive and reproductive performances without any adverse effects on blood and metabolic profiles.
1. Introduction
The peripartum or transition period is the most critical for the productive and reproductive performances of mammalian pregnant species and their resulting offspring. It extends from 3 weeks prepartum to 3 weeks postpartum in small ruminants [1]. During this period, disruptions were observed in ovarian follicle development and the quality of the resulting oocytes and embryos, feed utilization and growth performance, milk production and composition, and blood metabolites [2,3,4,5,6,7,8]. The decrease in feed intake can reach 30–35%, especially during the summer season in subtropics where temperatures exceed 45.0 °C during the day. The decrease in feed intake or the lack of management resulted in severe deficiencies of productive and reproductive performances under such circumstances [2,5,6,9]. Therefore, the restoration of disruptions during the prepartum and postpartum periods might improve reproductive and productive performances of pregnant species.
Several approaches can be used to mitigate the disruptive effects of prepartum and postpartum periods in pregnant animals, such as diet composition and supplementation [2,4,5,6,7]. Because of the negative effects on animals’ health and the fertility of increased protein in the diet [10,11], it is important to find beneficial and non-traditional supplements for improving productive and reproductive performances [12]. There is an increased interest in using N. sativa as a diet supplement for ruminants and humans [4,13,14,15,16,17,18].
N. sativa or black seeds are obtained from an annual plant cultivated in Asia and the Middle East. The N. sativa seeds contain protein (20.0–27.0%), carbohydrates (23.50–33.20%), fat (34.5–38.7%), crude fiber (8.4%), and ash (4.8%), in addition to vitamins, minerals, and carotene [4]. In addition, the N. sativa seeds contain active materials known as thymohydroquinone, thymoquinome, and nogelleone, which are known to give antitoxic, antimicrobial, and pharmacological properties that improve the defense system [4]. The N. sativa seed and their purified constituents have been widely used in the treatment of different diseases. Because N. sativa plays an important role as a natural antioxidant and immune stimulant [4,13], it might be used for anti-stress during the elevation of ambient temperature and humidity.
Several studies have been designed using different animal species to explore the effect of N. sativa on growth and reproductive performance [19,20,21]. The supplementation of animals with black seeds or black seed oil resulted in the improvement of growth performance and milk production [22], blood profiles, plasma metabolites [20,23], and reproductive performance [21,24,25]. There is inadequate information on the effects of N. sativa on ovarian follicle development, growth and feed utilization, as well as the biochemical profiles of Ardi goats during the peripartum period in subtropics. Therefore, our hypothesis is that the supplementation of N. sativa seeds would alleviate the negative effects of the peripartum and postpartum periods through improving feed utilization, blood and metabolic profiles, ovarian follicles’ development, and milk composition.
2. Materials and Methods
2.1. Animals’ Diets, Management, and Experimental Management
This study was carried out in the Research and Training Station of King Faisal University and approved by the scientific research ethical committee (Ref. No. KFU-REC-2022-JAN-EA000130). Twenty-one healthy pregnant Ardi goats of 101.66 ± 0.4 kg body weight, aged 2.0–2.5 years, were allocated using complete random design to three groups: a control group and two N. sativa groups (10 and 20 g/kg diet). The goats were given the routine vaccination of the farm station. The goats of each group during the experimental period were kept free in pens at a stocking rate of 2.00 m2/head, and they were fed individually. The average ambient temperature ranged between 34.0 to 45.0 °C, and the relative humidity ranged between 29.5 and 40.0%. The goats were offered daily 2.0 kg concentrate diet for the control group (Table 1) and concentrate diet supplemented with the recommended doses of 10.0 and 20.0 g N. sativa seeds/kg diet per head in addition to ad libitum berseem hay [4].

Table 1.
Chemical composition of experimental diets on dry matter basis (%).
The goats were randomly assigned into three groups: two control groups and 1.0% and 2.0% N. sativa groups. The periods for the study included approximately 4 weeks prepartum and continued to 4 weeks postpartum with N. sativa supplementation. The doses of N. sativa seeds were mixed with the concentrate diet and given daily at 08:00 a.m. to each goat. The diet was prepared according to the guidelines from the National Research Council for goats to meet the requirements [26]. Fresh water was available ad libitum. The recorded feed intake was calculated through the difference between the daily offered diet and its respective ort. The diet and ort samples were collected daily, transformed into composites, and stored for chemical analysis at the end of the experiment. The body weight gain (kg) of the goats was recorded 4 weeks prepartum and 4 weeks postpartum.
2.2. Collection of Ruminal Fluid
A stomach tube with sufficient diameter and length was used to collect ruminal fluid from the control and N. sativa (1.0 and 2.0 g/kg diet) animal groups [27]. The tube was inserted into the rumen to suck out the ruminal fluid. The tube was inserted into the mouth to the pharynx and enabled the animal to swallow the tube. The ruminal samples were aspirated 4 h after morning feeding. The ruminal samples were filtered through two layers of cheesecloth to count the protozoal [28] and bacterial numbers [29]. The filtrate portion was immediately used for pH measurement using a digital pH meter (Eacam, China) [30].
2.3. Rectal Body Temperature (RT), Heart Rate (HR), and Partial Pressure of Oxygen (SPO2)
The physiological parameters (RT, HR, and SPO2) of N. sativa (1.0 and 2.0 g/kg diet) and control groups were recorded biweekly. Rectal body temperature was recorded using a digital thermometer (Citizen Flex Digital Thermometer CTA303, Citizen, Stuttgart, Germany). The partial pressure of oxygen (PO2) and pulse rate were recorded using a pulse oximetry apparatus (CMS60D-VET, Contec Medical Systems Co., Ltd., Qinhuangdao (Hebei), China). The goats were kept in a pen, restrained, and the heart rate and PO2 were recorded by the proper sensor that was put on the upper lip of the goat [6].
2.4. Ovarian Follicle Development
Goats were investigated postpartum by a real-time B-mode ultrasound scanner (ContecTM B-Ultrasound Diagnostic System Model CMS 600 P2VET, Qinhuangdao (Hebei), China) with a 3.5 MHz transducer (C3.5-80R20-A16A IPX7). Ovaries of N. sativa and control goats were examined at days 3, 6, 9, 12, 15, and 18 postpartum to record the numbers and sizes of ovarian follicles. The ovarian follicles were categorized according to diameter into small—(diameter 2–2.9 mm), medium—(diameter 3–4.9 mm), and large-sized (diameter ≥ 5 mm) follicles [6].
2.5. Milk Chemical Analyses
One hundred milliliters of milk (three samples) were collected through hand milking from the control and N. sativa (1.0% and 2.0%) animal groups weekly (weeks 2, 3, and 4 postpartum) in flasks for the chemical analysis of protein, lactose, solids not fat, fat, and ash (MilkoScan™ Mars, Hilleroed, Denmark). Milk energy was calculated as described by Economides [31].
2.6. Blood Samples’ Collection and Analysis
Blood samples were collected biweekly through jugular vein puncture from each goat of N. sativa (10.0 and 20 g/kg diet) and control groups in a sterile tube containing anticoagulant (EDTA K3 Australia). The collected blood samples were analyzed for hematological profiles using a hematology analyzer (Abaxis Vetscan HM5, Union City, CA, United States) and biochemistry parameters using a chemistry analyzer (Skyla VB1, Hsinchu, Taiwan). The measured hematological parameters included red blood cells, hematocrit, and hemoglobin values, in addition to white blood cells and their differentiation and platelets. The measured biochemistry plasma parameters included total protein, glucose, urea, liver functions, and mineral values.
2.7. Statistical Analysis
The statistical analysis of variances was conducted through the general linear model of the Proc Mixed SAS package version 9.2 [32]. Differences between N. sativa (1.0 and 2.0 g/kg diet) and control groups were tested for body weight, rumen parameters, milk traits, ovarian follicle development, as well as blood and metabolic profiles by one-way ANOVA. A comparison between the means of N. sativa and control groups and the level of significance (p < 0.05) was set using Duncan’s test [33]. The statistical model was Yij = μ + Ti + Eij, where Yij = the observation ij, μ = the overall mean, Ti = the effect due to N. sativa supplementation (10.0 and 20 g/kg diet), and Eij = the experimental error.
3. Results
3.1. Feed Intake, Body Weight, Ruminal Parameters, and Physiological Parameters
Feed intake, body weight, ruminal traits, and physiological parameters are shown in Table 2. Treatments with N. sativa (10.0 and 20 g/kg diet) resulted in higher (p > 0.05) dry matter intake and body weight (kg) 4 weeks postpartum. Regarding ruminal parameters, ruminal pH and total bacterial count increased, whereas the total protozoal count decreased in N. sativa groups compared to the control group. The highest values were recorded in the 2.0% N. sativa group followed by 1.0% N. sativa and control groups, respectively. The body temperature and partial pressure of oxygen were insignificantly increased in the 2.0% N. sativa when compared to 1.0% N. sativa and control groups. The lowest pulse rate (beats/min) was recorded in 2.0% N. sativa group (p < 0.05) compared to other groups.

Table 2.
Effects of N. sativa (10.0 and 20 g/kg diet) on body weight, ruminal, and physiological parameters of Ardi goats in subtropics.
3.2. Ovarian Follicle Development and Milk Composition
The results of small, medium, and large ovarian follicles’ development recorded 3, 6, 9, 12, 15, and 18 days postpartum indicated earlier ovarian follicle resumption in N. sativa groups compared to the control group (Figure 1). The numbers of small, medium, and large follicles were higher (p < 0.05) in the N. sativa groups during the postpartum periods (Figure 1A–C). N. sativa effects (10.0 and 20 g/kg diet) on the milk composition of Ardi goats after kidding are presented in Table 3. The N. sativa (10.0 and 20 g/kg diet) supplementation increased (p < 0.05) solids not fat, protein, lactose, and ash compared to the control group.
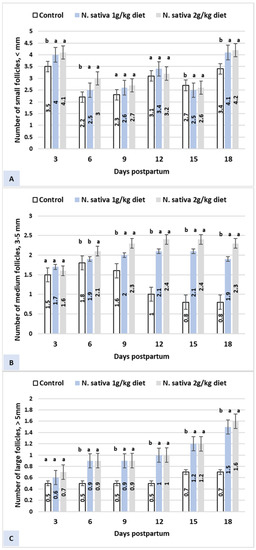
Figure 1.
Effects of N. sativa (10.0 and 20 g/kg diet) on ovarian follicle development postpartum of Ardi goats. a, b Values with different superscripts between groups significantly differ at p < 0.05. (A) Number of small follicles < 3 mm; (B) Number of medium follicles 3–5 mm; (C) Number of large follicles > 5 mm.

Table 3.
Effects of N. sativa (10.0 and 20 g/kg diet) on milk constituents of Ardi goats in subtropics.
3.3. Hematological and Biochemistry Profiles
Hematological indices are presented in Table 4. Hematological indices showed a significant increase in the values of RBCs (106/μL) and Hb (g/dL), in addition to WBCs (103/μL), lymphocytes, and neutrophils (103/μL) in N. sativa groups (10.0 and 20 g/kg diet), when compared to the control one. The highest values were observed in 2.0% N. sativa group when compared to 1.0% N. sativa and control groups, respectively. The blood biochemistry indices of N. sativa (10.0 and 20 g/kg diet) and control groups are presented in Table 5. The results indicated the highest values (p < 0.05) of total protein (g/dL), albumin (g/dL), globulin (g/dL), and glucose (mg/dL) recorded in the 2.0% N. sativa group when compared to the other groups. Urea nitrogen (p < 0.05) and liver enzymes (p > 0.05) were lowered due to 2.0% N. sativa supplementation when compared to 1.0% N. sativa and control feeding, respectively. Furthermore, the mineral concentrations (calcium, phosphorus, and magnesium) were improved (p < 0.05) due to N. sativa supplementation when compared to the control one.

Table 4.
Effects of N. sativa (10.0 and 20 g/kg diet) on blood indices of Ardi goats in the subtropics.

Table 5.
Effects of N. sativa (10.0 and 20 g/kg diet) on blood biochemistry of Ardi goats in subtropics.
4. Discussion
The beneficial effects of feed additives, especially N. sativa seeds on feed utilization, metabolic conditions, and reproductive functions, have been reported in several studies [13,21,34,35,36]. Our present study was designed to restore the negative effects of the peripartum period on feed intake and rumen parameters, milk traits, postpartum ovarian follicle development, and the related blood and plasma metabolites of Ardi goats in the Eastern subtropical area of KSA. The significant effects of N. sativa seeds and extract on body health status, as well as productive and reproductive performances, were confirmed earlier in several studies [13,21,25]. In the current study, the improvement in body health, as well as productive and reproductive performances, was the highest in the N. sativa (20 g/kg diet) group when compared with the N. sativa (10.0 g/kg diet) and control groups.
4.1. Body Weight, Ruminal, and Physiological Parameters
Body weight gain was improved in N. sativa groups (10.0 and 20 g/kg diet) when compared to the control group, as indicated in previous studies [12,13,16,19]. This might be attributed to increased feed intake, in addition to significant changes in rumen microbes, including increased bacteria and decreased protozoa values (Table 2), which might improve digestibility coefficients. It was found that N. sativa inclusion (12 g/day) significantly improved the digestibility coefficients of dry matter, organic matter, crude protein, and crude fiber [12]. Collectively, earlier reports concluded that N. sativa seeds or their extract have positive effects on body weight gain and nutrient digestibility [12,13,14,15,16,19]. The stimulation of appetite and increased peristaltic action of the stomach and bowels have been recorded due to N. sativa actions [37,38].
Ruminal pH and total bacterial counts increased, while total protozoal counts decreased in N. sativa groups (10.0 and 20 g/kg diet) when compared to the control group (Table 2). The changes in the rumen environment due to the 10.0 and 20 g/kg diet N. sativa treatments might be favorable for bacterial species growth [12,39]. It is reported that nitrogen retention and ruminal ammonia nitrogen values were improved (p < 0.05) due to N. sativa supplementation [12]. In addition, the pulse rate decreased in N. sativa groups, as previously indicated [40], and this might be consequently useful in the treatment of hypertension [41]. The higher partial pressure of oxygen (PO2) in N. sativa groups could be attributed to the increase in RBCs in those groups compared to control one (Table 4) [42].
4.2. Ovarian Follicle Development and Milk Composition
Feed additives must be safe for the health and well-being of pregnant and lactating goats to support their ovarian structures’ development, milk production, and milk quality [5,6,7,8,34]. N. sativa seed supplementation (10.0 and 20 g/kg diet) resulted in an increase in small, medium, and large ovarian follicles compared to the control diet. This might be attributed to the significant increase in glucose (p < 0.05) and the decrease in urea (p < 0.05) levels in N. sativa groups (Table 5). The positive energy balance in the N. sativa group might lead to an increase in insulin concentration and glucose uptake [43] (Nielsen and Ingvartsen, 2004). This change appears to stimulate the ovary and is associated with increased folliculogenesis. This explanation could be confirmed through our supplementation of N. sativa oil to female mice upon ovarian transplantation. Our results indicated an increased number of aspirated oocytes and quality from ovarian follicles [21]. Furthermore, the decreased level of blood urea nitrogen is associated with increased fertility [10,11]. The other metabolic factors attributed to the beneficial effects of N. sativa seeds and their extracts on ovarian follicle development and reproductive performance include essential amino and fatty acids and reproductive hormone values [4,11,13,21,24,25,36,44,45,46,47,48,49]. The improvement in metabolic factors was due to the effects of N. sativa seeds on the digestive system, including nutrient digestibility, better absorption, and body weight gain [13,19,20,45], as indicated in this study through higher total protein and body weight gain.
The N. sativa inclusion in the diets (10.0 and 20 g/kg diet) significantly improved milk constituents. Earlier studies coinciding with our study indicated the significant effects of N. sativa on improving milk production and composition [13,16]. The main factors of N. sativa that are involved in milk traits’ improvement are the improvement in nutrient digestibility and blood metabolic profiles [12,16,50] (Table 3 and Table 4), which lead to the availability of nutrients required for milk secretion. Additionally, N. sativa contains several nutrients, which might be attributed to the observed increase in milk production and composition [13,14,15].
4.3. Hematological and Biochemistry Indices
Of note, in the current experiment, the changes in not only the blood indices were obtained by the supplementation of N. sativa to pregnant or lactating goats’ feed, but also in the blood plasma composition. Hematological profiles (RBCs, Hb, and PCV) showed significant beneficial changes between the N. sativa and control groups, as previously indicated [13,21,51]. Blood profiles are indicative of the body’s health status. The immune stimulation of N. sativa seeds recorded in earlier reports [52], in addition to antioxidant activity [53,54,55,56], plays a crucial role in the protection of the body against inflammation or infection. Therefore, the improvement in blood profiles in N. sativa groups (10.0 and 20 g/kg diet) compared to the control might be attributed to the increase in feed conversion and body weight gain [4].
Metabolic profiles were improved in N. sativa groups if compared to control one. The increased activity of hepatic function is suggested when N. sativa seeds were fed [16], which resulted in a higher concentration of total proteins as recorded in the present study. Furthermore, supplementation with N. sativa seeds enhanced glucose concentration as a result of improved nutrient digestibility and greater total volatile fatty acids production [16]. Propionate is considered as the primary gluconeogenic volatile fatty acid used for glucose biosynthesis [57]. The present data of lower blood urea nitrogen and creatinine in N. sativa-treated groups were the same as previous reports [13,16]. Therefore, it can be assumed that N. sativa supplementation might improve the protein balance in goats.
Measurements of AST, GGT, and ALP hepatic enzymes are considered reliable indicators of liver function in ruminant animals [58,59], and the liver AST enzyme values were lowered (p < 0.05) due to N. sativa inclusion in the diet of goats. Moreover, CK, which has been used as a screening diagnostic parameter for endometritis muscular damage or hypocalcemia in dairy cattle [60], was unchanged due to N. sativa supplementation. There were lower values (p > 0.05) of liver enzymes with feeding N. sativa seeds to calves and goats [20], indicating their probable protective roles against liver dysfunction [61,62,63] or renal tissue damage [64].
The calcium, phosphorus, magnesium, and potassium values were increased in N. sativa groups when compared to control one, as indicated in several previous studies [13]. Calcium, phosphorus, and magnesium minerals in the blood provide an indication of the animals’ health, and they are important for animals’ production [65]. Herein, the aforementioned mineral values were in the normal range and reflected the adequacy of minerals of in the N. sativa and control diets [66]. The increase in aforementioned minerals in N. sativa groups could be attributed to their presence in N. sativa seeds [4]. Calcium, phosphorus, and magnesium minerals are essential elements for muscle contraction, skeletal building, the production of energy, and anti-viral and anti-inflammatory agents [67,68]. Collectively, N. sativa seed components lead to a significant improvement in the functions of the gastrointestinal tract and liver, leading to an increase in body health, body weight gain, milk production, and ovarian structures’ development during gestation and lactation periods of Ardi goats in subtropics.
5. Conclusions
The supplementation of pregnant Ardi goats with N. sativa seeds (1.0 and 2.0 g/kg diet) during the peripartum period is an effective strategy for improving feed utilization, milk traits, and blood and metabolic profiles, in addition to higher ovarian follicle development in subtropics. Further studies may be designed to explore the effects of N. sativa bioactive compounds to give proof of their possible applications for treatments of metabolic dysfunctions.
Author Contributions
Conceptualization, A.E.-N.M. and S.A.-S.; methodology, A.E.-N.M. and S.A.-S.; software, S.A.-S.; validation, S.A.-S.; formal analysis, S.A.-S.; investigation, S.A.-S.; resources, A.E.-N.M.; data curation, S.A.-S.; writing—original draft preparation, A.E.-N.M.; writing—review and editing, A.E.-N.M. and S.A.-S.; visualization, S.A.-S.; supervision, A.E.-N.M.; project administration, A.E.-N.M.; funding acquisition, A.E.-N.M. and S.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding this research work [INST130].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets used in this research are available from the corresponding author upon reasonable request.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding this research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tharwat, M.; Ali, A.; Al-Sobayil, F. Hematological and biochemical profiles in goats during the transition period. Comp. Clin. Path. 2013, 24, 1–7. [Google Scholar] [CrossRef]
- Hussein, H.A.; Abdel-Raheem, S.M.; Abd-Allah, M.; Senosy, W. Effects of propylene glycol on the metabolic status and milk production of dairy buffaloes. Tierarztliche Prax. Ausg. G Grosstiere Nutztiere 2015, 43, 25–34. [Google Scholar] [PubMed]
- Bragança, G.M.; Souza-Fabjan, J.M.G.; Ribeiro, L.S.; Brair, V.L.; Brandão, F.Z. Exogenous progestogen does not affect first-wave follicle populations and oocyte quality during ovarian stimulation with FSH in sheep. Domest. Anim. Endocrinol. 2020, 72, 106369. [Google Scholar] [CrossRef]
- Ali, M.A.; Alshaheen, T.; Senosy, W.; Kassab, A.; Mohammed, A.A. Effects of feeding green microalgae and Nigella sativa on productive performance and metabolic profile of Boer goats during peripartum period in subtropics. Fresen. Environ. Bull. 2021, 30, 8203–8212. [Google Scholar]
- Al Mufarji, A.; Mohammed, A.A. Organic Moringa oleifera Leaves Chemical Composition and Fatty Acid Profiles and its Effect on Modulation of Blood and Plasma Parameters of Ewes in Subtropics. Adv. Anim. Vet. Sci. 2022, 10, 1227–1232. [Google Scholar] [CrossRef]
- Al Mufarji, A.; Mohammed, A.A.; Al-Zeidi, R.; Al-Masruri, H.; Mohammed, A. Effects of Moringa oleifera on follicular development, blood and metabolic profiles of subtropical ewes during peripartum. Adv. Anim. Vet. Sci. 2022, 10, 1706–1712. [Google Scholar] [CrossRef]
- Al Mufarji, A.; Mohammed, A.A.; Al Zeidi, R.; Al Masruri, H. Modulation impacts of Moringa oleifera on thermo tolerance parameters and blood indices in subtropical ewes under heat stress. Adv. Anim. Vet. Sci. 2022, 10, 1641–1648. [Google Scholar] [CrossRef]
- Al-Mufarji, A.; Mohammed, A.A.; Al-Masruri, H.; Al-Zeidi, R. Effects of dietary microalgae supplementation on mammals’ production and health. Adv. Anim. Vet. Sci. 2022, 10, 1718–1724. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, T.G.; Robinson, J.J.; Aitken, R.P.; Findlay, P.A.; Robertson, I.S. Dietary excesses of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Anim. Reprod. Sci. 1997, 47, 71–90. [Google Scholar] [CrossRef]
- Dawuda, P.M.; Scaramuzzi, R.J.; Leese, H.J.; Hall, C.J.; Peters, A.R.; Drew, S.B.; Wathes, D.C. Effect of timing of urea on the yield and quality of embryos in lactating dairy cows. Theriogenology 2002, 58, 1443–1455. [Google Scholar] [CrossRef]
- Cherif, M.; Ben Salem, H.; Abidi, S. Effect of the addition of Nigella sativa seeds to low or high concentrate diets on intake, digestion, blood metabolites, growth and carcass. traits of Barbarine lamb. Small Rum. Res. 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Al-Suwaiegh, S.B. Effects of Nigella sativa on Mammals’ Health and Production. Adv. Anim. Vet. Sci. 2016, 4, 630–636. [Google Scholar]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J. Agric. Sci. 2017, 155, 494–507. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Matloup, O.H.; Anele, U.Y.; Mohamed, A.G.; El-Sayed, A.B. Dietary Chlorella vulgaris microalgae improves feed utilization, milk production and concentrations of conjugated linoleic acids in the milk of Damascus goats. J. Agric. Sci. 2017, 155, 508–518. [Google Scholar] [CrossRef]
- Kholif, A.E.; Hamdon, H.E.; Kassab, A.Y.; Farahat, E.S.A.; Azzaz, H.H.; Matloup, O.H.; Mohamed, A.G.; Anele, U.Y. Chlorella vulgaris microalgae and/or copper supplementation enhanced feed intake, nutrient digestibility, ruminal fermentation, blood metabolites and lactational performance of Boer goat. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.S.; Kholif, A.E.; Kholif, A.M.; Salama, R.; El-Alamy, H.A.; Olafadehan, O.A. Sunflower oil and Nannochloropsis oculata microalgae as sources of unsaturated fatty acids for mitiga tion of methane production and enhancing diets’ nutritive value. J. Agric. Food Chemist. 2018, 66, 1751–1759. [Google Scholar] [CrossRef]
- Lamminen, M.; Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Vanhatalo, A. Comparison of microalgae and rapeseed meal as supplementary protein in the grass silage based nutrition of dairy cows. Anim. Feed Sci. Technol. 2017, 234, 295. [Google Scholar] [CrossRef]
- Abd El-Rahman, H.H.; Abedo, A.A.; Salman, F.M.; Mohamed, M.I.; Shoukry, M.M. Partial substitution of cumin seed meal by Jatropha meal as a potential protein source for feed. Afr. J. Biotechnol. 2011, 10, 15456–15461. [Google Scholar] [CrossRef]
- Khattab, H.M.; El -Basiony, A.Z.; Hamdy, S.M.; Marwan, A.A. Immune response and productive performance of dairy buffaloes and their offspring supplemented with black seed oil. Iran. J. Applied Anim. Sci. 2011, 1, 227–234. [Google Scholar]
- Mohammed, A.A. Nigella sativa oil improves physiological parameters, oocyte quality after ovarian transplantation, and reproductive performance of female mice. Pak. J. Zool. 2019, 51, 2225–2231. [Google Scholar] [CrossRef]
- Odhaib, K.J.; Adeyemi, K.D.; Ahmed, M.A.; Jahromi, M.F.; Jusoh, S.; Samsudin, A.A.; Alimon, A.R.; Yaakub, H.; Sazili, A.Q. Influence of Nigella sativa seeds, Rosmarinus officinalis leaves and their combination on growth performance, immune response and rumen metabolism in Dorper lambs. Tropical Anim.Health Prod. 2018, 50, 1011–1023. [Google Scholar] [CrossRef]
- Zeweil, H.S.; Ahmed, M.H.; El-Adawy, M.M.; Zaki, B. Evaluation of substituting nigella seed meal as a source of protein for soybean meal in diets of New Zealand white rabbits. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008; pp. 863–868. [Google Scholar]
- Parhizkar, S.; Latiff, L.A.; Parsa, A. Effect of Nigella sativa on reproductive system in experimental menopause rat model. AJP 2016, 6, 95–103. [Google Scholar]
- Mohammed, A.A.; Farghaly, M.M. Effect of Nigella sativa seeds dietary supplementation on oocyte maturation and embryo development in mice. Egypt. J. Anim. Prod. 2018, 55, 195–201. [Google Scholar]
- NRC. National Research Council (US). Nutrient Requirements of Sheep, 6th ed.; The National Academy Press: Washington, DC, USA, 1985. [Google Scholar]
- Pass, M.A. A stomach tube for the collection of ruminal fluid from sheep. Aust. Vet. J. 1991, 68, 312. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Naga, M.A. Some Metabolic Studies on Rumen Microorganisms. Ph.D. Thesis, University of Alexandria, Alexandria, Egypt, 1976. [Google Scholar]
- Newbold, C.J.; Wallace, R.J.; Chen, X.B.; Mclntosh, F.M. Different strains of Saccharomyces cerevisiae differ in their effects on ruminal bacterial numbers in vitro and in sheep. J. Anim. Sci. 1995, 73, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.J. Microdefusion Analysis and Volumetric Error, 5th ed.; Crosby- Lockwood and Sons Ltd.: London, UK, 1962. [Google Scholar]
- Economides, S. Comparative studies of sheep and goats milk yield and composition and growth rate of lambs and kids. J. Agric. Sci. 1986, 106, 477–484. [Google Scholar] [CrossRef]
- SAS. SAS User’s Guide: Basics; Statistical Analysis System Institute, Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Duncan, D.B. Multiple range and multiple Ftest. Biometrics 1955, 11, 1. [Google Scholar] [CrossRef]
- Al Masruri, H.; Al Zeidi, R.; Al Mufarji, A.; Mohammed, A.A.; Al Madani, A.; Mohammed, H. Leverage of Moringa oleifera supplementation on performances, biochemical, and milk profiles in mammals. Adv. Anim. Vet. Sci. 2022, 10, 2043–2050. [Google Scholar] [CrossRef]
- Al Masruri, H.; Al Zeidi, R.; Al Mufarji, A.; Mohammed, A.A. Moringa oleifera leaves supplementation to anaesthetized mice associated with changes of thermo-tolerance parameters, blood and plasma indices. Fresen. Environ. Bull. 2022, 8, 8179–8187. [Google Scholar]
- Mohammed, A.A.; Al-Hizab, F.; Al-Suwaiegh, S.; Alshaheen, T.; Kassab, A.; Hamdon, H.; Waleed Senosy, W. Effects of propylene glycol on ovarian restoration, reproductive performance, metabolic status and milk production of Farafra ewes in subtropics. Fresen. Environ. Bull. 2021, 30, 8192–8202. [Google Scholar]
- Inamul, H. Safety of medicinal plants. Pak. J. Med. Res. 2004, 43, 1–8. [Google Scholar]
- DerMarderosian, A.; Liberti, L.; Beutler, J.A.; Grauds, C.; Tatro, D.S.; Cirigliano, M.; DeSilva, D., Jr. Alfalfa. Review of Natural Products: Facts and Comparisons, Wolters Kluwer Health, 8th ed.; Lippincott Williams & Wilkins: Alphen aan den Rijn, The Netherlands, 2014. [Google Scholar]
- Febrina, D.; Haq, M.S.; Nurfitriani, R.A.; Barkah, N.N.; Sholikin, M.M.; Qomariyah, N.; Jayanegara, A.; Solfaine, R.; Irawan, A. Effect of dietary black cumin seed (Nigella sativa) on performance, immune status, and serum metabolites of small ruminants: A meta-analysis. Small Rum. Res. 2021, 204, 106521. [Google Scholar]
- Demir, H.; Kanter, M.; Coskun, O.; Uz, Y.H.; Koc, A.; Yildiz, A. Effect of black cumin (Nigella sativa) on heart rate, some hematological values, and pancreatic beta-cell damage in cadmium- treated rats. Biol. Trace Elem. Res. 2006, 110, 151–162. [Google Scholar] [CrossRef]
- Dehkordi, F.R.; Kamkhah, A.F. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam. Clin. Pharmacol. 2008, 22, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Crystal, G.J.; Pagel, P.S. The physiology of oxygen transport by the cardiovascular system: Evolution of knowledge. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1142–1151. [Google Scholar] [CrossRef]
- Nielsen, N.I.; Ingvartsen, K.L. Propylene glycol for dairy cows: A review of the metabolism of propylene glycol and its effects on physiological parameters, feed intake, milk production and risk of ketosis. Anim. Feed Sci. Tech. 2004, 115, 191–213. [Google Scholar] [CrossRef]
- Dupont, J.; Scaramuzzi, R.J.; Reverchon, M. The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants. Animal 2014, 8, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.M. Evaluation of growth performance for growing Maghraby camel fed on un-conventional feed. Int. J. Agric. Biol. 2007, 9, 18–21. [Google Scholar]
- Takruri, H.R.H.; Dameh, M.A.F. Study of the nutritional value of black cumin seeds (Nigella sativa L.). J. Sci. Food Agric. 1998, 76, 404–410. [Google Scholar] [CrossRef]
- Senosy, W.; Kassab, A.Y.; Hamdon, H.A.; Mohammed, A.A. Influence of organic phosphorus on reproductive performance and metabolic profiles of anoestrous Farafra ewes in subtropics at the end of breeding season. Reprod. Domest. Anim. 2018, 53, 904–913. [Google Scholar] [CrossRef]
- Senosy, W.; Kassab, A.Y.; Mohammed, A.A. Effects of feeding green microalgae on ovarian activity, reproductive hormones and metabolic parameters of Boer goats in arid subtropics. Theriogenology 2017, 96, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Marine Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Vargas-Beltran, F.; Tena-Sempere, M.; González-Bulnes, A.; Macias-Cruz, U.; Veliz-Deras, F.G. Short-term beta-carotene-supplementation positively affects ovarian activity and serum insulin concentrations in a goat model. J. Endocrinol. Investig. 2013, 36, 185–189. [Google Scholar]
- Justine, T.E.; Oluwatosin, K.Y. Some biochemical and haematological effects of black seed (Nigella sativa) oil on Trypanosoma brucei infected rats. Afr. J. Biotechol. 2008, 7, 153–157. [Google Scholar]
- El-Far, A.H.; Bazh, E.K.; Moharam, M.S. Antioxidant and Antinematodal Effects of Nigella sativa and Zingiber officinale Supplementations in Ewes. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 222–227. [Google Scholar]
- Badary, O.A.; Taha, R.A.; Gamal-el-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003, 26, 87–98. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Bazzaz, B.S.F.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its constituent (thymoquinone): A review on antimicrobial effects. Iran. J. Basic Med. Sci. 2014, 17, 929–938. [Google Scholar] [PubMed]
- Guler, T.; Ertas, O.N.; Kizil, M.; Dalkilic, B.; Ciftci, M. Effect of dietary supplemental black cumin seeds on antioxidant activity in broilers. Med. Wet. 2007, 63, 1060–1063. [Google Scholar]
- Ratz-Lyko, A.; Herman, A.; Arct, J.; Pytkowska, K. Evaluation of antioxidant and antimicrobial activities of Oenothera biennis, Borago officinalis and Nigella sativa seed cake extracts. Food Sci. Biotechnol. 2014, 23, 1029–1036. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Varvikko, T.; Huhtanen, P. Effects of various glucogenic sources on production and metabolic responses of dairy cows fed grass silage-based diets. J. Dairy Sci. 2003, 86, 3249–3259. [Google Scholar] [CrossRef]
- Liu, P.; He, B.; Yang, X.; Hou, X.; Han, J.; Han, Y.; Nie, P.; Deng, H.; Du, X. Bioactivity evaluation of certain hepatic enzymes in blood plasma and milk of Holstein Cows. Pak. Vet. J. 2012, 32, 601–604. [Google Scholar]
- Noro, M.; Cid, T.P.; Wagemann, F.C.; Arnés, V.V.; Wittwer, M.F. Valoración diagnóstica de enzimas hepáticas en perfiles bioquímicos sanguíneos de vacas lecheras. Rev. MVZ Córdoba 2013, 18, 3474–3479. [Google Scholar] [CrossRef]
- Sattler, T.; Fürll, M. Creatine kinase and aspartate aminotransferase in cows as indicators for endometritis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 132–137. [Google Scholar] [CrossRef]
- Elitok, B.; Kabu, M.; Elitok, Ö. Evaluation of liver function tests in cows during periparturient period. F.Ü. Sağlık Bil. Dergisi. 2006, 20, 205–209. [Google Scholar]
- Burdock, G.A. Assessment of black cumin (Nigella sativa L.) as a food ingredient and putative therapeutic agent. Reg. Toxicol. Pharmacol. 2022, 128, 105088. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elkareem, M.; Soliman, M.; Abd El-Rahman, M.A.M.; Abou Khalil, N.S. The protective effect of Nigella sativa seeds against monosodium glutamate-induced hepatic dysfunction in rats. Toxicol. Rep. 2022, 9, 147–153. [Google Scholar] [CrossRef]
- Hikmah, Z.; Endaryanto, A.; Ugrasena, D.G.; Rahaju, A.S.; Arifin, S. Nigella sativa L. as immunomodulator and preventive effect on renal tissue damage of lupus mice induced by pristine. Heliyon 2022, 8, e09242. [Google Scholar] [CrossRef]
- Nozad, S.; Ramin, A.-G.; Moghadam, G.; Asri-Rezaei, S.; Babapour, A.; Ramin, S. Relationship between blood urea, protein, creatinine, triglycerides and macro-mineral concentrations with the quality and quantity of milk in dairy Holstein cows. Vet. Res. Forum 2012, 3, 55–59. [Google Scholar]
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef]
- Ignatov, I. Anti inflammatory and anti viral effects of potassium (K) and chemical composition of Moringa. Asian J. Biol. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Ignatov, I.; Popova, T. Applications of Moringa oleifera Lam., Urtica dioica L., Malva sylvestris L. and Plantago major L. Containing potassium for recovery. Molecules 2021, 33, 14. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).