Effect of Foliar Application of Phosphorus, Zinc, and Silicon Nanoparticles along with Mineral NPK Fertilization on Yield and Chemical Compositions of Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
Characterization of Nanoparticles
2.2. Experiment Treatments
2.3. Studied Characteristics
- Phosphorus content (P%) in the grains and straw was extracted as described by [29] and measured by spectrophotometer (Spectrophotometer UV-Vis Biochrom Libra S-12, Cambridge, England), absorbance measurements were performed at wavelengths ranging from 800to 900 nm) using the ascorbic acid method [30].
- Potassium content (K%) in the grains and straw was determined using the Flame photometer method [31].
- Zinc content (Zn%) in the grains and straw was determined by the atomic absorption spectrophotometer, Model 153 [32].
- Silicon content (Si%) in the grains and straw [33].
2.4. Statistical Analyses
3. Results
3.1. Grain yield Attributes
3.2. Chemical Compositions
3.2.1. Nitrogen Uptake
3.2.2. Phosphorus Uptake
3.2.3. Potassium Uptake
3.2.4. Zinc Uptake
3.2.5. Silicon Uptake
3.3. Grain and Straw Yields
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elekhtyar, N.M.; Elsharnobi, D.E.; El-Mowafi, H.F. Evaluation of New Egyptian Japonica Green Super Rice Varieties Under Fertilization and Plant Spacing. J. Plant Prod. 2021, 12, 1015–1019. [Google Scholar] [CrossRef]
- Gu, H.H.; Zhan, S.S.; Wang, S.Z.; Tang, Y.T.; Chaney, R.L.; Fang, X.H.; Cai, X.D.; Qiu, R.L. Silicon-mediated amelioration of zinc toxicity in rice (Oryza sativa L.) seedlings. Plant Soil 2012, 350, 193–204. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Bashir, K.; Nishizawa, N.K. Zn uptake and translocation in rice plants. Rice 2011, 4, 21–27. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, R.A.; Nada, A.M.; Elekhtyar, N.M. Effect of different sources of nitrogen application on rice yield and soil health in saline sodic soil. J. Soil Sci. Agric. Eng. Mansoura Univ. 2022, 13, 371–380. [Google Scholar] [CrossRef]
- Wilson, M.A.; Tran, N.H.; Milev, A.S.; Kannangara, G.S.K.; Volk, H.; Lu, G.H.M. Nanomaterials in soils. Geoderma 2008, 146, 291–302. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 11, 2429–2437. [Google Scholar] [CrossRef]
- Naderi, M.; Shahraki, A.A.D.; Naderi, R. Application of nanotechnology in the optimization of formulation of chemical fertilizers. Iran. J. Nanotech. 2011, 12, 16–23. [Google Scholar]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef]
- Son, S.; Moon, S.J.; Kim, H.; Lee, K.S.; Park, S.R. Identification of a novel NPR1 homolog gene, OsNH5N16, which contributes to broad-spectrum resistance in rice. Biochem. Biophys. Res. Commun. 2021, 549, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Salas-Leiva, J.S.; Luna-Velasco, A.; Salas-Leiva, D.E. Use of magnesium nanomaterials in plants and crop pathogens. J. Nanoparticle Res. 2021, 23, 267. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Weir, B.L. Foliar fertilization of cotton. In Physiology of Cotton; Stewart, J.M.D., Ed.; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2010; pp. 272–288. [Google Scholar] [CrossRef]
- Banfield, J.F.; Zhang, H. Nanoparticles in the Environment. In Nanoparticles and the Environment; Banfield, J.F., Navorotsky, A., Eds.; Mineralogical Society of America: Washington, DC, USA, 2001; Chapter 1; pp. 1–58. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. A review. Sci. Total Envir. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadia, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2015, 22, 2837–2845. [Google Scholar] [CrossRef]
- Qureshi, A.; Singh, D.K.; Dwivedi, S. Nano-fertilizers: A Novel Way for Enhancing Nutrient Use Efficiency and Crop Productivity. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3325–3335. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Poll. 2007, 150, 243–250. [Google Scholar]
- Kim, S.G.; Kim, K.W.; Park, E.W.; Choi, D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Am. Phytopathol. Soc. 2002, 92, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Benzon, H.R.L.; Rubenecia, M.R.U.; Ultra, V.U.; Lee, J.S.C. Nano-fertilizer affects the growth, development, and chemical properties of rice. Int. J. Agron. Agric. Res. 2015, 7, 105–117. [Google Scholar]
- Saltzman, A.; Andersson, M.S.; Asare-Marfo, D.; Lividini, K.; De Moura, F.F.; Moursi, M.; Oparinde, A.; Taleon, V. Bioforti Cation Techniques to Improve Food Security; Elsevier: Amsterdam, The Netherlands, 2016; Reference Module in Food Science. [Google Scholar]
- Ding, Y.; Wang, Y.; Zheng, X.; Cheng, W.; Shi, R.; Feng, R. Effects of foliar dressing of selenite and siliconte alone or combined with different soil ameliorants on the accumulation of As and Cd and antioxidant system in Brassica campestris. Ecotoxicol. Environ. Saf. 2017, 142, 207–215. [Google Scholar] [CrossRef]
- Sabbe, W.E.; Hodges, S.C. Interpretation of plant mineral status. In Physiology of Cotton 2009; Stewart, J.M., Oosterhuis, D.M., Heitholt, J.J., Mauney, J.R., Eds.; National Cotton Council of America: Memphis, TN, USA; Springer: London, UK, 2009; pp. 266–272. [Google Scholar]
- Black, C.A.; Evans, D.D.; Ensminger, L.E.; Clark, F.E. Methods of Soil Analysis. In Part 2-Chemical and Microbiological Properties; American Society of Agronomy Inc.: Madison, WI, USA, 1965; (C. F. comp. Research). [Google Scholar]
- Dai, L.; Sun, C.X.; Wei, Y.; Mao, L.; Gao, Y.X. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Williams, B.; Carter, C. Transmission Electron Microscopy; Springer: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Hafez, A.A.R.; Mikkelson, D.S. Colorimetric determination of Nitrogen for evaluating the nutrition status of rice. Soil Sci. Plant Anal. 1981, 12, 61–69. [Google Scholar] [CrossRef]
- Chapman, H.D.; Part, P.F. Method of Analysis for Soils, Plant and Water; University of California: Oakland, CA, USA, 1961. [Google Scholar]
- Peterpurgski, A.V. Handbook of Agronomic Chemistry; Kolop Publishing House: Moscow, Russia, 1968; pp. 29–86. (In Russian) [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Method of Soil Analysis–Part 2; American Society of Agricultural and Biological Engineers: Madison, WI, USA, 1982. [Google Scholar]
- Jackson, M.L. A simplified assay for milled rice analysis. Cereal Sci. Today J. 1967, 334–338, 340–360. [Google Scholar]
- Emami, A. Methods of Plant Analysis; Soil and Water Res. Institute: Tehran, Iran, 1996; Volume 982. [Google Scholar]
- Wei-min, D.; Ke-qin, Z.; Bin-wu, D.; Cheng-Xiao, S.; Kang, Z.; Run, C.; Jie-yun, Z. Rapid determination of silicon content in rice. Rice Sci. 2005, 12, 145–147. [Google Scholar]
- A.O.A.C Association of Official Analytical Chemists. Official Methods of Analysis Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Adair, C.R. The Mc Gill Miller method for determining the milled quality of small samples of rice. Rice J. 1952, 55, 21–23. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley Sons: New York, NY, USA, 1984. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Freed, R. MSTATC version 1.2. In Department of Crop and Soil Sciences; Michigan State University: East Lansing, MI, USA, 1986. [Google Scholar]
- Elekhtyar, N.M. Statistical Analysis of Experiments Using MSTAT-C Computer Software; Academy of Scientific Research and Technology (ASRT): Cairo, Egypt, 2018; No. 3486/2018; ISBN 978-977-268-723-7. [Google Scholar]
- Elekhtyar, N.M.; Awad-Allah, M.M.A.; Alshallash, K.S.; Alatawi, A.; Alshegaihi, R.M.; Alsalmi, R.A. Impact of Arbuscular Mycorrhizal Fungi, Phosphate Solubilizing Bacteria and Selected Chemical Phosphorus Fertilizers on Growth and Productivity of Rice. Agriculture 2022, 12, 1596. [Google Scholar] [CrossRef]
- Welch, R.M. Linkages between trace elements in food crops and human health. In Micronutrient Deficiencies in Global Crop Production Alloway; Springer Science: Berlin/Heidelberg, Germany, 2008; pp. 41–61. [Google Scholar]
- Aktas, H.; Abak, K.; Ozturk, L.; Cakmak, S. The effect of Zinc on growth and shoot concentrations of sodium and potassium in pepper plants under salinity stress. Turk. J. Agric. For. 2006, 30, 407–412. [Google Scholar]
- Zoz, T.; Steiner, F.; Vitor, J.; Paulo Testa, E.; Pereira Seidel, R.; Fey, D.; Dalazen, C.; Zoz, A. Foliar fertilization with molybdenum in wheat. J. Ciência Rural St. Maria 2012, 42, 78. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Elkhoby, W.M.; Zidan, A.A. Prospects of using rhizobium as supplements for mineral nitrogen fertilizer on rice production in Egypt. J. Agric. Res. Kafr El-Sheikh Univ. 2015, 41, 875–884. [Google Scholar]
- Cuong, T.X.; Ullah, H.; Datta, A.; Han, T.C. Effects of silicon- based fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam. Rice Sci. 2017, 24, 283–290. [Google Scholar] [CrossRef]
- Elekhtyar, N.M. Influence of different plant growth promoting rhizobacteria (PGPR) strains on rice promising line. In Proceedings of the Sixth Field Crops Conference, FCRI, ARC, Giza, Egypt, 22–23 November 2016; Volume 6, pp. 327–335. [Google Scholar]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Farahat, M.M.; Ibrahim, S.; Taha, L.S.; El-Quesni, F.E.M. Response of vegetative growth and some chemical constituents of Cupressus sempervirens L. to foliar application of ascorbic acid and zinc at Nubaria. World J. Agric. Sci. 2007, 3, 496–502. [Google Scholar]
- Sasson, Y.; Levy-Ruso, G.; Toledano, O.; Ishaaya, I. Nanosuspensions: Emerging Novel Arochemical Formulations. In Insecticides Design Using Advanced Technologies Netherlands; Ishaaya, I., Nauen, R., Horowitz, A.R., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2007; pp. 1–32, Schaad NW, Opgenn. [Google Scholar]
- Lavinsky, A.O.; Detmann, K.C.; Reis, J.V.; Avila, R.T.; Sanglard, M.L.; Pereira, L.F.; Sanglard, L.M.V.P.; Rodrigues, F.; Araujo, W.L.; DaMatta, F.M. Silicon improves rice grain yield and photosynthesis specifically when supplied during the reproductive growth stage. J. Plant Physiol. 2016, 206, 125–132. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Mikhael, B.B.; Wissa, M.T. Utilization of compost and compost tea for improving Egyptian hybrid rice one cultivar. J. Sustain. Agric. Sci. 2017, 43, 141–149. [Google Scholar] [CrossRef]
- Elekhtyar, N.M. Effect of Bio and Mineral Nitrogen Fertilizer on Growth, Yield and Chemical Composition of Rice. Ph.D. Thesis, Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, Egypt, 2011. [Google Scholar]
- Elekhtyar, N.M. Impact of three strains of Bacillus as bio NPK fertilizers and three levels of mineral NPK fertilizers on growth, chemical compositions and yield of Sakha 106 rice cultivar. Int. J. Chem. Tech. Res. 2015, 8, 2150–2156. [Google Scholar]
- Naik, S.K.; Das, D.K. Effect of split application of zinc on yield of rice (Oryza sativa L.) in an inceptisol. Arch. Agron. Soil Sci. 2007, 53, 305–313. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Q.; Shen, Z.; Ma, T. Effects of silicon on salinity tolerance of two barley cultivars. Plant Nutr. 1996, 19, 173–183. [Google Scholar] [CrossRef]
- Norollah, K.; Hossein, A.N.; Hamid, R.M.; Benjamin, T. Effects of Silicon and Zinc Nanoparticles on Growth, Yield, and Biochemical Characteristics of Rice. Agron. J. 2019, 111, 3084–3090. [Google Scholar]
- Singh, K.R.; Singh, J.P.; Singh, Y.; Singh, K.K. Effect of level and time of silicon application on growth, yield and its uptake by rice (Oryza sativa). Indian J. Agric. Sci. 2006, 76, 410–413. [Google Scholar]
- Korndorfer, G.H.; Snyder, G.H.; Ulloa, M.; Datnoff, L.E. Calibration of soil and plant silicon for rice production. J. Plant Nutr. 2001, 24, 1071–1084. [Google Scholar] [CrossRef]
- Abou-Baker, N.H.; Abd-Eladl, M.; Abbas, M. Use silicate and different cultivation practices in alleviating salt stress effect on Bean Plants. Aust. J. Basic Appl. Sci. 2011, 5, 769–781. [Google Scholar]
- Wissa, M.T.; Awad-Allah, M.M.A.; Elekhtyar, N.M. Response of Egyptian hybrid rice one cultivar to times of nitrogen application and foliar spraying of ascobien compound. J. Plant Prod. 2016, 7, 567–574. [Google Scholar] [CrossRef]
- Ma, I.F. Role of silicon enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Lux, A.; Luxov, M.; Abe, J.; Tanimoto, E.; Hattori, T.; Inanaga, S. The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 2003, 158, 437–441. [Google Scholar] [CrossRef]
- Imran, M.; Rehim, A. Zinc fertilization approaches for agronomic biofortification and estimated human bioavailability of zinc in maize grain. Arch. Agron. Soil Sci. 2016, 63, 410–413. [Google Scholar] [CrossRef]
- Tahir, M.A.; Rahmatullah, A.T.; Ashraf, M.; Kanwal, S.; Magsood, M.A. Beneficial effects of silicon in wheat (Triticum aestivum L.) under salinity stress. Pak. J. Bot. 2006, 38, 1715–1722. [Google Scholar]
- Fageria, N.K.; Dos Santos, A.B.; Cobucci, T. Zinc nutrition of lowland rice. Commun. Soil Sci. Plant Anal. 2011, 42, 1719–1727. [Google Scholar] [CrossRef]
- Mabesa, R.L.; Impa, S.M.; Grewal, D.; Johnson-Beebout, S.E. Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. Field Crops Res. 2013, 149, 223–233. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mobasser, H.R.; Asadimanesh, H.; Gholizadeh, A. Investigating the effect of potassium, zinc and silicon on grain yield, yield components and their absorption in grain rice (Oryza sativa L.). Elect. J. Soil Manag. Sustain. 2014, 4, 1–24. [Google Scholar]
- Farooq, M.; Ullah, A.; Rehman, A.; Nawaz, A.; Nadeem, A.; Wakeel, A.; Nadeem, F.; Siddique, K.H.M. Application of zinc improves the productivity and biofortification of fine grain aromatic rice grown in dry seeded and puddle transplanted production systems. Field Crops Res. 2018, 216, 53–62. [Google Scholar] [CrossRef]
- Mehrabanjoubani, P.; Abdolzadeh, A.; Sadeghipour, H.R.; Aghdasi, M. Impacts of silicon nutrition on growth and nutrient status of rice plants grown under varying zinc regimes. Theor. Exp. Plant Physiol. 2015, 27, 19–29. [Google Scholar] [CrossRef]
- Singh, N.B.; Nimisha, A.; Kavita, Y.; Deepti, S.; Pandey, J.K.; Singh, S.C. Zinc Oxide Nanoparticles as Fertilizer for the Germination, Growth and Metabolism of Vegetable Crops. J. Nanoeng. Nanomanufacturing 2013, 3, 353–364. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Poll. 2011, 159, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.N.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Raja, K.; Reddy, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Rodrigues, F.A.; Benhamou, N.; Jones, J.B.; Belanger, R.R. Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology 2003, 93, 535–546. [Google Scholar]
- Savant, N.K.; Datnoff, L.E.; Snyder, G.H. Depletion of plant available silicon in soils: A possible cause of declining rice yields. Commun. Soil Sci. Plant Anal. 1997, 28, 1245–1252. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Metwally, T.F.; Nour El-Din, M. Evaluation of bio-NPK and compost tea on seedling vigor and yield of rice. In Proceedings of the 1st International Conference of Applied Microbiology, Cairo, Egypt, 1–3 March 2016; Volume 1, pp. 8–20. [Google Scholar]
- Basu, S.; Roychoudhury, A.; Sanyal, S.; Sengupta, D.N. Carbohydrate content and antioxidative potential of the seed of the edible indica rice (Oryza sativa L.) cultivars. Indian J. Biochem. Biophys. 2012, 49, 115–123. [Google Scholar]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna radiata) and Gram (Cicer arietinum) Seedlings Using Plant Agar Method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef]
- Sirisena, D.N.; Dissanayake, D.M.N.; Somaweera, K.A.T.N.; Karunaratne, V.; Kottegoda, N. Use of Nano-K Fertilizer as a Source of Potassium in Rice Cultivation. Ann. Sri Lanka Dep. Agric. 2013, 15, 257–262. [Google Scholar]
- Tarafdar, J.C.; Agrawal, A.; Raliya, R.; Kumar, P.; Burman, U.; Kaul, R.K. ZnO nanoparticles induced synthesis of polysaccharides and phosphatases by Aspergillus fungi. Adv. Sci. Eng. Med. 2012, 4, 324–328. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R.; Rathore, I. Microbial synthesis of phosphorus nanoparticles from Tri-calcium phosphate using Aspergillus tubingensis TFR-5. J. Bionanosci. 2012, 6, 84–89. [Google Scholar] [CrossRef]
- Sorour, S.G.R.; Elekhtyar, N.M.; El Rewainy, I.M.; Ibrahim, M.H.; Taha, H.A. Potential Use of Bio-Fertilizer and Stimulating Growth Compounds to Promote Rice Productivity. J. Plant Prod. 2018, 9, 559–565. [Google Scholar] [CrossRef]
- Elekhtyar, N.M. Response of Rice Yield to Application of Nitrogen from Different Sources and Forms. Master’s Thesis, Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, Egypt, 2007. [Google Scholar]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef]
- Tamai, K.; Ma, J.F. Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 2008, 307, 21–27. [Google Scholar] [CrossRef]
- Ma, J.; Nishimura, K.; Takahashi, E. Effect of silicon on the growth of rice plant at different growth stages. Soil Sci. Plant Nutr. 1989, 35, 347–356. [Google Scholar] [CrossRef]
- Elekhtyar, N.M.; Awad-Allah, M.M.A.; Zidan, A.A. Effect of Plant Growth-Promoting Rhizobacteria and Cyanobacteria on Physico-chemical and cooking characteristics of Giza 179 rice grains. Egypt. J. Agric. Res. 2022, 100, 22–29. [Google Scholar] [CrossRef]
- Naderi, M.R.; Danesh-Sharaki, A. Nanofertilizers and their role in sustainable agriculture. Int. J. Agric. Crop Sci. 2013, 5, 2229–2232. [Google Scholar]
- Mikael, B.B.; Ghazy, H.A.; Elekhtyar, N.M.; Aziz, M.A. Using of bio and organic fertilization to reduce mineral nitrogen fertilizer and improve Sakha 108 rice cultivar productivity. Menoufia J. Plant Prod. 2021, 6, 71–82. [Google Scholar] [CrossRef]
- Xie, Y.; Li, B.; Zhang, Q.; Zhang, C. Effects of nano-silicon dioxide on photosynthetic fluorescence characteristics of Indocalamus barbatus McClure. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2012, 2, 59–63. [Google Scholar]
- Zidan, A.A.; Elekhtyar, N.M. Response of two rice cultivars to bio and inorganic fertilization. J. Agric. Res. Kafrelsheikh Univ. 2015, 41, 863–874. [Google Scholar]
- Awad-Allah, M.M.A.; Elekhtyar, N.M.; Said, M.M.; Abdein, M.A.; Shamseldin, S.A.M. Gene action and genetic improvement of parental lines in hybrid rice for developing new hybrids. J. Appl. Sci. 2022, 22, 55–67. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A.; Elekhtyar, N.M.; El-Abd, M.A.-E.-M.; Abdelkader, M.F.M.; Mahmoud, M.H.; Mohamed, A.H.; El-Diasty, M.Z.; Said, M.M.; Shamseldin, S.A.M.; Abdein, M.A. Development of New Restorer Lines Carrying Some Restoring Fertility Genes with Flowering, Yield and Grains Quality Characteristics in Rice (Oryza sativa L.). Genes 2022, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Elekhtyar, N.M. Efficiency of Pseudomonas fluorescence as Plant Growth-Promoting Rhizobacteria (PGPR) for the enhancement of seedling vigor, nitrogen uptake, yield and its attributes of rice (Oryza sativa L.). Int. J. Sci. Res. Agric. Sci. 2015, 2, 57–67. [Google Scholar]
- AL-Huqail, A.A.; Kumar, P.; Eid, E.M.; Adelodun, B.; Abou Fayssal, S.; Singh, J.; Arya, A.K.; Goala, M.; Kumar, V.; Širi´c, I. Risk Assessment of Heavy Metals Contamination in Soil and Two Rice (Oryza sativa L.) Varieties Irrigated with Paper Mill Effluent. Agriculture 2022, 12, 1864. [Google Scholar] [CrossRef]

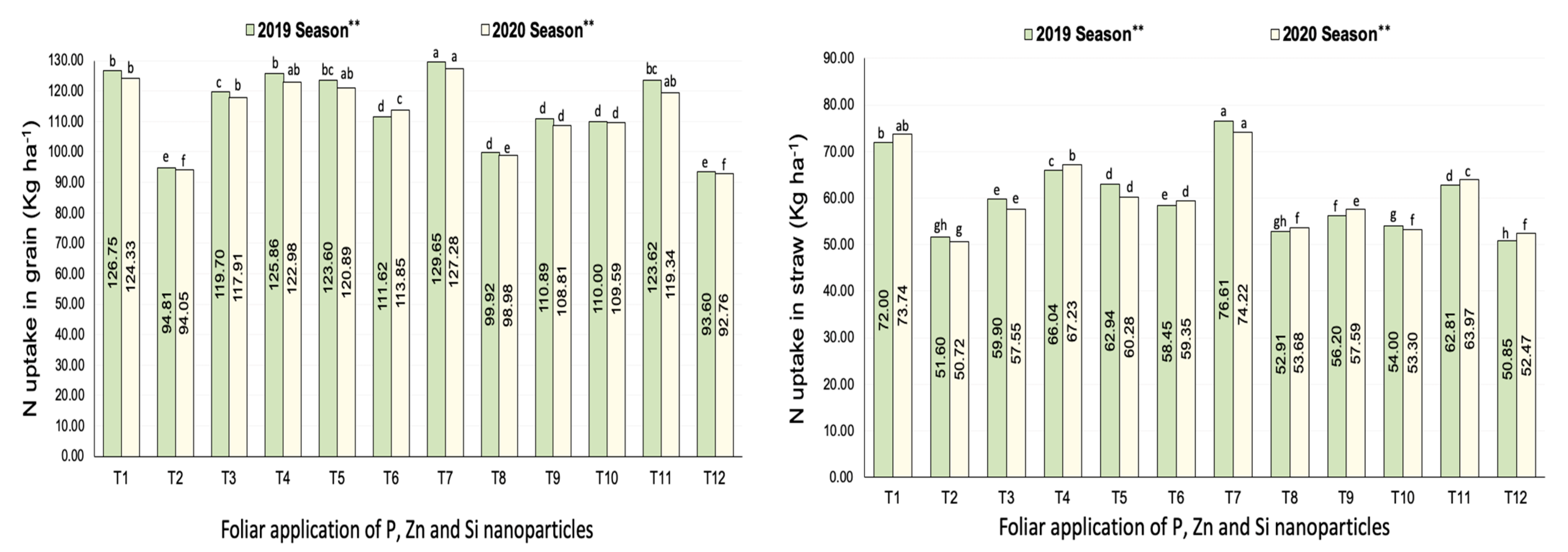
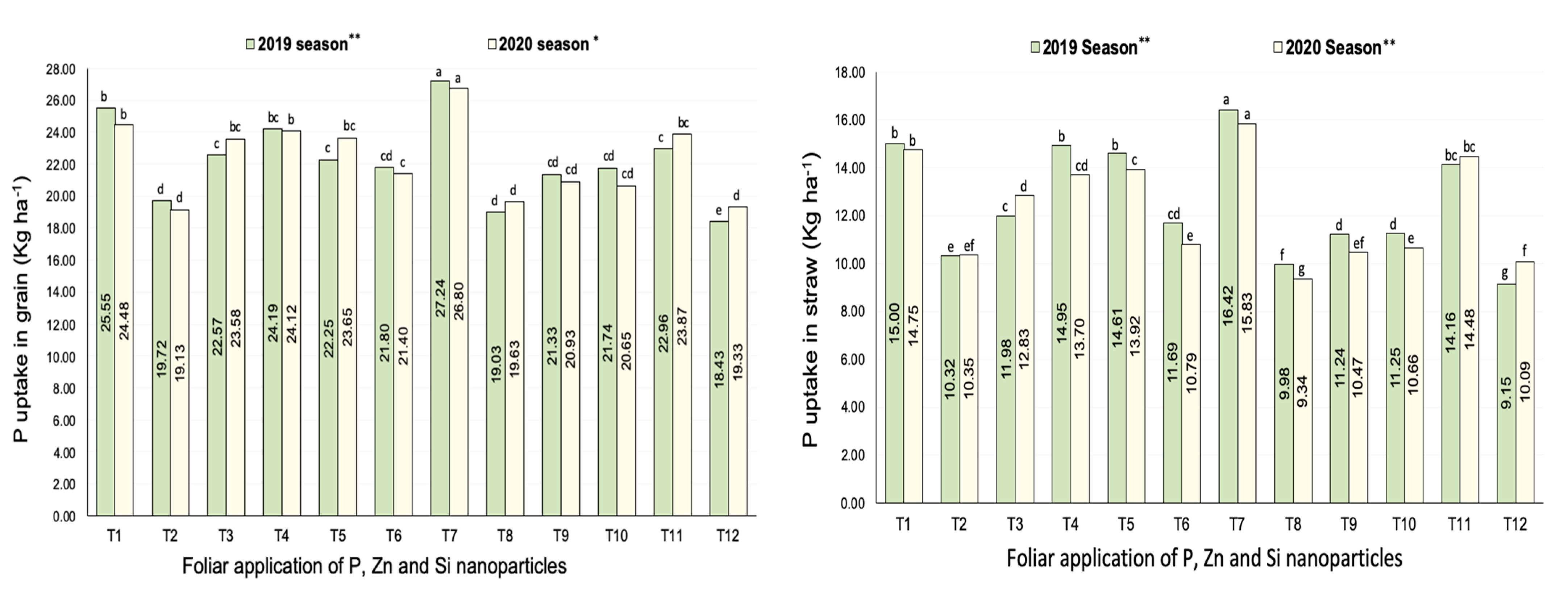
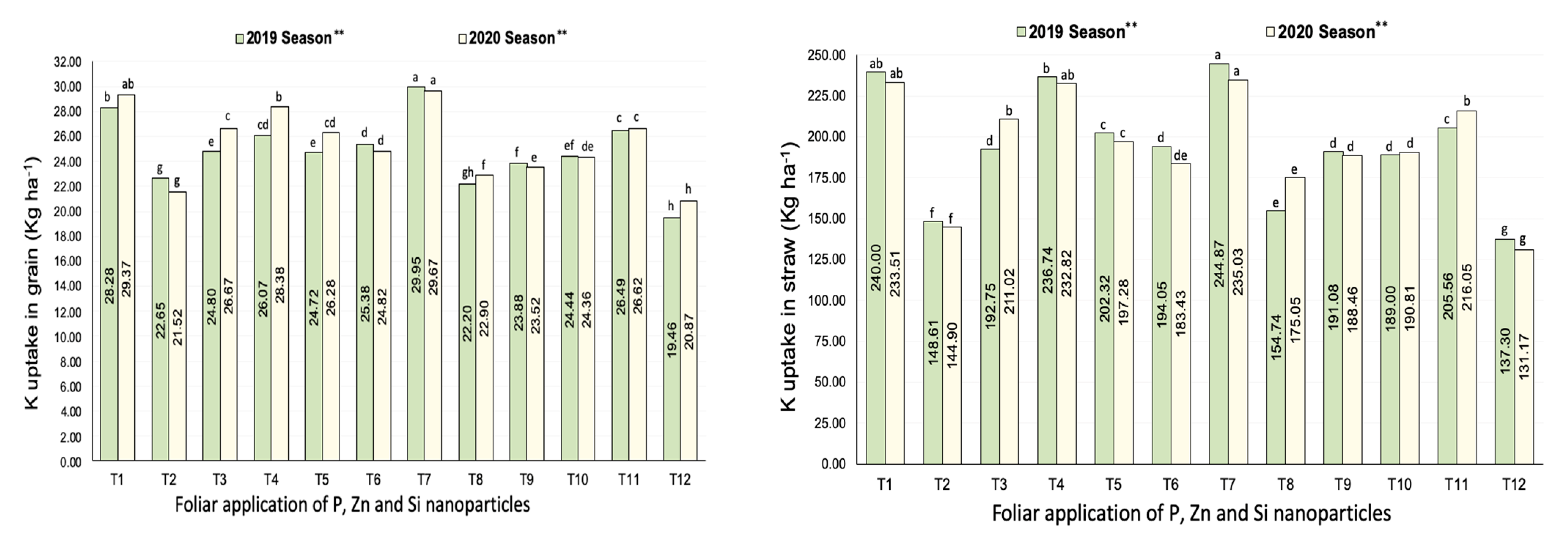

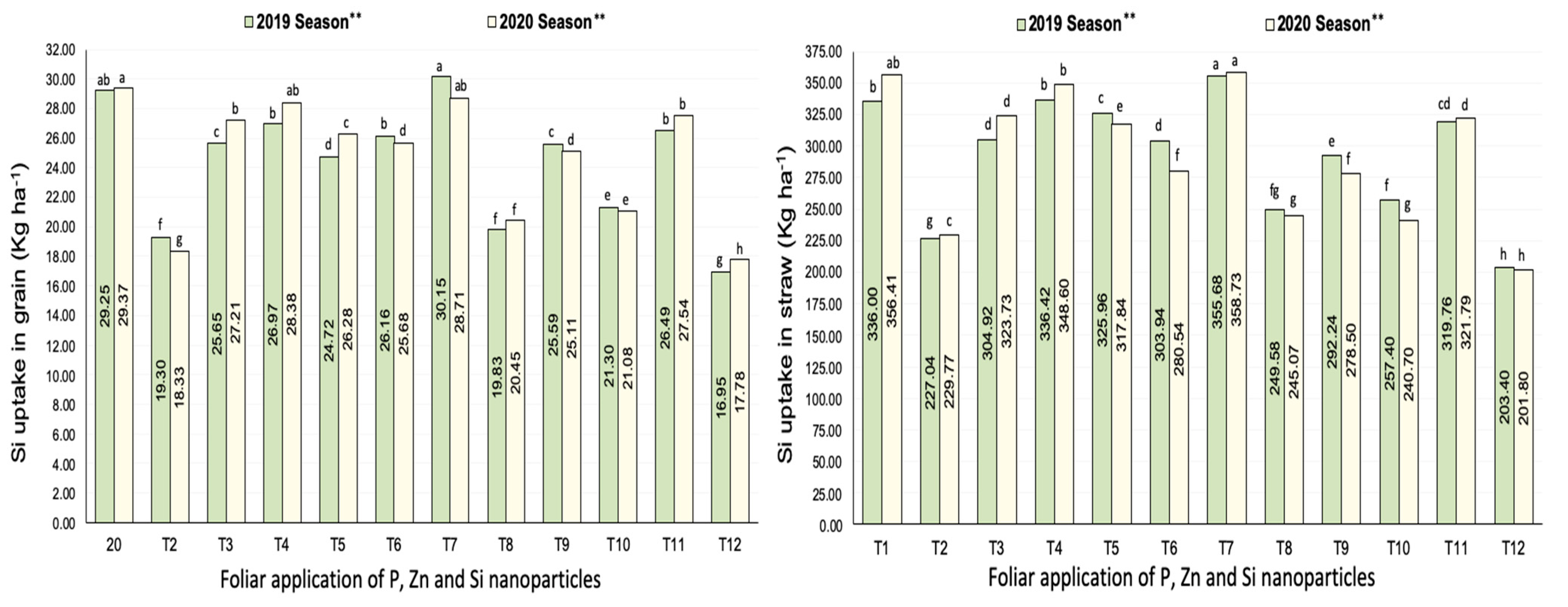

| Treatments | No. of Panicles m−2 | Filled Grains wt. Panicle−1 (g) | Panicle Length (cm) | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| N165:P36:K60 (NPK) | 458.3 b | 458.3 b | 2.03 a | 2.33 a | 19.33 ab | 19.17 |
| N110:P24:K40 (2/3 NPK) | 408.3 bc | 416.7 h | 1.70 f | 1.88 ef | 17.33 bc | 17.67 |
| 2/3 NPK + PNPs1000 | 441.7 bc | 433.3 e | 1.83 c-e | 1.92 d-f | 18.67 a-c | 19.00 |
| 2/3 NPK + PNPs3000 | 455.0 b | 458.3 b | 1.95 a-c | 2.13 b | 18.33 a-c | 18.33 |
| 2/3 NPK + PNPs5000 | 441.7 bc | 425.0 f | 1.88 b-d | 1.97 c-e | 17.67 bc | 18.67 |
| 2/3 NPK + ZnNPs25 | 450.0 b | 433.3 e | 1.78 d-f | 1.83 fg | 18.67 a-c | 18.33 |
| 2/3 NPK + ZnNPs50 | 541.7 a | 466.7 a | 2.00 ab | 2.27 ab | 20.33 a | 17.67 |
| 2/3 NPK + ZnNPs100 | 425.0 bc | 433.3 e | 1.72 ef | 1.85 fg | 17.33 bc | 18.33 |
| 2/3 NPK + SiNPs50 | 441.7 bc | 418.3 g | 1.77 d-f | 1.92 d-f | 18.33 a-c | 18.00 |
| 2/3 NPK + SiNPs100 | 450.0 b | 441.7 d | 1.92 a-c | 2.00 cd | 18.67 a-c | 17.67 |
| 2/3 NPK + SiNPs200 | 450.0 b | 450.0 c | 1.93 a-c | 2.03 c | 18.00 bc | 18.67 |
| N0:P0:K0 (0 NPK) | 391.7 c | 400.0 i | 1.52 g | 1.78 g | 17.00 c | 17.33 |
| F. test | ** | ** | ** | ** | ** | NS |
| Treatments | No. of Grains Panicle−1 | Filled Grains (%) | 1000-Grains wt.(g) | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| N165:P36:K60 (NPK) | 81.67 a | 85.00 a | 86.33 ab | 86.33 ab | 25.00 a–c | 25.00 ab |
| N110:P24:K40 (2/3 NPK) | 70.00 cd | 71.67 d | 82.00 bc | 81.67 bc | 26.33 a | 26.00 a |
| 2/3 NPK + PNPs1000 | 71.67 cd | 75.00 cd | 83.00 bc | 82.67 a–c | 24.33 b–d | 25.00 ab |
| 2/3 NPK + PNPs3000 | 76.67 a–c | 81.67 ab | 84.33 a-c | 84.00 a–c | 23.00 c–e | 23.00 ab |
| 2/3 NPK + PNPs5000 | 71.67 cd | 73.33 cd | 85.67 a-c | 85.00 a–c | 23.00 c–e | 23.00 ab |
| 2/3 NPK + ZnNPs25 | 70.00 cd | 73.33 cd | 85.33 a-c | 84.67 a–c | 22.67 de | 22.33 b |
| 2/3 NPK + ZnNPs50 | 80.00 ab | 81.67 ab | 88.00 a | 87.00 a | 21.33 e | 22.33 b |
| 2/3 NPK + ZnNPs100 | 71.67 cd | 71.67 d | 83.00 bc | 83.67 a–c | 24.33 b–d | 25.00 ab |
| 2/3 NPK + SiNPs50 | 71.67 cd | 71.67 d | 83.33 a–c | 84.00 a–c | 23.33 c–e | 24.00 ab |
| 2/3 NPK + SiNPs100 | 73.33 b–d | 71.67 d | 85.00 a–c | 84.67 a–c | 23.67 cd | 24.33 ab |
| 2/3 NPK + SiNPs200 | 75.00 a–d | 78.33 bc | 85.67 a–c | 86.00 ab | 22.67 de | 22.00 b |
| N0:P0:K0 (0 NPK) | 68.33 d | 70.00 d | 81.33 c | 80.67 c | 25.67 ab | 25.67 a |
| F. test | ** | ** | ** | ** | ** | * |
| Treatments | Hulling (%) | Milling (%) | Broken Rice (%) | Protein Content (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| N165:P36:K60 (NPK) | 79.80 b | 78.68 b | 71.00 b | 71.02 ab | 33.96 gh | 33.42 d | 8.855 ab | 8.614 ab |
| N110:P24:K40 (2/3 NPK) | 75.70 g | 75.14 fg | 66.48 g | 66.23 e | 35.63 c | 35.96 ab | 6.724 d | 7.021 e |
| 2/3 NPK + PNPs1000 | 76.31 f | 76.53 e | 69.27 e | 69.49 c | 33.82 h | 34.80 c | 7.735 c | 7.557 c |
| 2/3 NPK + PNPs3000 | 78.99 c | 78.43 bc | 70.80 bc | 70.83 ab | 34.21 fg | 33.73 d | 8.110 bc | 7.735 bc |
| 2/3 NPK + PNPs5000 | 76.50 f | 76.37 e | 69.07 e | 69.90 bc | 34.99 d | 35.13 c | 8.330 b | 7.431 cd |
| 2/3 NPK + ZnNPs25 | 77.18 e | 76.92 de | 69.97 d | 69.52 c | 35.44 c | 34.87 c | 7.616 c | 7.514 c |
| 2/3 NPK + ZnNPs50 | 80.56 a | 79.83 a | 71.70 a | 71.15 a | 34.56 ef | 34.10 d | 8.925 a | 8.511 a |
| 2/3 NPK + ZnNPs100 | 75.44 gh | 75.52 f | 67.37 f | 66.67 de | 36.20 b | 36.32 ab | 7.497 cd | 7.200 d |
| 2/3 NPK + SiNPs50 | 76.33 f | 76.37 e | 68.02 f | 67.63 d | 36.58 a | 35.83 b | 7.735 c | 7.810 b |
| 2/3 NPK + SiNPs100 | 77.36 e | 77.36 d | 70.18 cd | 69.92 bc | 34.92 de | 33.89 d | 7.497 cd | 7.735 bc |
| 2/3 NPK + SiNPs200 | 77.95 d | 77.93 c | 70.58 b-d | 70.61 a-c | 34.68 de | 34.07 d | 8.214 bc | 7.110 d |
| N0:P0:K0 (0 NPK) | 74.97 h | 74.90 g | 65.60 h | 66.04 e | 36.50 ab | 36.53 a | 6.670 d | 6.840 f |
| F. test | ** | ** | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elekhtyar, N.M.; AL-Huqail, A.A. Effect of Foliar Application of Phosphorus, Zinc, and Silicon Nanoparticles along with Mineral NPK Fertilization on Yield and Chemical Compositions of Rice (Oryza sativa L.). Agriculture 2023, 13, 1061. https://doi.org/10.3390/agriculture13051061
Elekhtyar NM, AL-Huqail AA. Effect of Foliar Application of Phosphorus, Zinc, and Silicon Nanoparticles along with Mineral NPK Fertilization on Yield and Chemical Compositions of Rice (Oryza sativa L.). Agriculture. 2023; 13(5):1061. https://doi.org/10.3390/agriculture13051061
Chicago/Turabian StyleElekhtyar, Nehal M., and Arwa A. AL-Huqail. 2023. "Effect of Foliar Application of Phosphorus, Zinc, and Silicon Nanoparticles along with Mineral NPK Fertilization on Yield and Chemical Compositions of Rice (Oryza sativa L.)" Agriculture 13, no. 5: 1061. https://doi.org/10.3390/agriculture13051061
APA StyleElekhtyar, N. M., & AL-Huqail, A. A. (2023). Effect of Foliar Application of Phosphorus, Zinc, and Silicon Nanoparticles along with Mineral NPK Fertilization on Yield and Chemical Compositions of Rice (Oryza sativa L.). Agriculture, 13(5), 1061. https://doi.org/10.3390/agriculture13051061







