Abstract
Purpose of the study: to substantiate the prospects of using various species, varieties and forms of the genus Robinia in protective afforestation and landscaping of settlements in dry-steppe and semi-desert zones of southern Russia. It is established that the main limiting factors affecting the growth, development and condition of representatives of the genus Robinia in the Volgograd region are winter temperatures up to −37 °C, as well as poor moisture availability and uneven distribution of precipitation during the growing season in combination with extremely low temperatures in the autumn–winter period. All representatives of the generic complex belong to the group of plants that start late and finish the vegetative period late. Phenological atypicity is in the lower half of the normal range, with indicators from +1 °C to 0, which indicates a high level of adaptation to the climatic conditions of the region. In the process of long-term acclimatization, many introduced species have developed a number of genotypic adaptations and are currently able to tolerate extreme winter temperatures up to −37 °C. An assessment of potential drought resistance based on the water-retaining ability of the leaves showed that higher rates of water-resistance capacity (76.8% water loss) are typical of R. neomexicana, which has a natural distribution area in the western arid part of the North American continent. R. viscosa var. hartwegii, with a natural distribution in the areas of the monsoon subtropical climate of eastern North America, is distinguished by low indicators (94.1% water loss), as are clonal decorative forms of R. pseudoacacia: f. pyramidalis and f. umbraculifera (97.6–95.8% water loss), which are common only in culture and characterized by a whole complex of low indicators of bioecological stability. The data obtained by us allow us to recommend the following assortment of species and forms of Robinia for protective afforestation and landscaping in the arid territories of southern Russia: R. pseudoacacia, R. neomexicana var. neomexicana, R. neomexicana var. rusbyi and R. pseudoacacia x R. neomexicana.
1. Introduction
The problems of preserving Russian forests, especially in the context of increasing changes in the global climate, have been attracting increasing attention both domestically and internationally in recent decades. Already occurring changes in air temperature, an increase in the number of extreme weather events, the risk of forest fires and the possibility of the beginning of active destruction of permafrost can have a catastrophic impact on Russian forests, which account for 21% of all forest plantations on the planet [1,2,3]. The severe natural conditions of the arid region of the European part of Russia cause an unsatisfactory state of protective plantings, of which there are currently about 500 thousand hectares. In this regard, the problem of increasing their stability and durability is very relevant. The way to solve the problem at the present stage is through the development and application of a complex of environmental, biological, technological and organizational measures. Their main components are the improvement of the assortment of trees and shrubs used for afforestation in arid conditions; differentiation of afforestation depending on the forest suitability of soils; organization of a seed base based on the selection of the most resistant species; use of different forms and hybrids of trees and shrubs; and improvement of the quality of the used planting material.
It is noteworthy that the effect of various factors of influence, especially meteorological factors, on the growth of forest plantations is not very clear. Therefore, an assessment of the factors affecting the growth of tree and shrub plantations, in particular the effects of climate change, is of great importance for overcoming global changes and expanding forest resources [4]. In the difficult conditions of the dry steppes and semi-deserts of the south-eastern European part of Russia, afforestation has become possible; currently, there are 170 thousand hectares of man-made forests in the Forest Fund of the Volgograd region. The Volgograd region belongs to a sparsely wooded region; it has the richest forestlands in the Lower Volga region, and in terms of forest area, 680.8 thousand hectares in the Southern Federal District is second only to the Krasnodar Territory [5]. The basis for the preservation and multiplication of forest plantations in the region is the reproduction of forests. If we take into account the new forest cover that has arisen on abandoned agricultural lands, but has thus far been ignored by the authorities, the area of forests in Russia is increasing. At the same time, negative changes in their species composition are noted. Due to fires, logging, exposure to diseases and pests, the areas of typical coniferous plantations (spruce, pine) are somewhat reduced, and these species are replaced by secondary species, namely birch and aspen.
In order to create sustainable, long-lasting landscape plantings in difficult forest-growing conditions of the Volgograd region, it is necessary to mobilize new promising species, varieties and forms of woody plants. The genus Robinia L. is of great practical and scientific interest in this regard. Currently, only one species has become widespread: Robinia pseudoacacia. The remaining representatives of the generic complex are used much less frequently, although an analysis of their bioecological and decorative features indicates that they are not only not inferior, but in fact superior to R. pseudoacacia in many respects. The selection and adaptation of representatives of the ancestral complex will provide new opportunities to increase the biodiversity, as well as the productivity, durability and the environmental role of protective strips and decorative landscaping tree plantations [6,7,8,9,10,11,12].
Robinia pseudoacacia is a well-known forest species native to eastern North America, from where it has been widely introduced worldwide [13]. The species was introduced to Europe in the early 17th century and currently has a significant distribution from the sub-Mediterranean to the warm continental climate [14,15]. Robinia pseudoacacia L. is widely distributed in protective forest stands in the south of Russia. Of scientific interest are plantings of the genus Robinia in the dry-steppe zone to combat soil degradation and desertification. These species can also be used as low-fertility crops, in protective belts and for decorative purposes with a high degree of drought resistance, as well as in forest restoration given their relatively easy degree of reproduction by seed. Moreover, Robinia species are valued as honey-bearing and nitrogen-fixing species, and their wood has several practical applications, including as poles, sleepers, timber, furniture, floor coverings, decorative items and various finishing materials. Robinia species are resistant to maximum high or low temperatures and to frequent fluctuations from negative to positive temperatures. The main disadvantage of Robinia pseudoacacia L. is low frost resistance [16,17].
The purpose of this study was to substantiate the prospects of using various species, forms and varieties of the genus Robinia in protective afforestation and landscaping of settlements in dry-steppe and semi-desert zones of southern Russia.
2. Materials and Methods
2.1. Plant Resources
Species, varieties and forms of the genus Robinia L., growing in cluster dendrological collections of the Federal Research Center of Agroecology of the Russian Academy of Sciences, were used in the landscaping of settlements in the Volgograd region and in protective forest plantations. The study was conducted from 2017 to 2022 (Table 1).

Table 1.
The same-age, introduced populations of representatives of the genus Robinia in the landscaping plantings of the Volgograd region.
To measure the taxation specifications of growing stock, same-age, introduced populations of representatives of the genus Robinia were determined.
The taxation specifications of growing stock were determined by the method of mass observations using arithmetic averages. The total number of individual trees studied was 1150.
A survey of the plantings for taxonomic identification was carried out by the route-expedition method. Landscaping and forest protection plantings in various districts of the Volgograd region and the city of Volgograd were subject to the survey. The nomenclature of registered species, varieties and forms of plants was verified according to the system proposed by Peabody [18].
2.2. Location of the Experimental Site and Conditions
The research objects are located in typical conditions for the region. The soils on the territory of the nursery of the Federal Research Center of Agroecology of the Russian Academy of Sciences are light chestnut, medium loamy and medium-thick, formed on a deluvial sand deposit lying at a depth of one meter. The content of mobile forms of nitrogen, phosphorus and potassium is typical for light chestnut soils. The depth of groundwater is 4.0–5.0 m. The humus content is 0.8–1.2%. The collection plantations of the Kirov forestry of the Volgograd Forestry are established on light chestnut, medium loamy soils with a humus content of 1.2–1.5%.
On the territory of the nursery of woody plants of the Lower Volga station for the selection of forest crops of the Federal Research Center of Agroecology of the Russian Academy of Sciences (Kamyshin), the soils are more or less homogeneous, dark chestnut, light loamy and slightly saline, with a humus content of 1.5–2.5%, N–0.22%, P2O5–0.17% and K2O–1.3%.
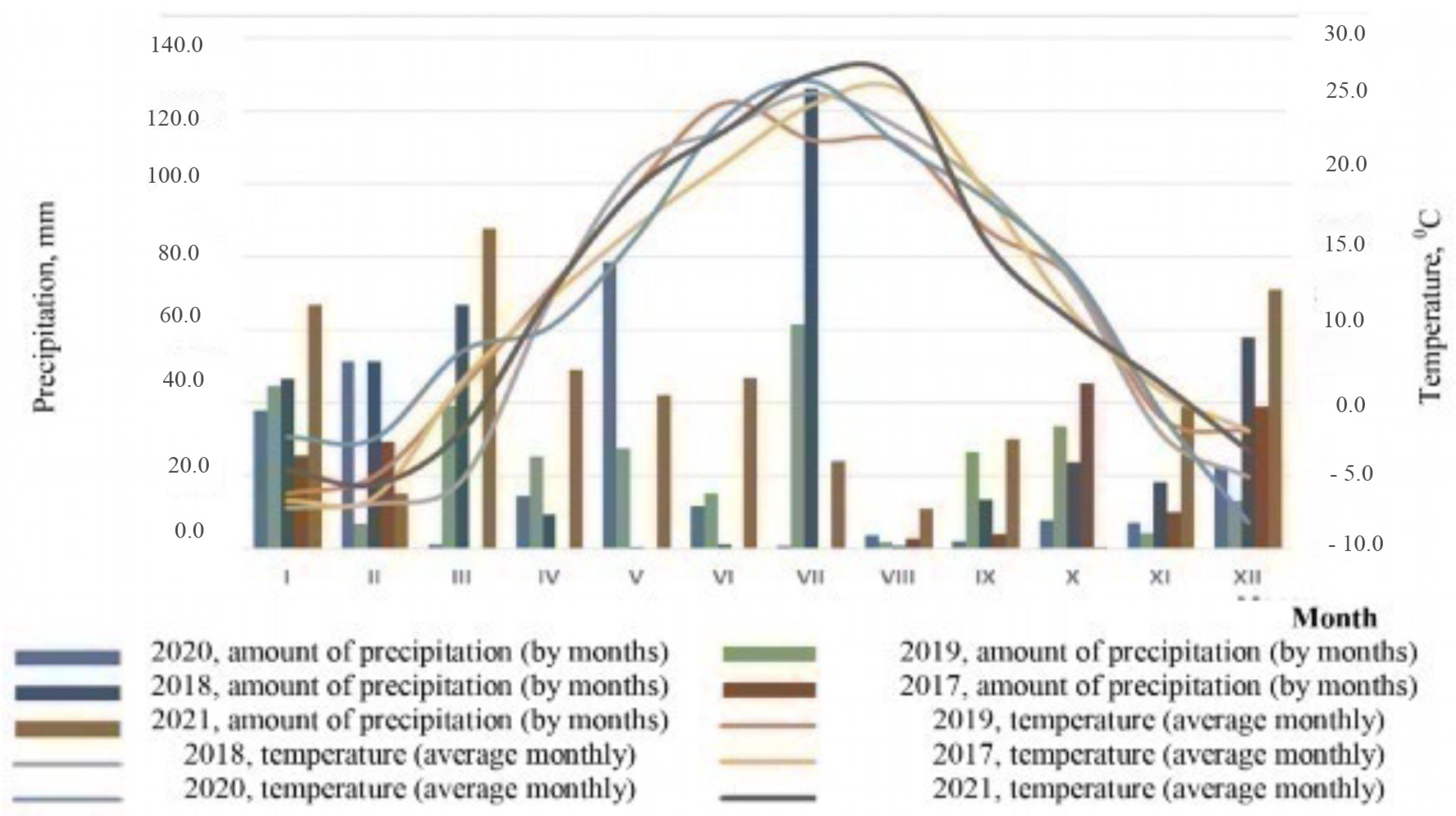
In general, the average annual temperatures during the research period ranged from 8.9 °C in 2018 to 9.9 °C in 2021 (Figure 1).
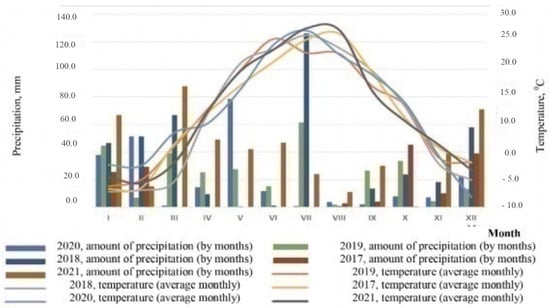
Figure 1.
Climatogram of the research period (according to the weather station of Volgograd).
These indicators are within two standard deviations (σ = 1.24) from the average annual temperature (8.3 °C) calculated from long-term data for the entire period of observations at the Volgograd weather station, and within one standard deviation (σ = 1.03) from the average annual temperature (8.9 °C) calculated from data for the last 30 years of meteorological observations. In both cases, deviations from the norm are observed towards an increase in average annual temperatures.
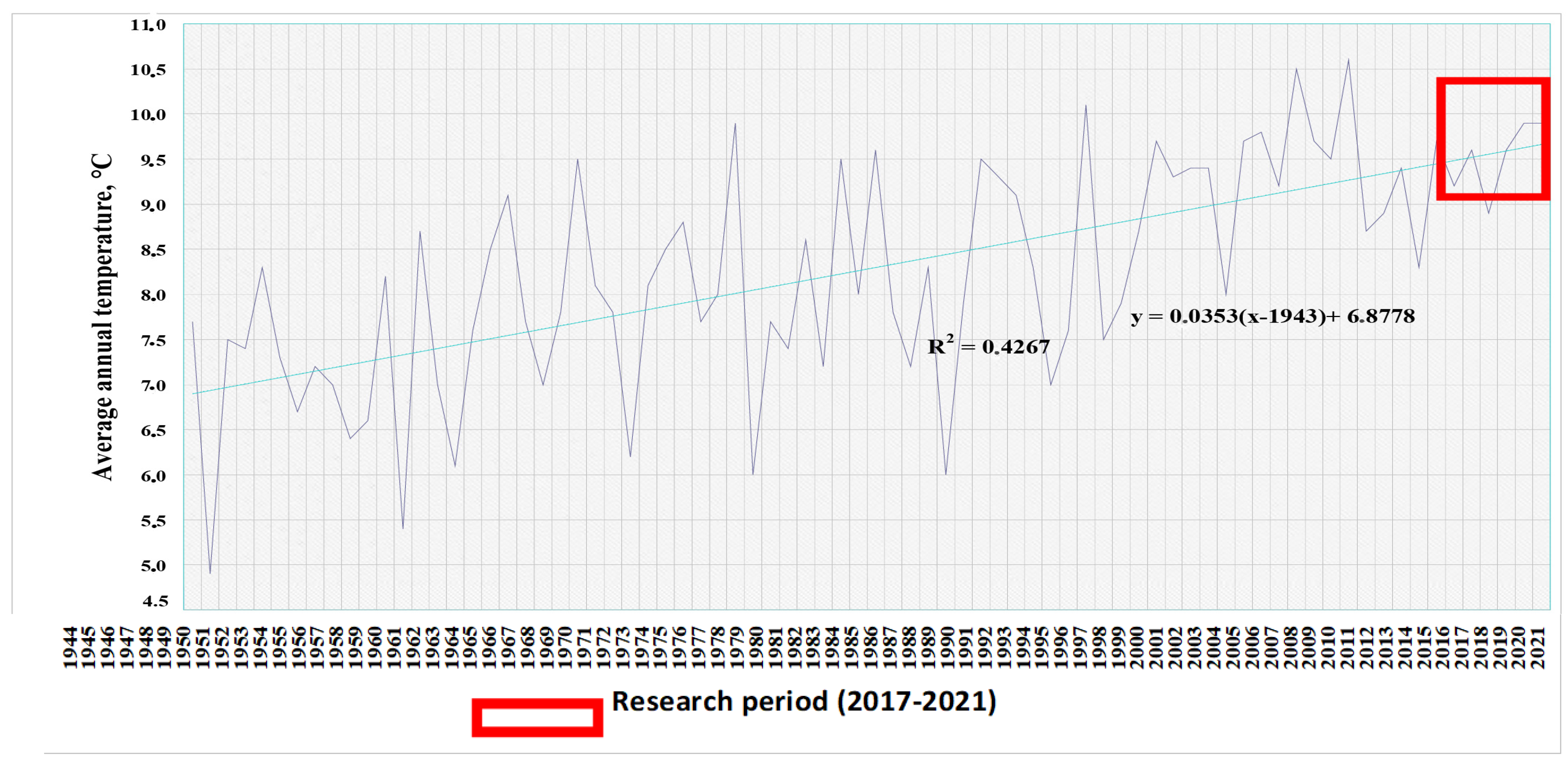
The graph constructed for the entire period of meteorological observations from 1944 to 2021 illustrates these processes well (Figure 2). The trend line of this chart is calculated using Equation (1):
where x is the analyzed year, and y is the predicted average annual temperature.
y = 0.0353 (x − 1943) + 6.8778
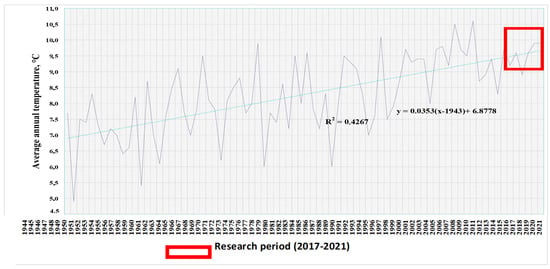
Figure 2.
Average annual temperatures according to the Volgograd weather station for 1944–2021. The red box highlights the research period (2017–2021).
In accordance with the general trends towards climate warming calculated over a 76-year period of meteorological observations, the climatic norm of average annual temperatures for the city of Volgograd was 9.49 °C in 2017 and 9.80 °C in 2021.
Calculations show that the average annual temperatures in 2017–2021 were close to the trend line and even fell below the average; consequently, the temperature regime of the research period can be characterized as typical for a region with a general tendency to increase average annual temperatures.
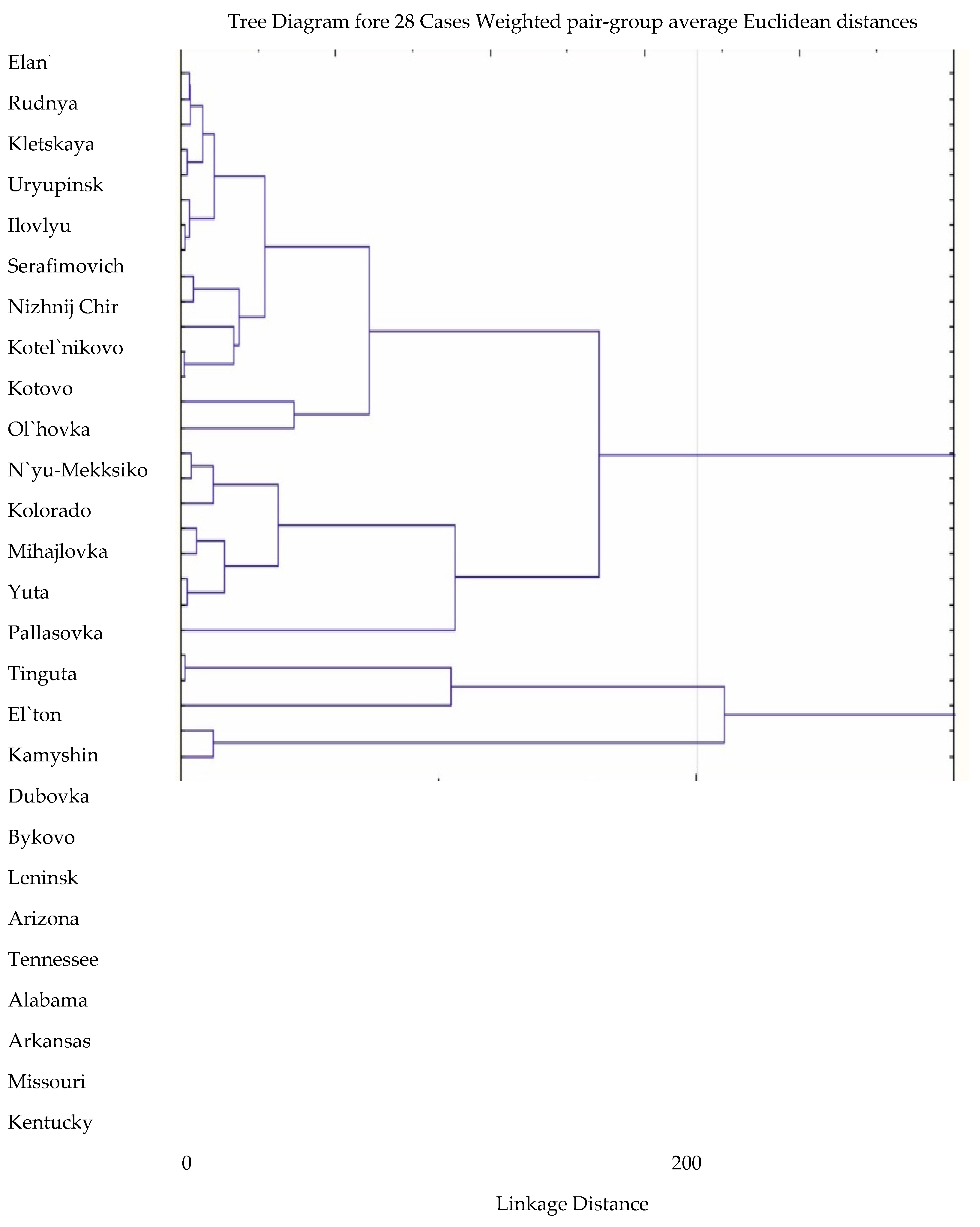
Agro-climatic conditions in various agroforestry and administrative districts of the Volgograd region have different degrees of similarity to natural conditions in the areas of natural distribution of species in North America. Cluster analysis based on the calculation of Euclidean distances made it possible to group points by similarity of climatic characteristics.
The comparison used meteorological indicators from US states with spatial distribution of certain species of the genus Robinia [19], with meteorological indicators of various districts of the Volgograd region [20]. The calculations took into account the average temperature of the coldest month, the average annual precipitation and the average annual temperature. The dendrogram of the similarity of climatic characteristics is shown in Figure 3.
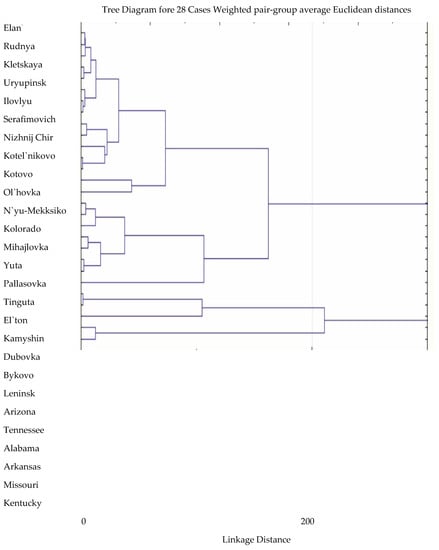
Figure 3.
Dendrogram of similarity of climatic indicators of natural and secondary distribution areas of species of the genus Robinia L.
2.3. Maintaining the Prototype, Records and Observations
The evaluation of the prospects of the studied species, varieties and forms was carried out according to the methodology of the All-Russian Research Institute of Agroforestry [21]. To analyze biological stability (winter hardiness, drought resistance, reproductive ability, vitality), we used data from our own observations, as well as data from long-term visual observations conducted on the basis of cluster dendrological collections of the Federal Research Center of Agroecology of the Russian Academy of Sciences [22]. The study of drought resistance was carried out according to visual observations in natural conditions, as well as by an indirect method for the water-retaining ability of leaves during the growing season (June–August), expressed as a percentage of the ratio of lost water to its primary content. The material for analysis (leaves from the annual increments of the middle part of the plant) was collected in the morning and quickly transported to the laboratory to determine the water retention capacity by the weight method according to Equation (2):
where B is the initial mass, mg; b is the weight after withering (after 1, 2, 3, 4, 5, 6, 24 h), mg; and A is the initial water content in the leaves, mg.
At the same time, the content of pigments in the leaves of trees was determined. To do this, 10 leaf samples were taken from each tree. To measure the content of chlorophyll, flavonoids, anthocyanins and the nitrogen balance index (NBI) in the epidermis of leaves of woody plants, a “Dualex Scientific+” device (“Force-A”, France) was used. The nitrogen balance index (NBI) shows the ratio of the amount of chlorophylls and flavonoids (nitrogen/carbon) and is presented in conventional units.
Laboratory analysis was performed in the Laboratory of Bioecology of Woody Plants of the Federal Research Center of Agroecology of the Russian Academy of Sciences. All samples were dried at the same temperature (20–23 °C), which was recorded by thermometers, under the same conditions of illumination and humidity (one laboratory), using the same containers for drying each sample and other equal conditions for each sample. Weighing was carried out on electronic laboratory scales (VK-600) with an accuracy of 0.001 g.
The habitat conditions and the state of the forest stand were studied. In the absence of direct or weak indicators of frost resistance, indirect phenological and morphological features of Robinia pseudoacacia were taken into account, which should be decisive if several signs are equal.
A 3-point scale was used to assess the vitality of plants. The winter hardiness of plants was assessed on a 6-point Pyatnitsky scale and a 7-point scale developed by the Main Botanical Garden of the Academy of Sciences (MBG RAS): I—the plant does not freeze; II—freezes no more than 50% of the length of annual shoots; III—freezes from 50 to 100% of the length of annual shoots; IV—freezes not only annual, but also older shoots; V—the aboveground part freezes to the snow cover; VI—the entire aboveground part freezes; VII—the plant freezes completely. Potential frost resistance was studied by freezing shoots in a climatic chamber (KHT-0.22). To assess the frost resistance of tree species, the freezing index was determined and the data obtained were compared with the following scale of the MBG RAS: 0—the plant does not freeze; 0.1–0.9—the plant freezes slightly; 1.0–7.9—the plant freezes moderately; 8.0–69.9—the plant freezes significantly; 70.0–100.0—the plant freezes completely. Here, 0–100.0 are the values of the freezing index, in % [23].
Reproductive ability was studied on a 5-point scale. The periodicity, duration and intensity of flowering, yield, entry into the generative phase of development, seed quality, weight of 1000 seeds, germination and germination energy were taken into account. According to the monitoring and mapping of introduced populations, a comprehensive assessment of invasive activity was carried out and conclusions were made based on meta-analysis. The status of invasive activity is assigned to alien species, according to the classification adopted in the project of European botanical gardens: 1—alien species that are massively widespread both on the territory of dendrological collections and beyond; 2—species that actively settle on the territory of the arboretum that is not occupied by collections and expositions; 3—species that have formed local naturalizing populations outside collections or expositions, and in the case of vegetative overgrowth—stable clones, those that have lost their physical connection with the mother plants; 4—species marked at least once outside the number of sites [24,25].
The prediction of the adaptive capabilities of introduced representatives of the genus Robinia was carried out by the method of climatic analogues, the main provisions of which were formulated in the works of Mayr and subsequently refined by Pavari, Selyaninov, Maleev and Guda [22]. The climatic conditions of the regions of natural growth of the studied species and the areas of introduction were compared according to the following indicators: absolute minimum and absolute maximum air temperature, average annual temperatures and average annual precipitation. To identify patterns, multivariate analysis was used by the method of hierarchical clustering with the construction of dendrograms.
The taxation specifications of plantings were determined by the method of mass observations using arithmetic mean values and standard deviation (σ). For the taxational characteristics of the life form and the general habitus of plants, the authors additionally proposed a multi-stem index—the average number of trunks per plant in the sample population.
The state of the nodule structure was determined by Keller light microscopy. A microtome was used to obtain a cross section up to 8 microns thick from a root nodule embedded in paraffin.
2.4. Statistical Analysis
Statistical processing of the obtained data was carried out according to the method of Zaitsev [26]. Mathematical processing of the results was carried out in the MS Excel application program using standard algorithms: arithmetic mean with absolute and relative errors (significance of differences between individual indicators), coefficient of variation and standard deviation. The main statistical data were calculated by the method of dispersion analysis of data. Classification analysis was carried out by the method of hierarchical clustering using the computer programs “Statistica” and “MS Excel”.
3. Results
The analysis of the similarity of the climatic indicators of the donor regions and the points of introduction allowed us to determine that Robinia neomexicana, growing in the states of Colorado, New Mexico, Arizona and Utah, has an advantage over other species (Figure 3). It should be noted that the meteorological conditions of these states have the maximum similarity with the Volgograd region both in terms of temperature indicators and the total amount of precipitation [27,28,29,30,31,32,33,34,35,36].
A comprehensive assessment of the prospects in terms of vitality, winter hardiness, drought resistance and reproductive ability, including the features of flowering and fruiting [37], allowed us to identify typical representatives of Robinia pseudoacacia and its interspecific hybrids with Robinia neomexicana with maximum reproductive ability (4.5–4.6 points) (Table 2). According to a 3-point scale of vitality, all representatives in the dry-steppe conditions of the Volgograd region are in the range from 1.2 to 2.7 points. Relatively low vitality is observed in the decorative form of Robinia pseudoacacia f. pyramidalis (2.5–2.7 points). The remaining representatives are characterized by high indicators of vitality from 1.2 to 1.5 points.

Table 2.
Comprehensive assessment of the indicators of the prospects of introduction according to visual observations.
Despite the relatively high degree of biological resistance, plants of this group have different potential winter hardiness. Thus, for the northern steppe regions, the interspecific hybrids R. pseudoacacia x R. neomexicana are more promising in comparison with R. pseudoacacia. R. viscosa also has a relatively low winter hardiness. This feature, along with low reproductive properties, allows us to recommend R. viscosa as an additional species useful in the southern regions but with limited use in the northern regions of the Volgograd region.
As our studies have shown, in the vegetatively propagated form of Robinia pseudoacacia f. pyramidalis (Pepin) Rehd. there is still an annual freezing of shoots by 25–50% of the length of the previous year’s growth (Figure 4).

Figure 4.
Annual freezing of shoots of Robinia pseudoacacia f. pyramidalis by 25–50% of the length of annual growth (Volgograd, seed plantations of the Federal Research Center of Agroecology of the Russian Academy of Sciences). Red arrows mean dry shoots as a result of freezing.
A series of unfavorable conditions in 2017–2022 had a destructive effect on the viability of various representatives of the genus Robinia. One of the main damaging factors during this period were the low average monthly temperatures and their distribution during the winter period. In December, the average monthly temperature was −7.9 °C, which is 3.3 °C lower than the climatic norm calculated for the entire period of observations at the weather station of Volgograd. According to the results of annual visual observations in the spring of 2021, the lowest winter hardiness was recorded during the research period from 2017 to 2022.
Despite periodic damage in severe winters, most representatives of the genus Robinia in the conditions of the Volgograd region have good long-term average winter hardiness. The most thermophilic was the decorative form of Robinia pseudoacacia pyramidalis, the annual shoots of which freeze annually. In some years, two-year-old shoots or more than half the length of the annual growth were damaged. All forms and typical representatives of Robinia neomexicana, as well as hybrid forms of R. pseudoacacia x R. neomexicana, turned out to be more winter hardy. Insufficient winter hardiness of decorative forms of Robinia pseudoacacia is explained by the fact that their reproduction in culture occurs mainly in a vegetative manner, which does not allow them to adapt to changing environmental conditions [38].
The potential winter hardiness of representatives of the genus Robinia was also confirmed by the data of the in-house study of frost resistance by freezing shoots in the climatic chamber KHT-0.22. Studies showed that exposure to a temperature of −37 °C for 24 h does not cause significant damage to shoots in typical representatives of R. neomexicana Gray, R. pseudoacacia L.; Robinia viscosa Vent. var. hartwegii and (Kene) ash.
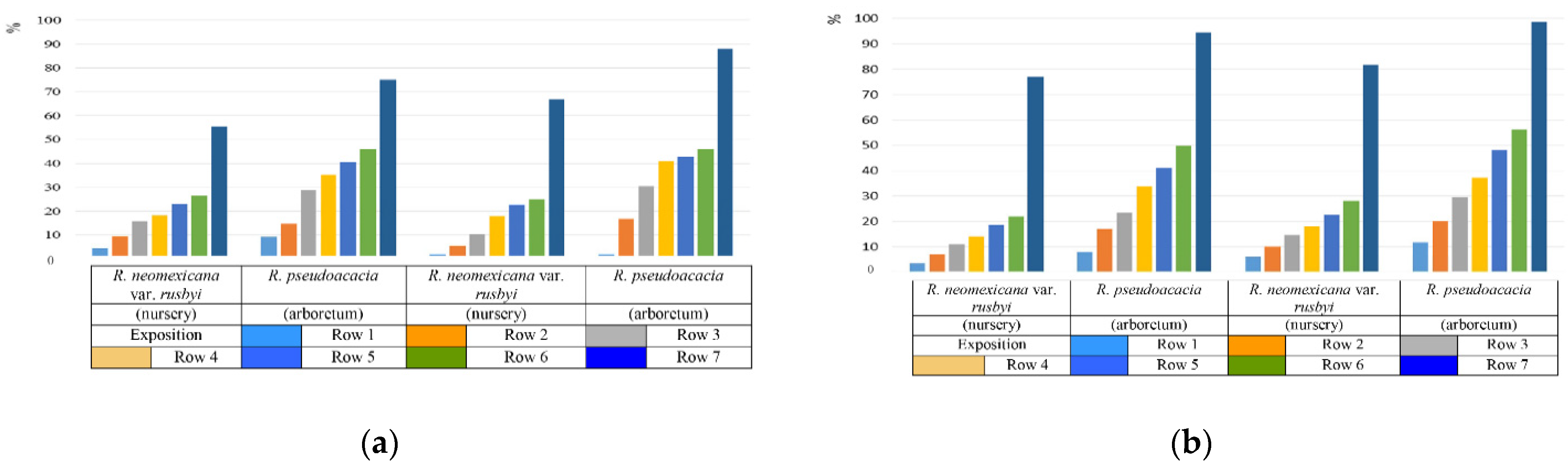
The study of drought resistance was carried out according to visual observations in full-scale conditions, as well as by an indirect method for the water-retaining ability of leaves. It has been established that the water-retaining ability of the leaves of the genus Robinia is significantly influenced by the cultivation conditions [39,40,41,42,43,44,45,46,47,48]. Comparative characteristics of the water-retaining ability of the leaves of R. pseudoacacia and R. neomexicana var. rusbyi allowed us to determine that the best indicators of the water regime are observed in plants growing in the nursery of woody plants of the Federal Research Center of Agroecology of the Russian Academy of Sciences. This feature is associated with the cultivation conditions, according to which the plantings on the territory of the nursery have a better moisture supply (Figure 5a).
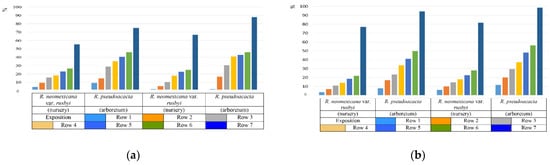
Figure 5.
Water–holding capacity of leaf tissues ((a)—July, (b)—September, on average 2019–2021) of R. pseudoacacia and R. neomexicana var. rusbyi under various cultivation conditions, expressed in % ratio of lost water to the original content.
By the beginning of September, against the background of a general decrease in water-retention capacity, plants growing in different conditions maintain a difference in the values of water-retention capacity (Figure 5b).
Comparison of water-retention capacity indicators during the growing season with the main climatic characteristics of the research period showed that the fastest water loss was observed during prolonged atmospheric and soil droughts. In accordance with the main meteorological indicators of the Volgograd region, the average monthly precipitation in the summer period decreased from June to August, which led to a deterioration in the conditions of moisture supply and a decrease in the water-holding capacity of the leaves (Table 3).

Table 3.
The water-retaining ability of the leaf tissues of representatives of the genus Robinia, expressed in % ratio of lost water to the initial content after exposure for 24 h.
A comparative analysis of the water-holding capacity of various species of the genus Robinia revealed the following patterns. Regardless of the time of the study, the maximum rates of water loss were observed in the decorative form of R. pseudoacacia f. pyramidalis. R. viscosa var. hartwegii is also distinguished by its low water-holding capacity.
With an increase in the air temperature, the magnitude of the real water deficit in the leaves increases. All representatives of the genus Robinia are very drought-resistant. Comparison of the indicators of the water-holding capacity of the leaves with the data of observations obtained under field conditions allowed us to identify a number of adaptations to the effects of prolonged summer atmospheric and soil drought:
- rhythmic nictinastic movements of the leaflets on a complex leaf—this mechanism is active and is not associated with the general loss of leaf turgor during exposure to high temperatures (Figure 6);
 Figure 6. Folding of leaflets of a complex leaf of R. neomexicana under the influence of high temperature and light.
Figure 6. Folding of leaflets of a complex leaf of R. neomexicana under the influence of high temperature and light. - summer leaf fall is a decrease in the number of leaves without reducing the overall vitality of the plant [49]. Leaf fall in R. neomexicana and its forms is observed in the second–third decade of July; in R. pseudoacacia, this occurs during the third decade of July through the first decade of August.
Comparison of visual observation data with indirect assessment methods indicates the presence of an inverse relationship between the beginning of summer leaf fall and water-retention capacity. The earliest summer leaf fall begins in R. neomexicana with high water-retention capacity.
Thus, the differences in the water-holding capacity of the leaves of representatives of the genus Robinia are relatively constant and change only slightly over the years.
A reaction to the cultivation conditions of R. pseudoacacia and R. neomexicana var. rusbyi was also noted at the physiological level in the functioning of the photosynthetic apparatus (Table 4).

Table 4.
The pigment system of R. pseudoacacia and R. neomexicana var. rusbyi in various cultivation conditions, mcg/cm2 of raw mass.
The content of chlorophyll and flavonoids in the leaves of plants growing in the nursery was higher than that of plants in the arboretum, while the content of anthocyanins and the level of the nitrogen balance index (NBI) were lower. An increase in the content of anthocyanins and the NBI in plants growing in the arboretum may be associated with a specific protective reaction to a complex of unfavorable growing conditions.
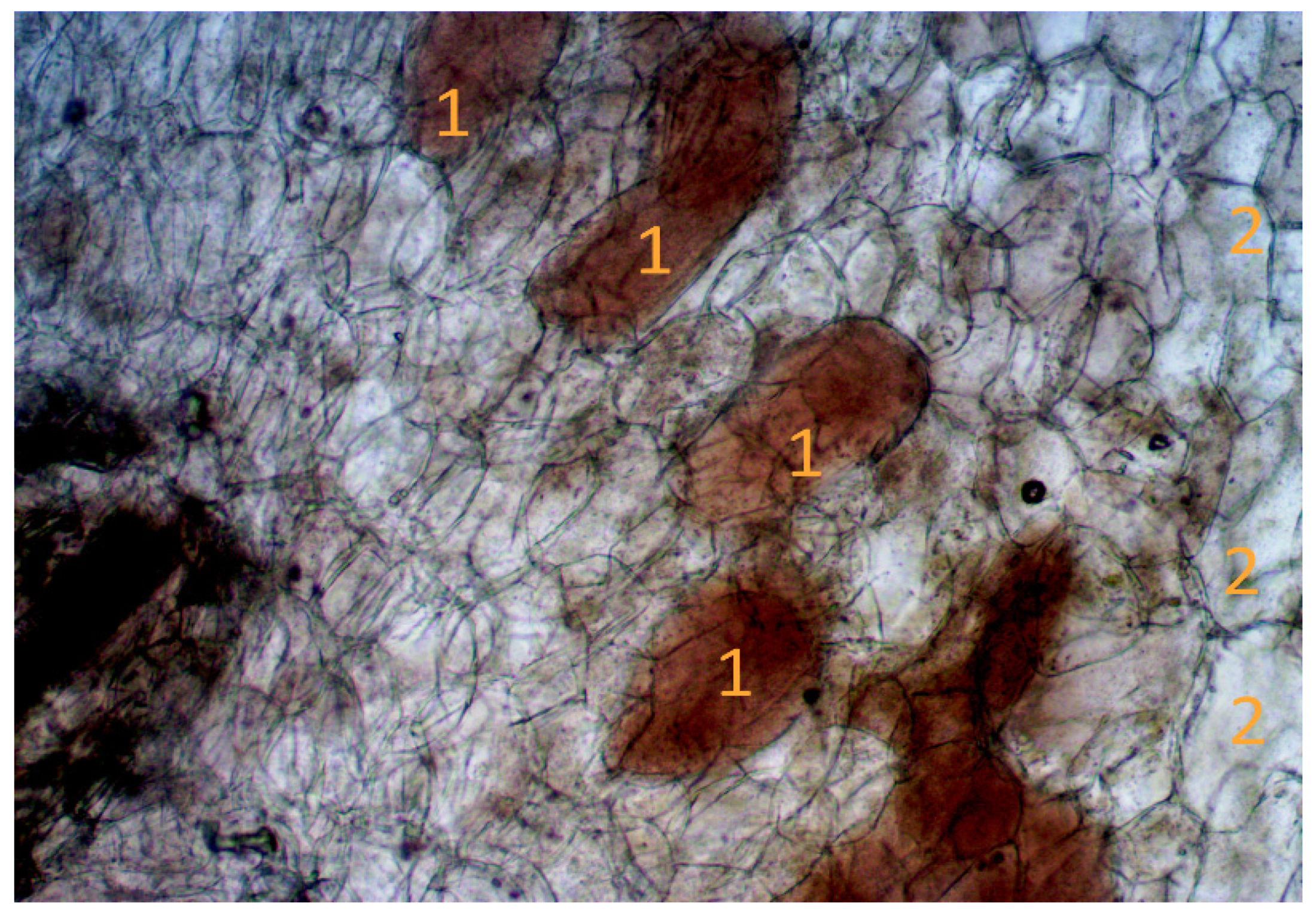
Methods of light microscopy allowed us to establish the relationship between the conditions of the place of growth and the state of the nodule structure during the growing season (Figure 7).
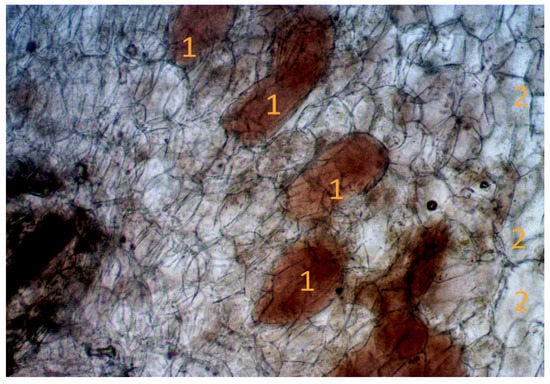
Figure 7.
Cross section of the root nodule. Note: 1—zone with infected cells; 2—zone with uninfected cells. Scale: 1 cm = 100 microns.
The nodules are perennial, and new blades are formed annually, developing throughout the growing season. The cross-section shows areas with infected and areas with non-infected cells. Darker infected areas contain a large number of vesicles.
To differentiate the plants of the first cluster into ‘leading’ and ‘additional’ members of the recommended assortment, a point assessment of bioecological indicators, as well as the availability of conditions for the mass production of planting material, showed that R. viscosa var. hartwegii has the lowest yield [49]. Small tree arrays in the conditions of the Volgograd region are represented by mother plantations of a typical variety of R. neomexicana var. neomexicana, color forms of R. neomexicana and hybrids of R. pseudoacacia x R. neomexicana. The limited stock of seed material of these plants at this stage of introduction allows us to recommend them for use as an additional assortment. Plants of the second cluster, i.e., Robinia pseudoacacia f. pyramidalis and Robinia pseudoacacia f. umbraculifera, have the lowest degree of bioecological stability. These forms reproduce only vegetatively and are characterized by low winter hardiness. Annual shoots freeze every year, and in some years, long-term shoots and more than half of the annual growth are damaged [50].
In accordance with the forest-reclamation zoning of the Volgograd region, it is advisable to recommend these forms for limited use in the southern semi-desert Ergeninsko-Sarpinsky district (Table 5).

Table 5.
The prospects of representatives of the generic complex for various agroforestry districts of the Volgograd region.
Taking into account the bioecological features of representatives of the generic complex, they can be recommended for different types of forest protection and landscaping plantings (Table 6).

Table 6.
Assortment of Robinia species and forms suitable for various types of protective afforestation and landscaping.
Typical representatives of Robinia pseudoacacia and its interspecific hybrids with Robinia neomexicana, characterized by high growth rates, are promising for protective plantings. Robinia neomexicana is advisable to use in ravine-beam plantings [12].
In garden and park construction, representatives of the generic complex are promising for the creation of irrigated and non-irrigated landscaping facilities of general, limited use and special purpose. The use of the thermophilic form of Robinia pseudoacacia f. pyramidalis is possible only in landscaping facilities of limited use. To grow these decorative forms, it is necessary to choose windproof areas with southern exposure. Robinia glutinosa, as the most decorative species of the genus Robinia, is advisable to use not only in groups, but also in solitary plantings.
4. Discussion
For most species of the genus Robinia L., a number of phylogenetic adaptations for growing in certain environmental conditions have developed in the process of evolution:
- anatomical and morphological adaptations to growing in arid regions with a highly branched root system [48] and xeromorphic structure of leaves [51]. The tracery and wind permeability of Robinia crowns is a clear adaptation for growing in regions with increased wind load;
- physiological and biochemical phylogenetic adaptations. R. pseudoacacia is able not only to easily tolerate prolonged droughts, reducing transpiration and photosynthesis intensity, but also to recover quickly, compensating for growth processes after exposure to adverse environmental factors [52,53];
- phenotypic ontogenetic adaptations, including changes in life form (tree–shrub, single-stemmed tree–multi-stemmed tree), slowing down the growth rate, reducing the overall size of plants, reducing the size of leaf blades, etc.;
- ecological and phytocenotic phylogenetic adaptations—adaptations that ensure the ability of plants to occupy ecological niches in the biocenosis—the ability to settle and enter plants into the natural ecosystems of the point of introduction (naturalization).
- genotypic adaptations are a comparative characteristic of the winter hardiness of varieties of R. pseudoacacia, whose reproduction occurs exclusively by vegetative methods, with typical representatives of this species, whose reproduction occurs mainly by the generative method. The absence of genetic heterogeneity in maple plantations excludes the possibility of natural or artificial selection and the formation of new genotypic adaptations in changing growing conditions. That is why generative reproduction and generational change are a prerequisite for the adaptation of plants in the process of introduction. The most thermophilic species is R. viscosa, and the most hardy is R. neomexicana (R. luxurians). The latter was used in breeding, in order to increase the winter hardiness of R. pseudoacacia by hybridization [16]. As our research has shown, at present the difference in winter hardiness of Robinia glutinosa, R. pseudoacacia and R. neomexicana has significantly decreased. Over the last century, in the process of natural and artificial selection, these species have developed a number of genotypic adaptations to low negative temperatures.
Like many plants of the Fabaceae family, all representatives of the genus Robinia have symbiotic relationships with nitrogen-fixing bacteria and are therefore able to grow on poor soils, enriching them with nitrogen.
Typical representatives of R. neomexicana are recommended for wide introduction into culture (the leading assortment), and color forms of this species as an additional assortment in all agroforestry areas of the Volgograd region.
A broad culture of R. pseudoacacia is appropriate in the southern part of the Volga–Don forest-steppe, Volga–Ural semi-desert and in the Ergenin–Sarpin semi-desert areas. In other areas, this type is promising only as an additional assortment.
Low frost resistance and reproductive capacity of R. viscosa var. hartwegii and decorative forms of R. pseudoacacia allow us to attribute them to a limited and additional assortment in various agroforestry areas of the southern part of the Volgograd region.
For the creation of tree massifs in the conditions of the Volgograd region, R. pseudoacacia is recommended; for groves: R. neomexicana and R. pseudoacacia x R. neomexicana; for alleys: R. pseudoacacia f. pyramidalis and R. pseudoacacia f. umbraculifera; and for solitary plantings: R. viscosa var. hartwegii.
5. Conclusions
It is established that the main limiting factors affecting the growth, development and condition of representatives of the genus Robinia in the Volgograd region are winter temperatures up to −37 °C, as well as poor moisture availability and uneven distribution of precipitation during the growing season in combination with extremely low temperatures in the autumn–winter period. Based on the analysis of the similarity of climatic indicators of donor regions and points of introduction, it was revealed that R. neomexicana has an advantage over other species, whose natural distribution area lies in the United States (Utah, New Mexico, Arizona and Colorado).
All representatives of the generic complex belong to the group of plants that start late and finish vegetative growth late. Phenological atypicity is in the lower half of the normal range with indicators from +1 °C to 0, which indicates a high level of adaptation to the climatic conditions of the region. In the process of long-term acclimatization, many introduced species have developed a number of genotypic adaptations and are currently able to tolerate extreme winter temperatures up to −37 °C.
The studied species have high indicators of viability in difficult growing conditions of the Volgograd region. An assessment of potential drought resistance based on the water-retaining ability of the leaves showed that higher rates of water-resistance capacity (76.8% water loss) are typical for representatives of R. neomexicana with a natural distribution area in the western arid part of the North American continent. R. viscosa var. hartwegii, with a natural distribution in the areas of the monsoon subtropical climate of eastern North America, is distinguished by low indicators (94.1% water loss), as are clonal decorative forms of R. pseudoacacia: f. pyramidalis and f. umbraculifera (97.6–95.8% water loss), which are common only in culture and are characterized by a whole complex of low indicators of bioecological stability.
The arid climatic regime of the Volgograd region imposes special requirements on introduced woody plants in terms of drought resistance and winter hardiness. The data obtained by us allow us to recommend the following assortment of species and forms of Robinia for protective afforestation and landscaping in the arid territories of southern Russia: R. pseudoacacia, R. neomexicana var. neomexicana, R. neomexicana var. rusbyi and R. pseudoacacia x R. neomexicana.
Author Contributions
The team of authors was directly involved in planning and conducting a pilot experiment and processing research results. Conceptualization, S.L. and E.K.; methodology, S.L.; validation, S.L. and E.K.; formal analysis, S.L.; investigation, S.L. and E.K.; writing—original draft preparation, S.L. and E.K.; writing—review and editing, S.L. and E.K.; project administration, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the topic of the state task of the Federal Scientific Center for Agroecology of the Russian Academy of Sciences No. FNFE-2021-0001 (registration number 121041200197-8) “Scientific foundations and technologies for enriching the dendroflora of forest reclamation complexes with economically valuable woody and shrubby plants in order to prevent degradation and desertification of territories”.
Institutional Review Board Statement
The expert commission reviewed the materials of the article “Increasing the biodiversity of the dendroflora of sparsely wooded regions by adapted representatives of the genus Robinia L.” regarding the absence of information constituting a state secret in them, and the possibility of their open publication.
Data Availability Statement
Not applicable.
Acknowledgments
The team of authors express their gratitude to the Federal State Budgetary Scientific Institution “Federal Scientific Center for Agroecology, Integrated Land Reclamation and Protective Afforestation of the Russian Academy of Sciences” and personally to Alexander Ivanovich for the opportunity to conduct research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Global Forest Resources Assessment 2020—Main Report; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Grigoriev, A.Y.; Laletin, A.P.; Pakhorukova, K.A.; Zabelin, S.I. Forests of Russia and climate change. RSoES 2021, 42. Available online: https://www.airclim.org/sites/default/files/documents/forests-of-russia-and-climate-change.pdf (accessed on 21 December 2022).
- FAO. The state of the world’s land and water resources for food production and agriculture. Systems are at the limit. In Summary Report 2021; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb7654ru/online/cb7654ru.html (accessed on 21 December 2022).
- Lipka, O.N.; Korzukhin, M.D.; Zamolodchikov, D.G.; Dobrolyubov, N.Y.; Krylenko, S.V.; Bogdanovich, A.Y.; Semenov, S.M. The role of forests in the adaptation of natural systems to climate change. Forestry 2021, 5, 531–546. [Google Scholar] [CrossRef]
- The Forestry Structure of the Volgograd Region Has Turned 85 Years Old. 2021. Available online: http://oblkompriroda.volgograd.ru/current-activity/cooperation/news/377753/?sphrase_id=805382 (accessed on 21 December 2022).
- Vítková, M.; Tonika, J.; Müllerová, J. Black locust-Successful invader of a wide range of soil conditions. Sci. Total Environ. 2015, 505, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Rédei, K.; Keseru, Z.; Rásó, J. Early evaluation of micropropagated black locust (Robinia pseudoacacia L.) clones in Hungary. For. Sci. Pract. 2013, 15, 81–84. [Google Scholar]
- Pepe, M.; Gratani, L.; Crescente, M.F.; Puglielli, G.; Varone, L. Daily Temperature Effect on Seedling Growth Dynamic of Three Invasive Alien Species. Front. Plant Sci. 2022, 13, 837449. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Yang, X.C.; Shen, Z.; Ma, L.Y.; Duan, J.; Li, Y. Properties and Distribution of Seed Banks in a Black Locust (Robinia pseudoacacia) Plantation in Central China. Nat. Environ. Pollut. Technol. 2022, 21, 367–376. [Google Scholar] [CrossRef]
- Wang, N.; Bi, H.; Cui, Y.; Zhao, D.; Hou, G.; Huiya, Y.U.N.; Liu, Z.; Lan, D.; Jin, C. Optimization of stand structure in Robinia pseudoacacia Linn. based on soil and water conservation improvement function. Ecol. Indic. 2022, 136, 108671. [Google Scholar] [CrossRef]
- Rusňák, T.; Halabuk, A.; Halada, L.; Hilbert, H.; Gerhátová, K. Detection of Invasive Black Locust (Robinia pseudoacacia) in Small Woody Features Using Spatiotemporal Compositing of Sentinel-2 Data. Remote Sens. 2022, 14, 971. [Google Scholar] [CrossRef]
- Lazarev, S.E.; Semenyutina, A.V. The prospects of species and forms of the genus Robinial For forest protection and landscaping plantings. Successes Mod. Nat. Sci. 2020, 8, 11–17. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Giuliani, C.; Lazzaro, L.; Calamassi, R.; Fico, G.; Foggi, B.; Mariotti Lippi, M. Induced water stress affects seed germination response and root anatomy in Robinia pseudoacacia (Fabaceae). Trees 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Morozova, E.V.; Iozus, A.P.; Kryuchkov, S.N. The main results of the breeding of robinia false acacia in the Lower Volga region. Successes Mod. Nat. Sci. 2018, 12, 290–295. [Google Scholar]
- Guo, Q.; Cao, S.; Dong, L.; Li, X.; Zhang, J.; Zhang, Y.; Zhang, Z.; Sun, Y.; Long, C.; Fan, Y.; et al. Genetic diversity and population structure of Robinia pseudoacacia from six improved variety bases in China as revealed by simple sequence repeat markers. J. For. Res. 2022, 33, 611–621. [Google Scholar] [CrossRef]
- Peabody, F.J. Revision of the Genus Robinia (Leguminosae: Papilionoideae). Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1984; 174p. [Google Scholar] [CrossRef]
- U.S. Climate Data [Electronic Resource]. Available online: https://www.usclimatedata.com (accessed on 17 February 2022).
- Sazhin, A.N.; Kulik, K.N.; Vasiliev, Y.I. Weather and Climate of the Volgograd Region; FRCARAS: Volgograd, Russia, 2010; 306p. [Google Scholar]
- Weather and Climate//Chronicle of Weather in Volgograd [Electronic Resource]. Available online: http://www.pogodaiklimat.ru/history/34560.htm (accessed on 17 February 2022).
- Semenyutina, A.V.; Dolgikh, A.A.; Khuzhakhmetova, A.S.; Zelenyak, A.K.; Danilina, D.V.; Kostyukov, S.M.; Bogdanov, A.V.; Solomentseva, A.S. Methodological Guidelines on Seed Science of Tree Introducers in Arid Zone Conditions; Agricultural Academy: Moscow, Russia, 2010; 56p. [Google Scholar]
- Khizhnyak, N.I.; Semenyutina, A.V. Trees and Shrubs of the Volgograd Arboretum All-Russian Research Institute of Agroforestry; Federal State Budgetary Scientific Institution “All-Russian Scientific Research Agroforestry Reclamation Institute”: Volgograd, Russia, 1984; p. 49. [Google Scholar]
- Vinogradova, Y.K.; Mayorov, S.R.; Bochkin, V.D. The influence of alien plant species on the dynamics of the flora of the territory of the Main Botanical Garden of the Russian Academy of Sciences. Russ. J. Biol. Invasions 2015, 4, 22–40. [Google Scholar]
- Vítková, M.; Sádlo, J.; Pergl, J.; Pyšek, P. Towards site-specific management of invasive alien trees based on the assessment of their impacts: The case of Robinia pseudoacacia. NeoBiota 2017, 35, 1–34. [Google Scholar] [CrossRef]
- Zaitsev, G.N. Mathematical Statistics in Experimental Botany; Nauka: Moscow, Russia, 1984; 424p. [Google Scholar]
- Sedykh, S.A.; Baboshko, O.I. The use of false activation robinia in protective afforestation of the Rostov region. Int. Stud. Sci. Bull. 2015, 2–3, 373–374. [Google Scholar]
- Nicolescu, V.-N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastien, J.-C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]
- Wojda, T.; Klisz, M.; Jastrzębowski, S.; Mionskowski, M.; Szyp-Borowska, I.; Szczygieł, K. The Geographical Distribution of the Black Locust (Robinia Pseudoacacia L.) in Poland and Its Role on Non-Forest Land. Pap. Glob. Chang. IGBP 2015, 22, 101–113. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Hernea, C.; Bakti, B.; Keserű, Z.; Antal, B.; Rédei, K. Black locust (Robinia pseudoacacia L.) as a multi-purpose tree species in Hungary and Romania: A review. J. For. Res. 2018, 29, 1449–1463. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, M.S.; Nam, J.I.; Song, J.H.; Kim, S.H. Analysis on floral nectar characteristics among the selected black locust (Robinia spp.) individuals. J. Apic. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Lazarev, S.E.; Semenyutina, A.V. Scientific principles of reconstruction of gardening robinia plantings. Sci. Thought Electron. Period. J. 2021, 12, 102–121. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Sedoy, R.G.; Babeshko, O.I.; Kurinskaya, L.V. Features of the growth of pseudoactation robinia in the conditions of the steppe zone. For. Sci. 2021, 3, 240–249. [Google Scholar] [CrossRef]
- Agapov, A.I.; Shakina, T.N. The use of North American introducents for green construction in 3–4 winter hardiness zones. Sci. Work. Cheboksary Branch N.V. Tsitsin Main Bot. Gard. Russ. Acad. Sci. 2019, 12, 96–99. [Google Scholar]
- Karpun, Y.N. Subtropical Decorative Dendrology: Handbook; Publishing House “VVM”: St. Petersburg, Russia, 2010; p. 580. [Google Scholar]
- Lazarev, S.E. Form diversity and decorative properties of representatives of the genus Robinia in the conditions of the dry steppe. Sci. Agron. J. 2020, 2, 42–50. [Google Scholar] [CrossRef]
- Koniakin, S.N.; Burda, R.I. The non-native woody species of the flora of Ukraine: Introduction, naturalization and invasion. Biosyst. Divers. 2019, 27, 276–290. [Google Scholar] [CrossRef]
- Andronova, M.M. Winter hardiness and frost resistance of tree species in the anthropogenic environment of the European north of Russia. Successes Mod. Nat. Sci. 2018, 5, 26–32. [Google Scholar]
- Winter Hardiness and Frost Resistance of Wood Species in the Anthropogenic Environment of European North of Russia. Available online: https://natural-sciences.ru/ru/article/view?id=36750 (accessed on 10 August 2022).
- Cook, B.I.; Mankin, J.S.; Anchukaitis, K.J. Climate change and drought: From past to future. Curr. Clim. Chang. Rep. 2018, 4, 164–179. [Google Scholar] [CrossRef]
- Li, L.; Song, X.; Zhao, X.; Meng, P.; Feng, D.; Fu, C.; Wang, L.; Jiao, R.; Wei, W.; Li, H. Modeling the impact of climate change and vegetation conversion on water budget: A case study in the Loess Plateau of China. J. Hydrol. Reg. Stud. 2022, 40, 101040. [Google Scholar] [CrossRef]
- Carlón Allende, T.; Mendoza, M.E.; Villanueva Díaz, J.; Li, Y. Climatic response of Pinus cembroides Zucc. radial growth in Sierra del Cubo, Guanajuato, Mexico. Trees 2018, 32, 1387–1399. [Google Scholar] [CrossRef]
- Pepe, M.; Crescente, M.F.; Varone, L. Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia. Plants 2022, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xue, Y.; Li, Z.; Wang, S.; Wu, X.; Gao, G.; Liu, G.; Fu, B. Soil moisture decline following the plantation of Robinia pseudoacacia forests: Evidence from the Loess Plateau. For. Ecol. Manag. 2018, 12, 62–69. [Google Scholar] [CrossRef]
- Kou, M.; Garcia-Fayos, P.; Hu, S.; Jiao, J. The effect of Robinia pseudoacacia afforestation on soil and vegetation properties in the Loess Plateau (China): A chronosequence approach. For. Ecol. Manag. 2016, 375, 146–158. [Google Scholar] [CrossRef]
- Wang, L.; Shao, M.A.; Li, Y.Y. Study on relationship between growth of artificial Robinia pseudoscacia plantation and soil desiccation in the Loess Plateau of northern Shannxi Province. Sci. Silvae Sin. 2004, 40, 84–91. [Google Scholar]
- Jiao, L.; An, W.; Li, Z.; Gao, G.; Wang, C. Regional variation in soil water and vegetation characteristics in the Chinese Loess Plateau. Ecol. Indic. 2020, 115, 106399. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Chen, L.; Chen, W.; Wang, J. Response of temporal variation of soil moisture to vegetation restoration in semi-arid Loess Plateau, China. Catena 2014, 115, 123–133. [Google Scholar] [CrossRef]
- Semenyutina, A.V.; Lazarev, S.E.; Melnik, K.A. Evaluation of the reproductive ability of representatives of generic complexes and features of their breeding seed science in dry-steppe conditions. Sci. Thought 2019, 9, 1–23. [Google Scholar]
- Dervishi, V.; Poschenrieder, W.; Rötzer, T.; Moser-Reischl, A.; Pretzsch, H. Effects of Climate and Drought on Stem Diameter Growth of Urban Tree Species. Forests 2022, 13, 641. [Google Scholar] [CrossRef]
- Burescu, L.; Cachiţa, D.; Craciun, C. Anatomical, morphological and cytological comparative study of leaves and cotyledons from forestry species or. II Comparison between the morpho-anatomical and cytological structures of cotyledons and leaves of Robinia pseudoacacia L. Stud. Univ. Vasile Goldis Arad Ser. Stiint. Vietii 2015, 25, 65–71. [Google Scholar]
- Nan, G.; Wang, N.; Jiao, L.; Zhu, Y.; Sun, H. A new exploration for accurately quantifying the effect of afforestation on soil moisture: A case study of artificial Robinia pseudoacacia in the Loess Plateau (China). For. Ecol. Manag. 2019, 433, 459–466. [Google Scholar] [CrossRef]
- Morozova, E.V.; Iozus, A.P.; Kryuchkov, S.N. Selective Seed Production of Tree Species in the Southeast of European Russia: Monograph; Volgograd State Technical University: Volgograd, Russia, 2016; 184p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).