White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses
Abstract
:1. Introduction
2. Materials and Methods
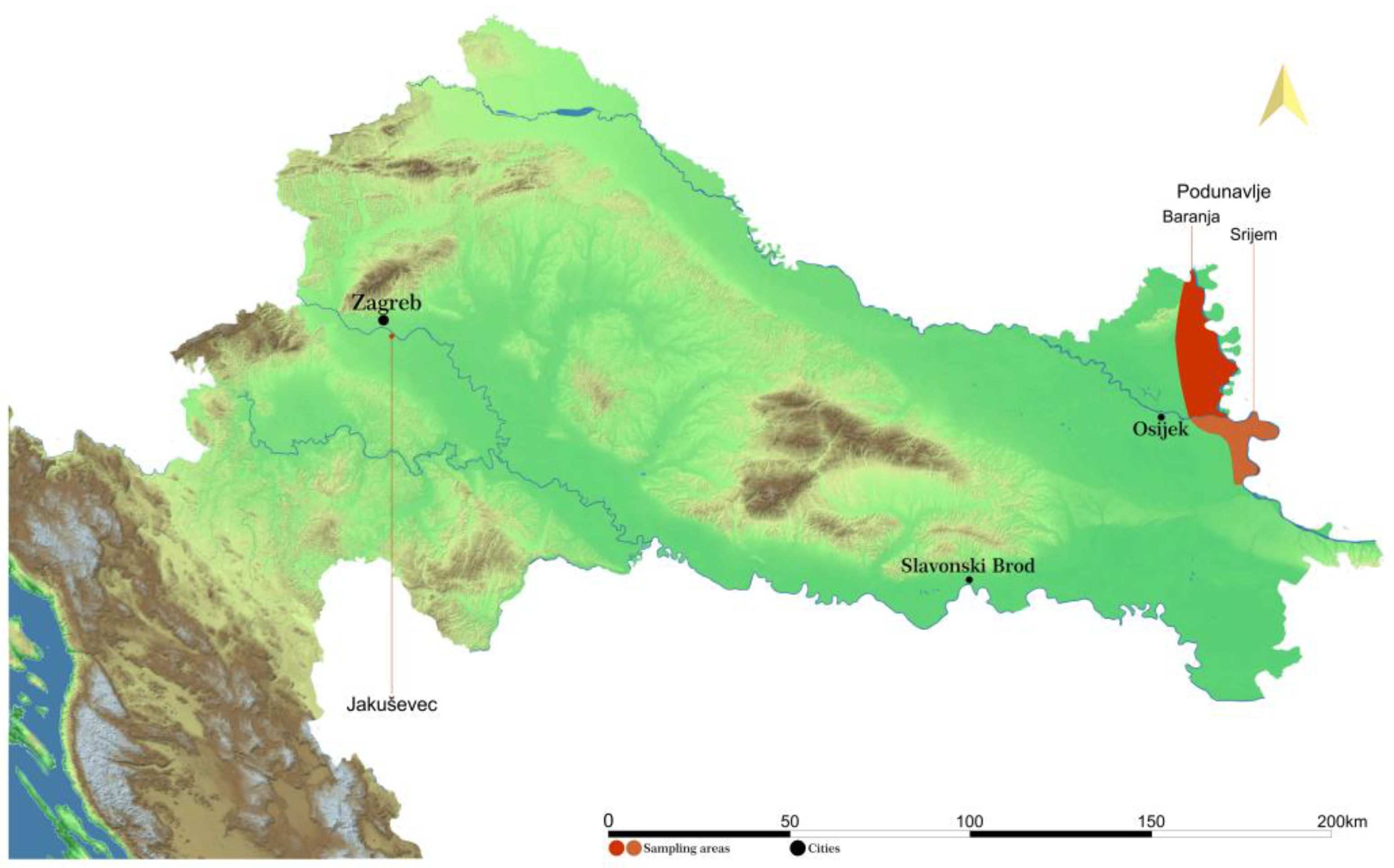
2.1. Study Site
2.2. Sampling Procedure and Blood Preparation
2.3. Chemicals
2.4. Esterase Activity
2.5. Oxidative Stress Biomarkers
2.6. Protein Content
2.7. Molecular Sex Determination
2.8. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sanderson, F.J.; Kloch, A.; Sachanowicz, K.; Donald, P.F. Predicting the effects of agricultural change on farmland bird populations in Poland. Agric. Ecosyst. Environ. 2009, 129, 37–42. [Google Scholar] [CrossRef]
- Lees, A.C.; Haskell, L.; Allinson, T.; Bezeng, S.B.; Burfield, I.J.; Renjifo, L.M.; Rosenberg, K.V.; Viswanathan, A.; Butchart, S.H. State of the World’s Birds. Annu. Rev. Environ. Resour. 2022, 47, 231–260. [Google Scholar] [CrossRef]
- Ratcliffe, D.A. Studies of the recent breeding success of the peregrine, Falco peregrinus. J. Reprod. Fertil. Suppl. 1973, 19, 377–389. [Google Scholar] [PubMed]
- Deweese, L.R.; McEwen, L.C.; Hensler, G.L.; Petersen, B.E. Organochlorine contaminants in passeriformes and other avian prey of the peregrine falcon in the western united states. Environ. Toxicol. Chem. 1986, 5, 675–693. [Google Scholar] [CrossRef]
- Ratcliffe, D.A. Changes Attributable to Pesticides in Egg Breakage Frequency and Eggshell Thickness in Some British Birds. J. Appl. Ecol. 1970, 7, 67. [Google Scholar] [CrossRef]
- Walker, C.H.; Hamilton, G.A.; Harrison, R.B. Organochlorine insecticide residues in wild birds in Britain. J. Sci. Food Agric. 1967, 18, 123–129. [Google Scholar] [CrossRef]
- Kamiński, P.; Jerzak, L.; Kasprzak, M.; Kartanas, E.; Bocheński, M.; Hromada, M.; Baszyński, J.; Kozera, W.; Woźniak, A.; Ulrich, W. Do agricultural environments increase the reproductive success of White Stork Ciconia ciconia populations in South-Western Poland? Sci. Total Environ. 2020, 702, 134503. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Man-Made Deserts: Desertization Processes and Threats. Arid. Land Res. Manag. 2002, 16, 1–36. [Google Scholar] [CrossRef]
- Azhar, B.; Puan, C.L.; Zakaria, M.; Hassan, N.; Arif, M. Effects of monoculture and polyculture practices in oil palm smallholdings on tropical farmland birds. Basic Appl. Ecol. 2014, 15, 336–346. [Google Scholar] [CrossRef]
- Flohre, A.; Fischer, C.; Aavik, T.; Bengtsson, J.; Berendse, F.; Bommarco, R.; Ceryngier, P.; Clement, L.W.; Dennis, C.; Eggers, S.; et al. Agricultural intensification and biodiversity partitioning in European landscapes comparing plants, carabids, and birds. Ecol. Appl. 2011, 21, 1772–1781. [Google Scholar] [CrossRef]
- Kirk, D.A.; Lindsay, K.E.; Brook, R.W. Risk of Agricultural Practices and Habitat Change to Farmland Birds. Avian Conserv. Ecol. 2011, 6, 5. [Google Scholar] [CrossRef]
- Vander Haegen, W.M. Fragmention by agriculture influences reproductive success of birds in a shrubsteppe landscape. Ecol. Appl. 2007, 17, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.I.; Correia, R.A.; Silva, J.P.; Pacheco, C.; Catry, I.; Atkinson, P.W.; Gill, J.A.; Franco, A.M.A. Are white storks addicted to junk food? Impacts of landfill use on the movement and behaviour of resident white storks (Ciconia ciconia) from a partially migratory population. Mov. Ecol. 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- BirdLife. White Stork (Ciconia ciconia)—BirdLife Species Factsheet. 2023. Available online: http://datazone.birdlife.org/species/factsheet/white-stork-ciconia-ciconia (accessed on 9 March 2023).
- Wuczyński, A.; Betleja, J.; Jerzak, L.; Król, W.; Mielczarek, P.; Profus, P.; Siekiera, A.; Siekiera, J.; Springer, S.; Sztwiertnia, H.; et al. Strong Declines of the White Stork Ciconia ciconia Population in South-Western Poland: A Differentiated Importance of Altitude and Land Use Changes. Acta Ornithol. 2022, 5, 255–271. [Google Scholar] [CrossRef]
- Boatman, N.D.; Brickle, N.W.; Hart, J.D.; Milsom, T.P.; Morris, A.J.; Murray, A.W.A.; Murray, K.A.; Robertson, P.A. Evidence for the indirect effects of pesticides on farmland birds. Ibis 2004, 146, 131–143. [Google Scholar] [CrossRef]
- Fry, D.M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ. Health Perspect. 1995, 103, 165–171. [Google Scholar]
- Buckingham, D.L.; Peach, W.J.; Fox, D.S. Effects of agricultural management on the use of lowland grassland by foraging birds. Agric. Ecosyst. Environ. 2006, 112, 21–40. [Google Scholar] [CrossRef]
- Zbyryt, A.; Sparks, T.H.; Tryjanowski, P. Foraging efficiency of white stork Ciconia ciconia significantly increases in pastures containing cows. Acta Oecol. 2020, 104, 103544. [Google Scholar] [CrossRef]
- Grue, C.E.; Shipley, B.K. Sensitivity of nestling and adult starlings to dicrotophos; an organophosphate pesticide. Environ. Res. 1984, 35, 454–465. [Google Scholar] [CrossRef]
- Bjedov, D.; Mikuška, A.; Lackmann, C.; Begović, L.; Mikuška, T.; Velki, M. Application of non-destructive methods: Biomarker assays in blood of white stork (Ciconia ciconia) nestlings. Animals 2021, 11, 2341. [Google Scholar] [CrossRef]
- Bjedov, D.; Velki, M.; Lackmann, C.; Begović, L.; Mikuška, T.; Jurinović, L.; Mikuška, A. Blood biomarkers in white stork (Ciconia ciconia) nestlings show different responses in several areas of Croatia. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, D.; Velki, M.; Toth, L.; Filipović Marijić, V.; Mikuška, T.; Jurinović, L.; Ečimović, S.; Turić, N.; Lončarić, Z.; Šariri, S.; et al. Heavy metal(loid) effect on multi-biomarker responses in apex predator: Novel assays in the monitoring of white stork nestlings. Environ. Pollut. 2023, 324, 121398. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, E.; Anyalogbu, E.; Ezejiofor, T.; Udensi, J. Determination of Reduced Glutathione and Glutathione S-transferase of Poultry Birds Exposed to Permethrin Insecticide. Am. J. Biochem. 2012, 2, 21–24. [Google Scholar] [CrossRef]
- Bartkowiak, D.J.; Wilson, B.W. Avian plasma carboxylesterase activity as a potential biomarker of organophosphate pesticide exposure. Environ. Toxicol. Chem. 1995, 14, 2149–2153. [Google Scholar] [CrossRef]
- Oropesa, A.L.; Gravato, C.; Sánchez, S.; Soler, F. Characterization of plasma cholinesterase from the White stork (Ciconia ciconia) and its in vitro inhibition by anticholinesterase pesticides. Ecotoxicol. Environ. Saf. 2013, 97, 131–138. [Google Scholar] [CrossRef]
- Koivula, M.J.; Eeva, T. Metal-related oxidative stress in birds. Environ. Pollut. 2010, 158, 2359–2370. [Google Scholar] [CrossRef]
- Osičková, J.; Banďouchová, H.; Kováčová, V.; Král, J.; Novotný, L.; Ondráček, K.; Pohanka, M.; Sedláčková, J.; Škochová, H.; Vitula, F.; et al. Oxidative stress and liver damage in birds exposed to diclofenac and lead. Acta Vet. Brno. 2014, 83, 299–304. [Google Scholar] [CrossRef]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef]
- Kamiński, P.; Kurhalyuk, N.; Kasprzak, M.; Jerzak, L.; Tkachenko, H.; Szady-Grad, M.; Klawe, J.J.; Koim, B. The impact of element-element interactions on antioxidant enzymatic activity in the blood of white stork (Ciconia ciconia) chicks. Arch. Environ. Contam. Toxicol. 2009, 56, 325–337. [Google Scholar] [CrossRef]
- Kamiński, P.; Kurhalyuk, N.; Jerzak, L.; Kasprzak, M.; Tkachenko, H.; Klawe, J.J.; Szady-Grad, M.; Koim, B.; Wiśniewska, E. Ecophysiological determinations of antioxidant enzymes and lipoperoxidation in the blood of White Stork Ciconia ciconia from Poland. Environ. Res. 2009, 109, 29–39. [Google Scholar] [CrossRef]
- Radović, A.; Tepić, N. Using Corine Land Cover habitat database for the analysis of breeding bird habitat: Case study of white storks (Ciconia ciconia) from northern Croatia. Biologia. Bratisl. 2009, 64, 1212–1218. [Google Scholar] [CrossRef]
- Mikuska, T. Distribution and population status of White Storks in Croatia. In Proceedings of the 12th Meeting of the European Stork Villages, Čigoč, Croatia, 25–27 June 2015. [Google Scholar]
- Kralj, J.; Barišić, S.; Tutiš, V.; Ćiković, D. Atlas Selidbe Ptica Hrvatske; HAZU: Zagreb, Croatia, 2013. [Google Scholar]
- Espin, S.; García-Fernández, A.; Herzke, D.; Shore, R.; van Hattum, B.; Martínez-López, E. Sampling and Contaminant Monitoring Protocol for Raptors. 2016. Available online: https://www.researchgate.net/publication/294885017_Sampling_and_contaminant_monitoring_protocol_for_raptors (accessed on 9 March 2023).
- Tryjanowski, P.; Sparks, T.H.; Bochenski, M.; Dabert, M.; Kasprzak, M.; Kaminski, P.; Mroczkowski, S.; Wisniewska, E.; Jerzak, L. Do males hatch first and dominate sex ratios in White Stork Ciconia ciconia chicks? J. Ornithol. 2011, 152, 213–218. [Google Scholar] [CrossRef]
- Barčić, D.; Ivančić, V. Impact of the Prudinec/Jakuševec landfill on environment pollution. Sumar. Lis. 2010, 137, 347–358. [Google Scholar]
- Romić, D.; Husnjak, S.; Mesić, M.; Salajpal, K.; Barić, K.; Poljak, M.; Romić, M.; Konjačić, M.; Vnučec, I.; Bakić, H. Utjecaj poljoprivrede na onečišćenje površinskih i podzemnih voda u Republici Hrvatskoj. Hrvatske vode. Elaborate/Study. 2015. Available online: https://www.bib.irb.hr/775043 (accessed on 9 March 2023).
- Zurell, D.; Eggers, U.; Kaatz, M.; Rotics, S.; Sapir, N.; Wikelski, M.; Nathan, R.; Jeltsch, F. Individual-based modelling of resource competition to predict density-dependent population dynamics: A case study with white storks. Oikos 2015, 124, 319–330. [Google Scholar] [CrossRef]
- Zurell, D.; von Wehrden, H.; Rotics, S.; Kaatz, M.; Groß, H.; Schlag, L.; Schäfer, M.; Sapir, N.; Turjeman, S.; Wikelski, M.; et al. Home Range Size and Resource Use of Breeding and Non-breeding White Storks Along a Land Use Gradient. Front. Ecol. Evol. 2018, 6, 79. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Hosokawa, M.; Satoh, T. Measurement of Carboxylesterase (CES) Activities. Curr. Protoc. Toxicol. 2001, 10, 4.7.1–4.7.14. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for Differentiation of Glutathione S-Transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar]
- Fridolfsson, A.K.; Ellegren, H. A Simple and Universal Method for Molecular Sexing of Non-Ratite Birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Begović, L.; Mihić, I.; Pospihalj, T.; Mikuška, T.; Mlinarić, S.; Mikuška, A. Evaluation of methods for molecular sex-typing of three heron species from different DNA sources. Turk. J. Zool. 2017, 41, 593–598. [Google Scholar] [CrossRef]
- Grubbs, F. Sample criteria for testing outlying observations. Ann. Math. Stat. 1950, 21, 37–58. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar]
- Girden, E. ANOVA: Repeated Measures; Sage Publications, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means; aka Least-Squares Means. 2019. Available online: https://cran.microsoft.com/snapshot/2018-01-13/web/packages/emmeans/emmeans.pdf (accessed on 9 March 2023).
- Mazerolle, M.J. Package “AICcmodavg.”; R Package: Vienna, Austria, 2017. [Google Scholar]
- Mazerolle, M.J. Model Selection and Multimodel Inference Using the AICcmodavg Package. R Vignette. 2020. Available online: https://mirror.marwan.ma/cran/web/packages/AICcmodavg/vignettes/AICcmodavg.pdf (accessed on 9 March 2023).
- Bailly, J.; Faivre, B.; Bernard, N.; Sage, M.; Crini, N.; Driget, V.; Garnier, S.; Rieffel, D.; Scheifler, R. Multi-element analysis of blood samples in a passerine species: Excesses and deficiencies of trace elements in an urbanization study. Front. Ecol. Evol. 2017, 5, 6. [Google Scholar] [CrossRef]
- Coeurdassier, M.; Fritsch, C.; Faivre, B.; Crini, N.; Scheifler, R. Partitioning of Cd and Pb in the blood of European blackbirds (Turdus merula) from a smelter contaminated site and use for biomonitoring. Chemosphere 2012, 87, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A. Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations. J. Wildl. Dis. 1997, 33, 383–384. [Google Scholar] [CrossRef]
- Hribšek, I.; Jovičić, K.; Karadžić, B.; Skorić, S. Allocation of Metals and Trace Elements in Different Tissues of Piscivorous Species Phalacrocorax carbo. Arch. Environ. Contam. Toxicol. 2017, 73, 533–541. [Google Scholar]
- Quinn, D.M. Acetylcholinesterase: Enzyme Structure, Reaction Dynamics, and Virtual Transition States. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Sanchez, B.M. Lizard cholinesterases as biomarkers of pesticide exposure: Enzymological characterization. Environ. Toxicol. Chem. 2002, 21, 2319–2325. [Google Scholar] [CrossRef]
- Thompson, H. Serum “B” esterases as indicators of exposure to pesticides. In Cholinesterase-Inhibiting Insecticides: Their Impact on Wildlife and the Environment; Mineau, P., Ed.; Elsevier Science Publishing Company Inc.: New York, NY, USA, 1991; pp. 110–125. [Google Scholar]
- Thompson, H.M. Esterases as markers of exposure to organophosphates and carbamates. Ecotoxicology 1999, 8, 369–384. [Google Scholar] [CrossRef]
- Herceg Romanić, S.; Krauthacker, B. Organochlorine Pesticides and Polychlorinated Biphenyls in Ambient Air Collected in Zagreb, Croatia. Bull. Environ. Contam. Toxicol. 2000, 64, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ahel, I.; Tepić, N.; Britvić, S.; Ahel, M. Assessment of organic pollution in the Jakuševec landfill (Croatia) by biomarker and chemical methods. Fresenius Environ. Bull. 2000, 9, 726–733. [Google Scholar]
- Krauthacker, B.; Herceg Romanić, S.; Wilken, M.; Milanović, Z. PCDD/Fs in ambient air collected in Zagreb, Croatia. Chemosphere 2006, 62, 1829–1837. [Google Scholar] [CrossRef]
- Musić, V.; Šikoronja, M.; Tomas, D.; Varat, M. Izvješće o stanju površinskih voda u 2019. godini. 2020. Available online: https://voda.hr/sites/default/files/2022-05/izvjesce_o_stanju_povrsinskih_voda_u_2020._godini_0.pdf (accessed on 9 March 2023).
- Scheuhammer, A.M.; Basu, N.; Burgess, N.M.; Elliott, J.E.; Campbell, G.D.; Wayland, M.; Champoux, L.; Rodrigue, J. Relationships among mercury, selenium, and neurochemical parameters in common loons (Gavia immer) and bald eagles (Haliaeetus leucocephalus). Ecotoxicology 2008, 17, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P. Responses of Acetylcholinesterase from Torpedo marmorata to Salts and Curarizing Drugs. Mol. Pharmacol. 1966, 2, 369–392. [Google Scholar]
- Coleman, M.H.; Eley, D.D. The inhibition of acetylcholinesterase by quaternary ammonium ions. Biochim. Biophys. Acta 1962, 58, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Blaney, L.; Kao, J.; Tyagi, R.D.; Zhang, T.C.; Surampalli, R.Y. Emerging contaminants in landfill leachate and their sustainable management. Environ. Earth Sci. 2015, 73, 1357–1368. [Google Scholar] [CrossRef]
- Bašić, F.; Bogunović, M.; Božić, M.; Husnjak, S.; Jurić, I.; Kisić, I.; Mesić, M.; Mirošević, M.; Romić, D.; Žugec, I. Regionalisation of Croatian agriculture. Agric. Conspec. Sci. 2007, 72, 27–38. [Google Scholar]
- Pandey, S.P.; Mohanty, B. The neonicotinoid pesticide imidacloprid and the dithiocarbamate fungicide mancozeb disrupt the pituitary-thyroid axis of a wildlife bird. Chemosphere 2015, 122, 227–234. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Fernie, K.J.; Shutt, J.L.; Mayne, G.; Hoffman, D.; Letcher, R.J.; Drouillard, K.G.; Ritchie, I.J. Exposure to Polybrominated Diphenyl Ethers (PBDEs): Changes in Thyroid, Vitamin A, Glutathione Homeostasis, and Oxidative Stress in American Kestrels (Falco sparverius). Toxicol. Sci. 2005, 88, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Heinz, G.H. Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ. Toxicol. Chem. 1998, 17, 161–616. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Stier, A.; Bize, P.; Schull, Q.; Zoll, J.; Singh, F.; Geny, B.; Gros, F.; Royer, C.; Massemin, S.; Criscuolo, F. Avian erythrocytes have functional mitochondria; opening novel perspectives for birds as animal models in the study of ageing. Front. Zool. 2013, 10, 33. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Arukwe, A.; Jaspers, V.L.B.; Eulaers, I.; Mennillo, E.; Ibor, O.R.; Frantz, A.; Covaci, A.; Malik, R.N. Oxidative stress responses in relationship to persistent organic pollutant levels in feathers and blood of two predatory bird species from Pakistan. Sci. Total Environ. 2017, 580, 26–33. [Google Scholar] [CrossRef]
- Hilscherova, K.; Blankenship, A.; Kannan, K.; Nie, M.; Williams, L.L.; Coady, K.; Bursian, S. Oxidative stress in laboratory-incubated double-crested cormorant eggs collected from the Great Lakes. Arch. Environ. Contam. Toxicol. 2003, 45, 533–546. [Google Scholar] [CrossRef]
- Herceg Romanić, S.; Marenjak, T.S.; Klinčić, D.; Janicki, Z.; Srebočan, E.; Konjević, D. Organochlorine compounds in red deer (Cervus elaphus L.) and fallow deer (Dama dama L.) from inland and coastal Croatia. Environ. Monit. Assess. 2012, 184, 5173–5180. [Google Scholar] [CrossRef]
- Klinčić, D.; Herceg Romanić, S.; Matek Sarić, M.; Grzunov, J.; Dukić, B. Polychlorinated biphenyls and organochlorine pesticides in human milk samples from two regions in Croatia. Environ. Toxicol. Pharmacol. 2014, 37, 543–552. [Google Scholar] [CrossRef]
- Bosnir, J.; Puntarić, D.; Smit, Z.; Klarić, M.; Grgić, M.; Kosanović, L.M. Organochlorine pesticides in freshwater fish from the Zagreb area. Arh. Hig. Rada Toksikol. 2007, 58, 187–193. [Google Scholar] [CrossRef]
- Lazarus, M.; Tariba Lovaković, B.; Orct, T.; Sekovanić, A.; Bilandžić, N.; Đokić, M.; Kolanović, B.S.; Varenina, I.; Jurič, A.; Lugomer, M.D.; et al. Difference in pesticides; trace metal(loid)s and drug residues between certified organic and conventional honeys from Croatia. Chemosphere 2021, 266, 128954. [Google Scholar] [CrossRef] [PubMed]



| Jakuševec | Baranja | Srijem | |||||
|---|---|---|---|---|---|---|---|
| Plasma | S9 | Plasma | S9 | Plasma | S9 | ||
| ChE (nmol min−1 mgPROT−1) | n | 10 | 10 | 24 | 24 | 19 | 20 |
| min. | 16.22 | 3.29 | 19.61 | 2.65 | 21.20 | 2.37 | |
| max. | 51.51 | 7.00 | 37.56 | 8.02 | 47.47 | 10.03 | |
| range | 35.29 | 3.70 | 17.95 | 5.36 | 26.26 | 7.66 | |
| mean | 27.31 | 4.76 | 27.83 | 4.63 | 32.49 | 4.22 | |
| SD | 10.02 | 1.42 | 5.43 | 1.50 | 7.97 | 1.88 | |
| CES (nmol min−1 mgPROT−1) | n | 10 | 10 | 24 | 24 | 19 | 20 |
| min. | 10.22 | 6.67 | 17.78 | 6.38 | 19.69 | 6.64 | |
| max. | 62.77 | 12.84 | 53.44 | 10.65 | 67.80 | 11.42 | |
| range | 52.55 | 6.18 | 35.66 | 4.28 | 48.11 | 4.78 | |
| mean | 32.89 | 8.31 | 37.33 | 8.25 | 36.07 | 8.55 | |
| SD | 16.43 | 1.73 | 9.52 | 1.16 | 11.68 | 1.29 | |
| GST (nmol min−1 mgPROT−1) | n | 10 | 10 | 24 | 24 | 17 | 19 |
| min. | 9.87 | 8.30 | 9.39 | 8.60 | 9.30 | 7.06 | |
| max. | 17.22 | 17.90 | 18.77 | 25.22 | 17.49 | 25.52 | |
| range | 7.35 | 9.61 | 9.38 | 16.62 | 8.19 | 18.46 | |
| mean | 13.17 | 13.25 | 13.67 | 14.64 | 12.65 | 13.94 | |
| SD | 2.63 | 3.65 | 2.31 | 3.74 | 2.18 | 5.02 | |
| GR (pmol min−1 mgPROT−1) | n | 10 | 10 | 24 | 23 | 19 | 20 |
| min. | 141.50 | 535.40 | 158.30 | 479.50 | 144.50 | 556.40 | |
| max. | 364.70 | 1534.00 | 605.80 | 1543.00 | 431.60 | 1623.00 | |
| range | 223.20 | 998.20 | 447.50 | 1064.00 | 287.10 | 1067.00 | |
| mean | 266.30 | 923.00 | 290.40 | 845.90 | 234.40 | 1028.00 | |
| SD | 79.16 | 285.20 | 97.18 | 265.40 | 72.17 | 250.20 | |
| GSH (RFU) | n | 10 | 10 | 24 | 23 | 18 | 17 |
| min. | 2062 | 17,294 | 2940 | 17,990 | 2012 | 15,363 | |
| max. | 6306 | 23,564 | 7426 | 25,407 | 4571 | 24,685 | |
| range | 4244 | 6270 | 4486 | 7418 | 2558 | 9323 | |
| mean | 4097 | 21,066 | 4055 | 21,955 | 3509 | 21,097 | |
| SD | 1116 | 1987 | 1026 | 1749 | 585 | 2752 | |
| ROS (RFU) | n | 10 | 10 | 24 | 24 | 19 | 20 |
| min. | 90.33 | 26.67 | 92.33 | 21.33 | 97.67 | 21.33 | |
| max. | 137.30 | 80.67 | 137.70 | 72.00 | 133.00 | 71.67 | |
| range | 47.00 | 54.00 | 45.33 | 50.67 | 35.33 | 50.33 | |
| mean | 118.50 | 52.13 | 111.80 | 39.19 | 118.50 | 38.65 | |
| SD | 14.11 | 16.84 | 10.50 | 13.98 | 8.22 | 17.77 | |
| Candidate Models | K | AICc | Δi | wi | Res. LL |
|---|---|---|---|---|---|
| ChE Plasma ~ sampling location + sex | 6 | 336.87 | 0.00 | 0.57 | −161.48 |
| ChE Plasma ~ sampling location | 5 | 337.54 | 0.67 | 0.41 | −168.10 |
| ChE Plasma ~ null | 3 | 343.76 | 6.89 | 0.02 | −168.63 |
| ChE S9 ~ null | 3 | 195.07 | 0.00 | 0.61 | −94.29 |
| ChE S9 ~ sampling location | 5 | 195.94 | 0.87 | 0.39 | −92.33 |
| ChE S9 ~ sampling location + sex | 6 | 209.58 | 14.51 | 0.00 | −97.86 |
| CES Plasma ~ sampling location + sex | 8 | 381.89 | 0.00 | 1.00 | −181.23 |
| CES Plasma ~ sampling location | 5 | 393.29 | 11.40 | 0.00 | −190.99 |
| CES Plasma ~ null | 3 | 398.61 | 16.73 | 0.00 | −196.06 |
| CES S9 ~ null | 3 | 173.55 | 0.00 | 0.76 | −83.53 |
| CES S9 ~ sampling location | 5 | 175.87 | 2.31 | 0.24 | −82.30 |
| CES S9 ~ sampling location + sex | 6 | 336.87 | 163.32 | 0.00 | −161.48 |
| GST Plasma ~ sampling location + sex | 6 | 234.15 | 0.00 | 0.67 | −110.10 |
| GST Plasma ~ null | 3 | 236.81 | 2.66 | 0.18 | −115.15 |
| GST Plasma ~ sampling location | 5 | 237.04 | 2.89 | 0.16 | −112.85 |
| GST S9 ~ sampling location + sex | 6 | 292.95 | 0.00 | 0.50 | −139.52 |
| GST S9 ~ sampling location | 5 | 294.22 | 1.27 | 1.27 | −141.46 |
| GST S9 ~ null | 3 | 294.54 | 1.60 | 0.23 | −144.02 |
| GR Plasma ~ sampling location | 5 | 332−89 | 0.00 | 1.00 | −160.79 |
| GR Plasma ~ null | 3 | 344.04 | 11.16 | 0.00 | −168.77 |
| GR Plasma ~ sampling location + sex | 6 | 578.28 | 245.40 | 0.00 | −282.19 |
| GR S9 ~ sampling location + sex | 6 | 678.64 | 0.00 | 1.00 | −332.36 |
| GR S9 ~ sampling location | 5 | 717.68 | 39.05 | 0.00 | −353.20 |
| GR S9 ~ null | 3 | 738.77 | 60.13 | 0.00 | −366.14 |
| GSH Plasma ~ sampling location | 5 | 794.85 | 0.00 | 0.94 | −391.76 |
| GSH Plasma ~ sampling location + sex | 6 | 800.47 | 5.61 | 0.06 | −393.28 |
| GSH Plasma ~ null | 3 | 820.31 | 25.45 | 0.00 | −406.90 |
| GSH S9 ~ sampling location + sex | 6 | 817.38 | 0.00 | 1.00 | −401.67 |
| GSH S9 ~ sampling location | 5 | 872.89 | 55.51 | 0.00 | −430.76 |
| GSH S9 ~ null | 3 | 899.66 | 82.28 | 0.00 | −446.57 |
| ROS Plasma ~ sampling location + sex | 6 | 377.38 | 0.00 | 1.00 | −181.73 |
| ROS Plasma ~ sampling location | 5 | 393.67 | 16.30 | 0.00 | −191.20 |
| ROS Plasma ~ null | 3 | 401.37 | 23.99 | 0.00 | −197.44 |
| ROS S9 ~ sampling location + sex | 6 | 424.95 | 0.00 | 1.00 | −206.54 |
| ROS S9 ~ sampling location | 5 | 445.28 | 20.33 | 0.00 | −217.02 |
| ROS S9 ~ null | 3 | 456.63 | 31.68 | 0.00 | −225.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjedov, D.; Velki, M.; Kovačić, L.S.; Begović, L.; Lešić, I.; Jurinović, L.; Mikuska, T.; Sudarić Bogojević, M.; Ečimović, S.; Mikuška, A. White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses. Agriculture 2023, 13, 1045. https://doi.org/10.3390/agriculture13051045
Bjedov D, Velki M, Kovačić LS, Begović L, Lešić I, Jurinović L, Mikuska T, Sudarić Bogojević M, Ečimović S, Mikuška A. White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses. Agriculture. 2023; 13(5):1045. https://doi.org/10.3390/agriculture13051045
Chicago/Turabian StyleBjedov, Dora, Mirna Velki, Lucija Sara Kovačić, Lidija Begović, Ivan Lešić, Luka Jurinović, Tibor Mikuska, Mirta Sudarić Bogojević, Sandra Ečimović, and Alma Mikuška. 2023. "White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses" Agriculture 13, no. 5: 1045. https://doi.org/10.3390/agriculture13051045
APA StyleBjedov, D., Velki, M., Kovačić, L. S., Begović, L., Lešić, I., Jurinović, L., Mikuska, T., Sudarić Bogojević, M., Ečimović, S., & Mikuška, A. (2023). White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses. Agriculture, 13(5), 1045. https://doi.org/10.3390/agriculture13051045








