A Comparative Study on Stability of Seed Characteristics in Vetch and Pea Cultivations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of Crops and Experimental Techniques
2.2. Measurements
2.3. Data Analysis
2.4. The Multi-Environment Evaluation AMMI Tool
3. Results
3.1. Vetch Seed Analysis
The AMMI Tool for Multi-Environment Evaluations in Common Vetch
3.2. Pea Seed Analysis
The AMMI Tool for Multi-Environment Evaluations in Peas
4. Stability Analysis, Comparative Results, and Discussion
4.1. Crude Protein (%DM) in Peas
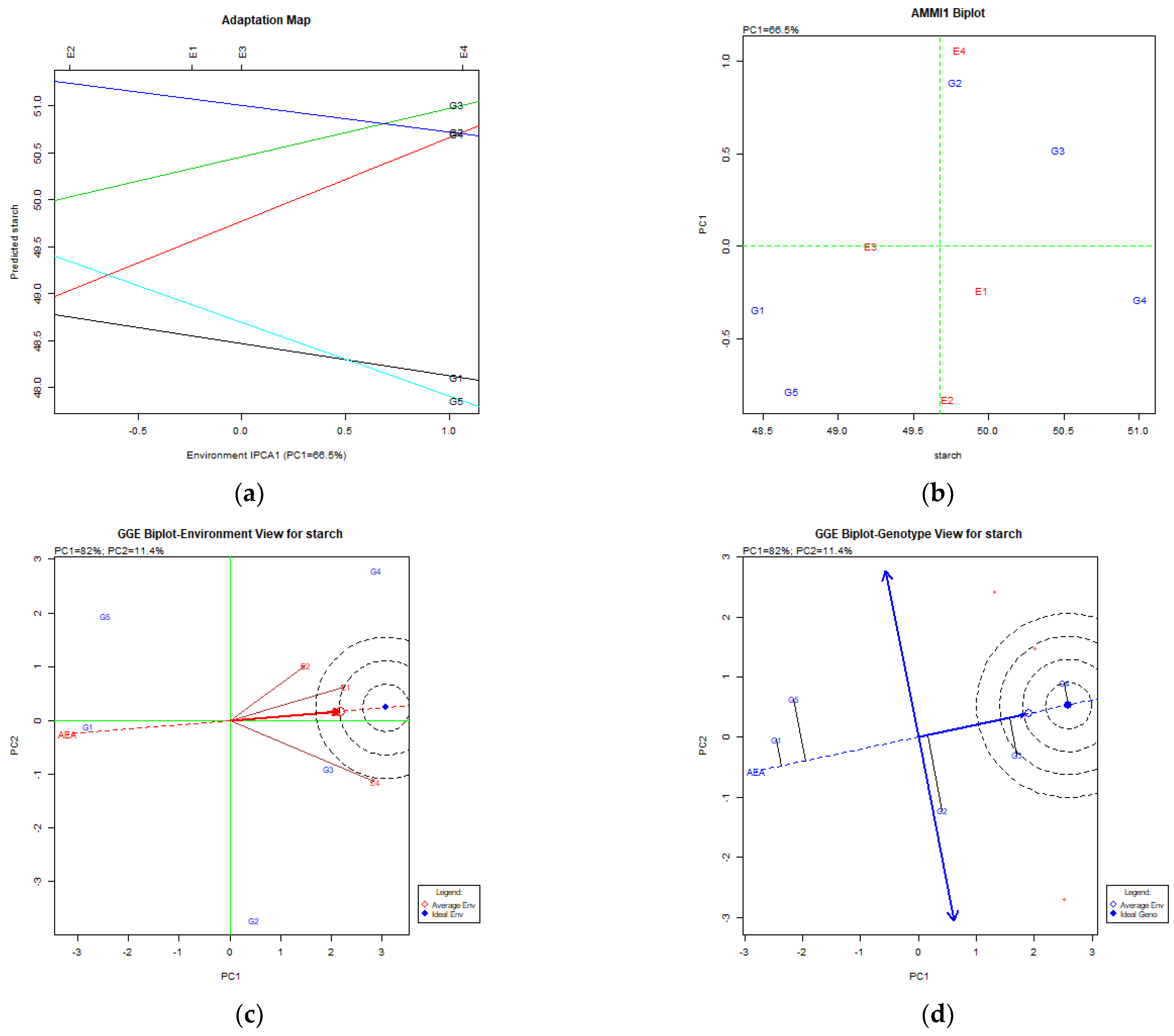
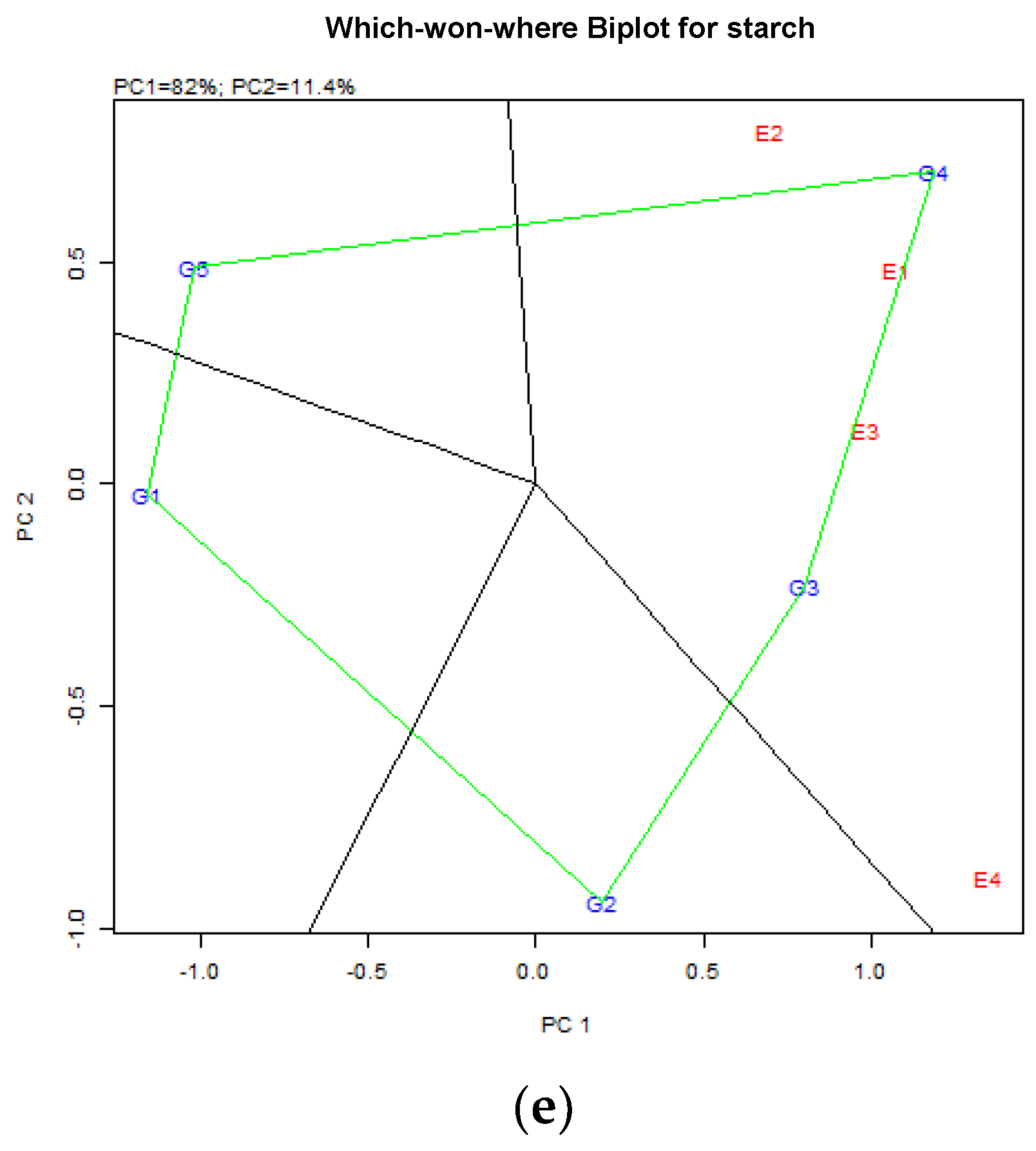
4.2. Starch Content (%DM) in Peas
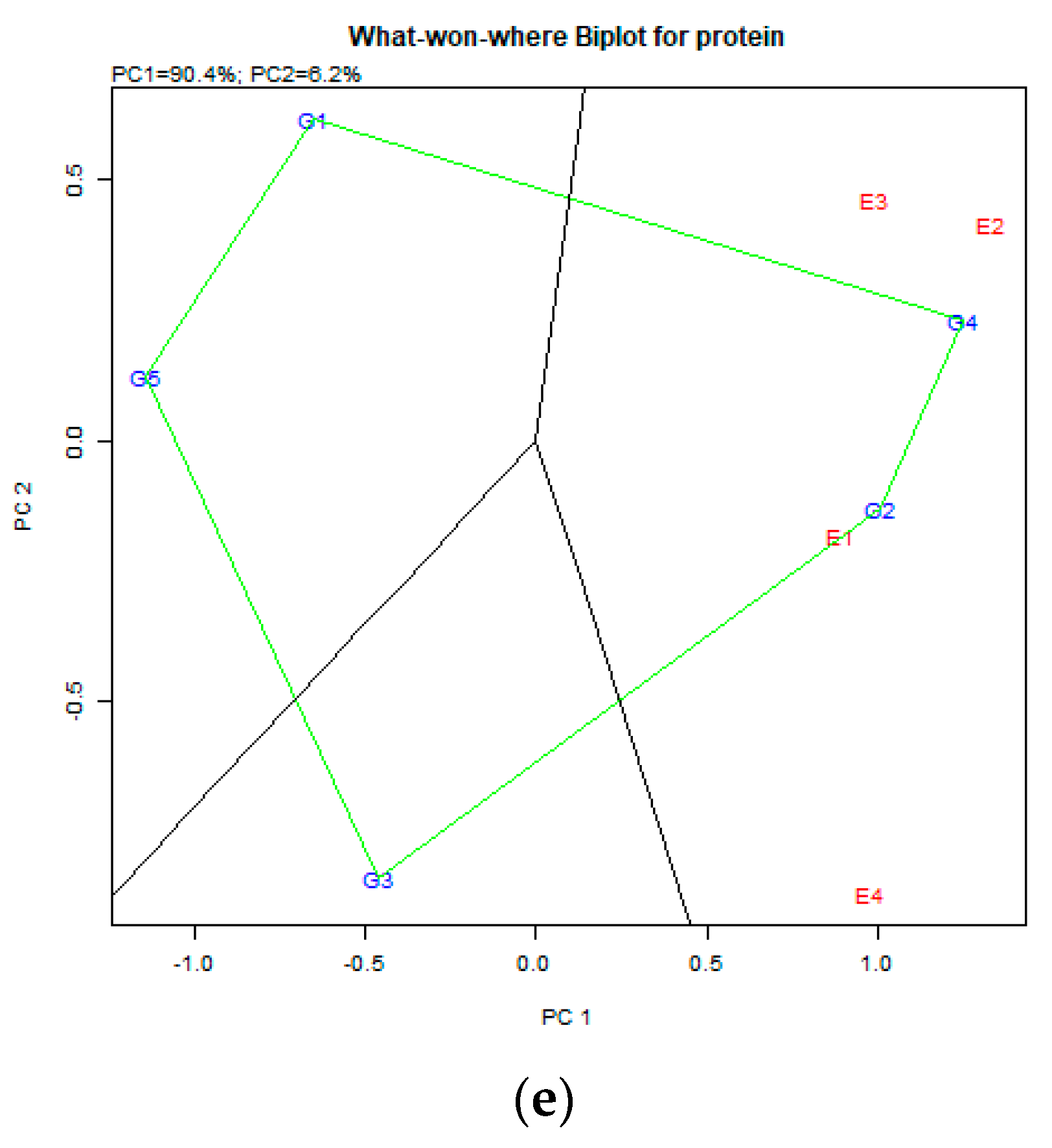
4.3. Crude Protein (%DM) in Common Vetch
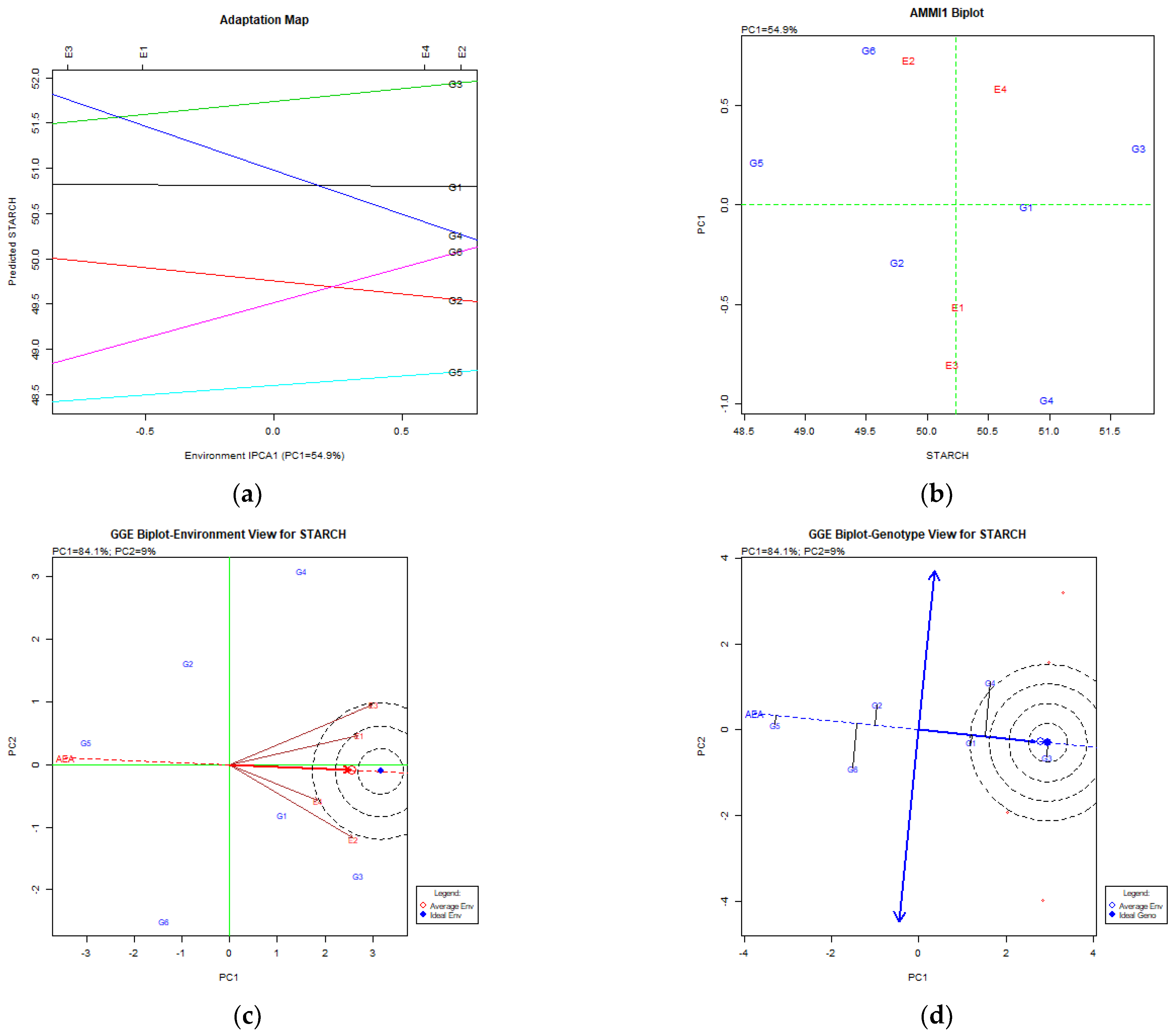
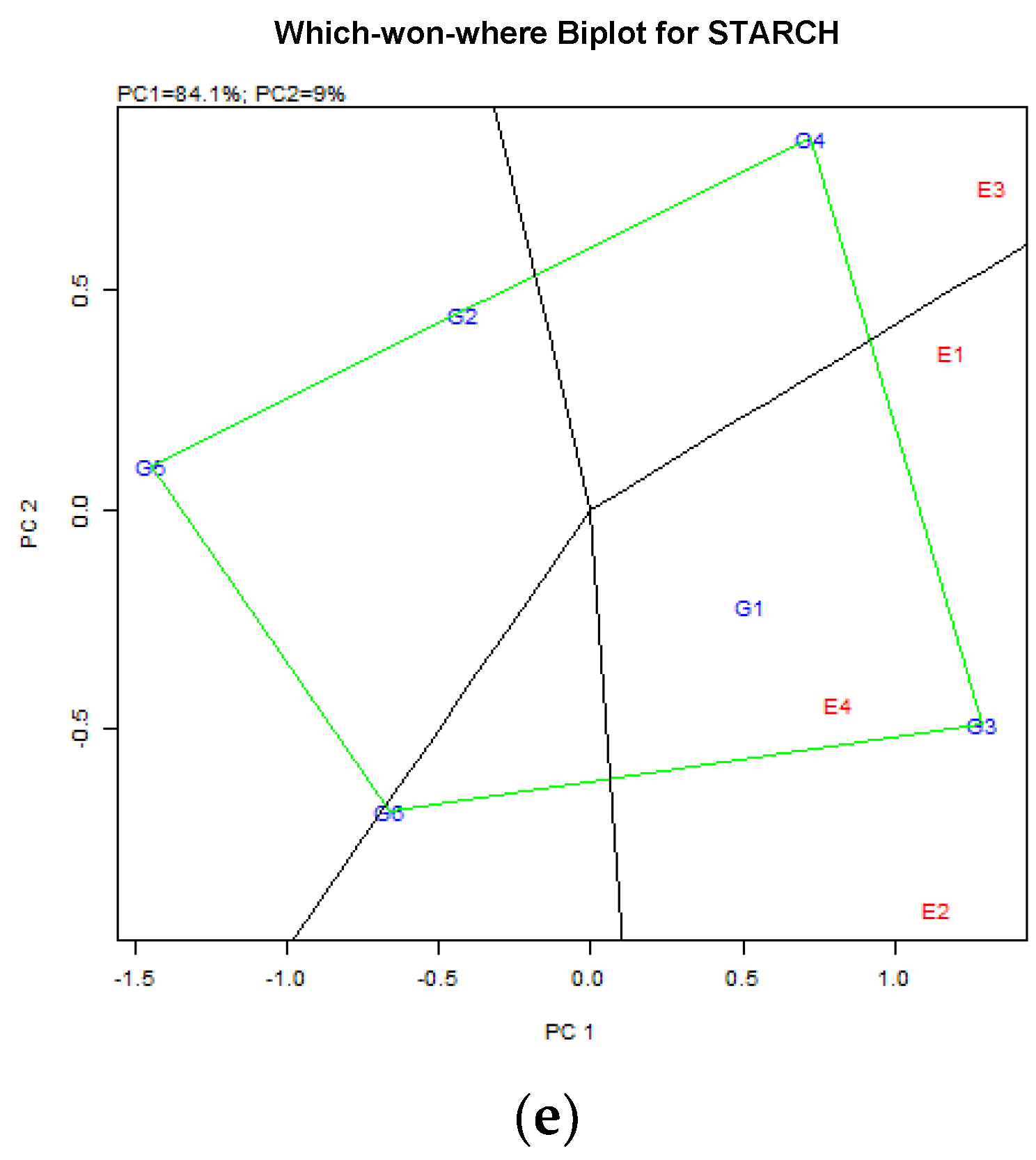
4.4. Starch Content (%DM) in Common Vetch
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cacan, E.; Kokten, K.; Bakoglu, A.; Kaplan, M.; Bozkurt, A. Evaluation of some forage pea (Pisum arvense L.) lines and cultivars in terms of herbage yield and quality. Harran Tarım Ve Gıda Bilim. Derg. 2019, 23, 254–262. [Google Scholar] [CrossRef]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. Yield components stability assessment of peas in conventional and low-input cultivation systems. Agriculture 2021, 11, 805. [Google Scholar] [CrossRef]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. Stability, the Last Frontier: Forage Yield Dynamics of Peas under Two Cultivation Systems. Plants 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Elzebroek, T.; Wind, K. Guide to Cultivated Plants; CAB International: Oxfordshire, UK, 2008. [Google Scholar]
- Tan, M.; Koc, A.; Dumlu, G.Z. Morphological characteristics and seed yield of East Anatolian local forage pea (Pisum sativum ssp. arvense L.) ecotypes. Turk. J. Field Crop. 2012, 17, 24–30. [Google Scholar]
- Elamine, Y.; Alaiz, M.; Girón-Calle, J.; Guiné, R.P.F.; Vioque, J. Nutritional Characteristics of the Seed Protein in 23 Mediterranean Legumes. Agronomy 2022, 12, 400. [Google Scholar] [CrossRef]
- Grela, E.R.; Rybinski, W.; Klebaniuk, R.; Matras, J. Morphological characteristics of some accessions in grass pea (Lathyrus sativus L.) grown in Europe and nutritional traits their seeds. Genet. Resour. Crop Evol. 2010, 57, 693–701. [Google Scholar] [CrossRef]
- Grela, E.R.; Kiczorowska, B.; Samolinska, W.; Matras, J.; Kiczorowski, P.; Rybinski, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—Content of basic nutrients; amino acids; phytochemical compounds; and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Grela, E.R.; Samolinska, W.; Kiczorowska, B.; Klebaniuk, R.; Kiczorowski, P. Content of Minerals and Fatty Acids and Their Correlation with Phytochemical Compounds and Antioxidant Activity of Leguminous Seeds. Biol. Trace Elem. Res. 2017, 180, 338–348. [Google Scholar] [CrossRef]
- Jeroch, H.; Lipiec, A.; Abel, H.; Zentek, J.; Grela, E.R.; Bellof, G. Körnerleguminosen als Futter- und Nahrungsmittel; DLG: Frankfurt am Main, Germany, 2016. [Google Scholar]
- Andueza, D.; Münoz, F.; Cardesa, C.; Delgado, I. Valor del nitritivo del forraje de doferentes cultivares de veza (Vicia sativa L.) en distans condiciones de medio de Aragon. In III Reunion Iberica de Pastos y Forraxes; Reunión Científica de la SEEP: Bragança, Portugal, 2000; pp. 485–491. [Google Scholar]
- Ballesta, A.; Lioveras, J.; Santiveri, P.; Torrent, D.; Vendrell, A. Varieties of vetch (Vicia sativa L.) for forage and grain production in Mediterranean areas. In Réhabilitation des Pâturages et des Parcours en Milieux Méditerranéen; Ferchichi, A., Ed.; CIHEAM: Zaragoza, Spain, 2004; pp. 103–106. [Google Scholar]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. Assessment of interactions between yield components of common vetch cultivars in both conventional and low-input cultivation systems. Agriculture 2021, 11, 369. [Google Scholar] [CrossRef]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. A Stability Analysis Using AMMI and GGE Biplot Approach on Forage Yield Assessment of Common Vetch in Both Conventional and Low-Input Cultivation Systems. Agriculture 2021, 11, 567. [Google Scholar] [CrossRef]
- Arif, U.; Ahmed, M.J.; Rabbani, M.A.; Arif, A.A. Assessment of genetic diversity in pea (Pisum sativum L.) landraces based on physic-chemical and nutritive quality using cluster and principal component analysis. Pak. J. Bot. 2020, 52, 575–580. [Google Scholar] [CrossRef]
- Vafias, B.; Goulas, C.; Lolas, G.; Ipsilandis, C.G. A triple stress effect on monogenotypic and multigenotypic maize populations. Asian J. Plant Sci. 2007, 6, 29–35. [Google Scholar] [CrossRef]
- Fasoulas, A.C. The Honeycomb Methodology of Plant Breeding; Department of Genetics and Plant Breeding, Aristotle University of Thessaloniki: Thessaloniki, Greece, 1988; p. 168. [Google Scholar]
- Fasoula, V.A. A novel equation paves the way for an everlasting revolution with cultivars characterized by high and stable crop yield and quality. In Proceedings of the 11th National Hellenic Conference in Genetics and Plant Breeding, Orestiada, Greece, 31 October–2 November 2006; pp. 7–14. [Google Scholar]
- Acikgoz, E.; Ustun, A.; Gul, İ.; Anlarsal, A.E.; Tekeli, A.S.; Nizam, İ.; Avcioglu, R.; Geren, H.; Cakmakcı, S.; Aydinoglu, B.; et al. Genotype × environment interaction and stability analysis for dry matter and seed yield in field pea (Pisum sativum L.). Span. J. Agric. Res. 2009, 7, 96–106. [Google Scholar] [CrossRef]
- Ceyhan, E.; Kahraman, A.; Ates, M.K.; Karadas, S. Stability analysis on seed yield and its components in peas. Bulg. J. Agric. Sci. 2012, 18, 905–911. [Google Scholar]
- Bocianowski, J.; Ksiezak, J.; Nowosad, K. Genotype by environment interaction for seeds yield in pea (Pisum sativum L.) using additive main effects and multiplicative interaction model. Euphytica 2019, 215, 191. [Google Scholar] [CrossRef]
- Rana, C.; Sharma, A.; Sharma, K.C.; Mittal, P.; Sinha, B.N.; Sharma, V.K.; Chandel, A.; Thakur, H.; Kaila, V.; Sharma, P.; et al. Stability analysis of garden pea (Pisum sativum L.) genotypes under North Western Himalayas using joint regression analysis and GGE biplots. Genet. Resour. Crop. Evol. 2021, 68, 999–1010. [Google Scholar] [CrossRef]
- Sayar, S.M. Additive Main Effects and Multiplicative Interactions (AMMI) analysis for fresh forage yield in common vetch (Vicia sativa L.) genotypes. Agric. For. 2017, 63, 119–127. [Google Scholar]
- Macák, M.; Candráková, E.; Ðalovic, I.; Prasad, P.V.V.; Farooq, M.; Korczyk-Szabó, J.; Kovácik, P.; Šimanský, V. The Influence of Different Fertilization Strategies on the Grain Yield of Field Peas (Pisum sativum L.) under Conventional and Conservation Tillage. Agronomy 2020, 10, 1728. [Google Scholar] [CrossRef]
- Uhlarik, A.; Ceran, M.; Živanov, D.; Grumeza, R.; Skøt, L.; Sizer-Coverdale, E.; Lloyd, D. Phenotypic and genotypic characterization and correlation analysis of pea (Pisum sativum L.) diversity panel. Plants 2022, 11, 1321. [Google Scholar] [CrossRef]
- Fasoula, V.A. Prognostic Breeding: A new paradigm for crop improvement. Plant Breed. Rev. 2013, 37, 297–347. [Google Scholar]
- Greveniotis, V.; Sioki, E.; Ipsilandis, C.G. Estimations of fiber trait stability and type of inheritance in cotton. Czech J. Genet. Plant Breed. 2018, 54, 190–192. [Google Scholar] [CrossRef]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. Estimations on Trait Stability of Maize Genotypes. Agriculture 2021, 11, 952. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists (AACC). Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Steel, R.G.D.; Torrie, H.; Dickey, D.A. Principles and Procedures of Statistics. A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- McIntosh, M.S. Analysis of Combined Experiments. Agron. J. 1983, 75, 153–155. [Google Scholar] [CrossRef]
- Johnson, H.W.; Robinson, H.E.; Comstock, R.E. Estimate of genetic and environmental variability in soybean. Agron. J. 1955, 47, 314–318. [Google Scholar] [CrossRef]
- Hanson, G.; Robinson, H.F.; Comstock, R.E. Biometrical studies on yield in segregating population of Korean Lespedeza. Agron. J. 1956, 48, 268–274. [Google Scholar] [CrossRef]
- Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani Publishers: New Delhi, India, 1977; p. 304. [Google Scholar]
- Koundinya, A.V.V.; Ajeesh, B.R.; Hegde, V.; Sheela, M.N.; Mohan, C.; Asha, K.I. Genetic parameters, stability and selection of cassava genotypes between rainy and water stress conditions using AMMI, WAAS, BLUP and MTSI. Sci. Hortic. 2021, 281, 109949. [Google Scholar]
- Broderick, G.A. Effects of varying dietary protein and energy levels on the production of lactating dairy cows. J. Dairy Sci. 2003, 86, 1370–1381. [Google Scholar] [CrossRef]
- Ipharraguerre, I.R.; Clark, J.H. Varying Protein and Starch in the Diet of Dairy Cows. II. Effects on Performance and Nitrogen Utilization for Milk Production. J. Dairy Sci. 2005, 88, 2556–2570. [Google Scholar] [CrossRef]
- Akhlaghi, B.; Ghasemi, E.; Alikhani, M.; Ghaedi, A.; Nasrollahi, S.M.; Ghaffari, M.H. Infuence of reducing starch in the diets with similar protein and energy contents on lactation performance, ruminal fermentation, digestibility, behaviour and blood metabolites in primiparous and multiparous dairy cows. Vet. Med. Sci. 2022, 8, 808–821. [Google Scholar] [CrossRef]
- Buryakov, N.P.; Aleshin, D.E.; Buryakova, M.A.; Zaikina, A.S.; Laptev, G.Y.; Ilina, L.A.; Petrov, A.S.; Kostomakhin, N.M.; Sheikh, A.I.E.; Sahwan, F.M.; et al. Influence of Using Various Levels of Protein Concentrate in Rations of Ayrshire Dairy Cows on Rumen Microbiome, Reproductive Traits and Economic Efficiency. Vet. Sci. 2022, 9, 534. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, X.L.; Nan, Z.B.; Zhang, Z.X. Potential value of the common vetch (Vicia sativa L.) as an animal feedstuff: A review. J. Anim. Physiol. Anim. Nutr. 2017, 101, 807–823. [Google Scholar] [CrossRef]
- Castell, A.G.; Guenter, W.; Igbasan, F.A. Nutritive value of peas for nonruminant diets. Anim. Feed Sci. Technol. 1996, 60, 209–227. [Google Scholar] [CrossRef]
- Bastianelli, D.; Grosjean, F.; Peyronnet, C.; Duparque, M.; Regnier, J.M. Feeding value of pea (Pisum sativum, L.)—1 Chemical composition of different categories of pea. Anim. Sci. 1998, 67, 609–619. [Google Scholar] [CrossRef]
- Hood-Niefer, S.D.; Warkentin, T.D.; Chibbar, R.N.; Vandenberg, A.; Tyler, R.T. Effect of genotype and environment on the concentrations of starch and protein in, and the physicochemical properties of starch from, field pea and fababean. J. Sci. Food Agric. 2011, 92, 141–150. [Google Scholar] [CrossRef]
- Yihunie, T.A.; Gesesse, C.A. GGE Biplot analysis of genotype by environment interaction in field pea (Pisum sativum L.) genotypes in North Western Ethiopia. J. Crop Sci. Biotechnol. 2018, 21, 67–74. [Google Scholar] [CrossRef]
- Sayar, M.S.; Han, Y. Forage Yield Performance of Forage Pea (Pisum sativum spp. arvense L.) Genotypes and Assessments Using GGE Biplot Analysis. J. Agric. Sci. Technol. 2016, 18, 1621–1634. [Google Scholar]
- Georgieva, N.; Nikolova, I.; Kosev, V. Association study of yield and its components in pea (Pisum sativum L.). Int. J. Pharmacogn. 2015, 2, 536–542. [Google Scholar]
- Sayar, M.S. Path coefficient and correlation analysis between forage yield and its affecting components in common vetch (Vicia sativa L.). Legume Res. 2014, 37, 445–452. [Google Scholar] [CrossRef]
- Tiryaki, G.Y.; Cil, A.; Tiryaki, I. Revealing seed coat colour variation and their possible association with seed yield parameters in common vetch (Vicia sativa L.). Int. J. Agron. 2016, 2016, 1804108. [Google Scholar]
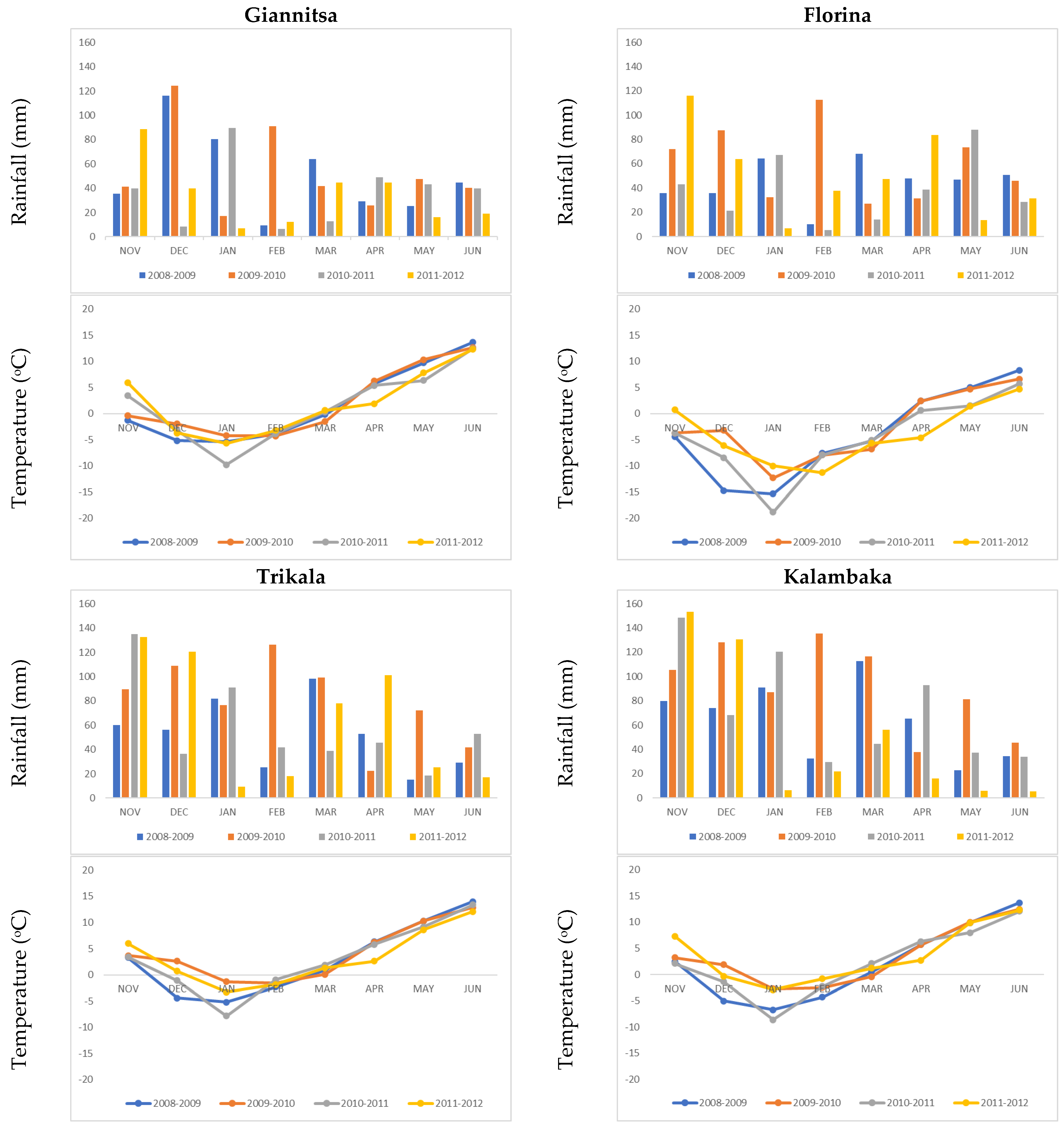



| Environments | Longitude | Latitude | Elevation (m) | Soil Texture |
|---|---|---|---|---|
| Northern Greece 1—Giannitsa | 22°39′ E | 40°77′ N | 10 | clay (C) |
| Northern Greece 2—Florina | 21°22′ E | 40°46′ N | 705 | sandy loam (SL) |
| Central Greece 1—Trikala | 21°64′ E | 39°55′ N | 120 | sandy clay loam (SCL) |
| Central Greece 2—Kalambaka | 21°65′ E | 39°64′ N | 190 | silty clay (SiC) |
| Source of Variation | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) |
|---|---|---|---|---|---|---|
| m.s. | m.s. | m.s. | m.s. | m.s. | m.s. | |
| Environments (E) | 35.116 ** | 0.635 ** | 0.801 ** | 13.218 ** | 2.574 ** | 5.291 ** |
| REPS/Environments | 0.087 ** | 0.003 ** | 0.001 ns | 0.157 * | 0.003 ns | 0.014 ns |
| Varieties (G) | 141.09 ** | 1.756 ** | 7.969 ** | 83.712 ** | 23.736 ** | 45.457 ** |
| Environments × Varieties (G × E) | 0.073 ** | 0.003 ** | 0.008 ** | 1.665 ** | 0.015 ** | 0.911 ** |
| Error | 0.050 | 0.001 | 0.004 | 0.107 | 0.003 | 0.014 |
| Environments | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) | |
|---|---|---|---|---|---|---|---|
| Conventional | Giannitsa | 230 | 54 | 112 | 1716 | 64 | 139 |
| Florina | 253 | 51 | 126 | 1247 | 52 | 104 | |
| Trikala | 277 | 57 | 122 | 1108 | 55 | 110 | |
| Kalambaka | 250 | 129 | 101 | 1764 | 45 | 89 | |
| Low-Input | Giannitsa | 292 | 65 | 126 | 1027 | 45 | 64 |
| Florina | 241 | 66 | 138 | 1385 | 49 | 116 | |
| Trikala | 305 | 64 | 132 | 1127 | 48 | 100 | |
| Kalambaka | 274 | 98 | 114 | 2520 | 43 | 89 | |
| Conventional and Low-Input | Giannitsa | 243 | 54 | 110 | 1263 | 42 | 88 |
| Florina | 229 | 49 | 121 | 1217 | 41 | 94 | |
| Trikala | 268 | 53 | 118 | 1057 | 44 | 106 | |
| Kalambaka | 247 | 102 | 100 | 1778 | 35 | 86 |
| Genotypes | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) | |
|---|---|---|---|---|---|---|---|
| Conventional | Filippos | 461 | 58 | 633 | 3276 | 344 | 303 |
| Omiros | 515 | 82 | 315 | 2940 | 1040 | 199 | |
| Alexandros | 508 | 38 | 642 | 4147 | 925 | 347 | |
| Tempi | 524 | 90 | 590 | 2730 | 864 | 96 | |
| Zefyros | 506 | 39 | 847 | 3543 | 732 | 424 | |
| Pigasos | 691 | 106 | 442 | 3582 | 577 | 460 | |
| Low-Input | Filippos | 630 | 103 | 711 | 3247 | 436 | 301 |
| Omiros | 736 | 87 | 362 | 2965 | 365 | 167 | |
| Alexandros | 616 | 59 | 711 | 4064 | 605 | 230 | |
| Tempi | 517 | 106 | 627 | 2715 | 592 | 458 | |
| Zefyros | 610 | 69 | 916 | 2355 | 660 | 191 | |
| Pigasos | 650 | 125 | 531 | 2679 | 625 | 624 | |
| Conventional and Low-Input | Filippos | 473 | 65 | 419 | 2770 | 132 | 303 |
| Omiros | 513 | 76 | 268 | 2530 | 111 | 146 | |
| Alexandros | 490 | 44 | 445 | 3369 | 201 | 275 | |
| Tempi | 461 | 85 | 427 | 2380 | 191 | 122 | |
| Zefyros | 458 | 47 | 585 | 2375 | 148 | 267 | |
| Pigasos | 574 | 96 | 339 | 2832 | 140 | 365 |
| Genotypes | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) | |
|---|---|---|---|---|---|---|---|
| Giannitsa | |||||||
| Conventional | Filippos | 650 | 737 | 960 | 5194 | 1098 | 409 |
| Omiros | 463 | 902 | 1071 | 5065 | 1560 | 682 | |
| Alexandros | 585 | 1168 | 1079 | 4678 | 1557 | 678 | |
| Tempi | 702 | 1005 | 1310 | 5743 | 1578 | 511 | |
| Zefyros | 612 | 883 | 1229 | 4967 | 2411 | 847 | |
| Pigasos | 625 | 1190 | 823 | 5796 | 1044 | 527 | |
| Low-Input | Filippos | 864 | 704 | 925 | 5006 | 1688 | 957 |
| Omiros | 1407 | 1142 | 1192 | 4900 | 2670 | 1018 | |
| Alexandros | 957 | 717 | 1182 | 4534 | 1727 | 940 | |
| Tempi | 780 | 924 | 1296 | 5493 | 1748 | 570 | |
| Zefyros | 907 | 855 | 1443 | 6790 | 2133 | 1095 | |
| Pigasos | 897 | 1215 | 921 | 4444 | 2288 | 530 | |
| Conventional and Low-Input | Filippos | 718 | 326 | 488 | 4114 | 97 | 530 |
| Omiros | 613 | 444 | 587 | 3981 | 92 | 446 | |
| Alexandros | 635 | 242 | 605 | 3788 | 213 | 787 | |
| Tempi | 628 | 417 | 656 | 4404 | 180 | 232 | |
| Zefyros | 583 | 230 | 758 | 5771 | 252 | 401 | |
| Pigasos | 653 | 412 | 472 | 5351 | 162 | 339 | |
| Florina | |||||||
| Conventional | Filippos | 725 | 764 | 906 | 5579 | 1929 | 980 |
| Omiros | 942 | 889 | 727 | 4059 | 1296 | 952 | |
| Alexandros | 689 | 782 | 985 | 4928 | 1468 | 901 | |
| Tempi | 502 | 1144 | 1113 | 4707 | 1340 | 1029 | |
| Zefyros | 691 | 789 | 1376 | 4597 | 2025 | 911 | |
| Pigasos | 923 | 1112 | 838 | 4824 | 1736 | 976 | |
| Low-Input | Filippos | 630 | 911 | 1070 | 5366 | 465 | 2860 |
| Omiros | 854 | 654 | 794 | 4202 | 729 | 2661 | |
| Alexandros | 774 | 1065 | 1047 | 4765 | 435 | 2200 | |
| Tempi | 475 | 843 | 1299 | 4477 | 472 | 2424 | |
| Zefyros | 761 | 761 | 1438 | 4035 | 453 | 2834 | |
| Pigasos | 822 | 996 | 1014 | 4742 | 525 | 1884 | |
| Conventional and Low-Input | Filippos | 519 | 223 | 535 | 4289 | 662 | 232 |
| Omiros | 661 | 327 | 487 | 3385 | 261 | 160 | |
| Alexandros | 633 | 139 | 562 | 3956 | 628 | 236 | |
| Tempi | 472 | 218 | 668 | 3759 | 95 | 234 | |
| Zefyros | 562 | 129 | 770 | 2898 | 421 | 126 | |
| Pigasos | 742 | 250 | 522 | 3869 | 182 | 118 | |
| Trikala | |||||||
| Conventional | Filippos | 560 | 666 | 929 | 4543 | 1144 | 443 |
| Omiros | 908 | 755 | 733 | 4513 | 1319 | 8201 | |
| Alexandros | 613 | 750 | 1057 | 6062 | 1039 | 575 | |
| Tempi | 766 | 1156 | 917 | 5417 | 2247 | 827 | |
| Zefyros | 741 | 1082 | 1780 | 4533 | 1949 | 727 | |
| Pigasos | 1580 | 1240 | 1007 | 4442 | 1461 | 1159 | |
| Low-Input | Filippos | 1085 | 736 | 1066 | 4403 | 2605 | 488 |
| Omiros | 1400 | 1285 | 914 | 4298 | 3442 | 513 | |
| Alexandros | 908 | 578 | 1176 | 5749 | 2647 | 685 | |
| Tempi | 948 | 1018 | 850 | 5209 | 2183 | 539 | |
| Zefyros | 1011 | 777 | 1994 | 4379 | 1603 | 434 | |
| Pigasos | 729 | 1264 | 1198 | 4301 | 1845 | 1010 | |
| Conventional and Low-Input | Filippos | 623 | 113 | 551 | 3671 | 204 | 456 |
| Omiros | 826 | 383 | 530 | 3620 | 183 | 948 | |
| Alexandros | 635 | 339 | 627 | 4600 | 303 | 644 | |
| Tempi | 697 | 367 | 561 | 4250 | 288 | 517 | |
| Zefyros | 658 | 312 | 911 | 3597 | 194 | 554 | |
| Pigasos | 792 | 358 | 590 | 3565 | 212 | 1146 | |
| Kalambaka | |||||||
| Conventional | Filippos | 483 | 645 | 957 | 4553 | 1322 | 958 |
| Omiros | 794 | 667 | 932 | 5540 | 2032 | 816 | |
| Alexandros | 742 | 1168 | 1173 | 5680 | 1692 | 1095 | |
| Tempi | 772 | 1183 | 1296 | 5567 | 1717 | 1021 | |
| Zefyros | 833 | 1106 | 1000 | 4545 | 1426 | 499 | |
| Pigasos | 971 | 728 | 689 | 5077 | 1652 | 1024 | |
| Low-Input | Filippos | 733 | 757 | 1034 | 4415 | 1316 | 445 |
| Omiros | 938 | 640 | 1024 | 5302 | 1135 | 440 | |
| Alexandros | 782 | 835 | 1263 | 5476 | 2338 | 454 | |
| Tempi | 792 | 681 | 1496 | 5462 | 2155 | 439 | |
| Zefyros | 825 | 1133 | 1141 | 7557 | 1614 | 533 | |
| Pigasos | 1093 | 673 | 885 | 4887 | 1356 | 607 | |
| Conventional and Low-Input | Filippos | 556 | 487 | 509 | 3692 | 212 | 248 |
| Omiros | 738 | 369 | 509 | 4248 | 85 | 182 | |
| Alexandros | 669 | 696 | 607 | 4415 | 173 | 499 | |
| Tempi | 675 | 437 | 683 | 4297 | 190 | 365 | |
| Zefyros | 675 | 885 | 666 | 2010 | 125 | 263 | |
| Pigasos | 841 | 381 | 441 | 3986 | 157 | 656 | |
| Traits | Min. | Max. | Mean | sd | GCV (%) | PCV (%) | H2 (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Crude protein content (%) | 22.13 | 30.87 | 26.74 | 1.809 | 2.2034 | 2.2045 | 5.551 | 5.553 | 99.95 |
| Fat content (%) | 0.96 | 1.95 | 1.34 | 0.222 | 0.0274 | 0.0275 | 12.351 | 12.362 | 99.83 |
| Ash content (%) | 3.04 | 4.76 | 3.81 | 0.374 | 0.1244 | 0.1245 | 9.257 | 9.262 | 99.90 |
| Starch content (%) | 46.75 | 53.65 | 50.23 | 1.422 | 1.2820 | 1.3080 | 2.254 | 2.277 | 98.01 |
| Crude fiber content (%) | 2.6 | 5.35 | 4.03 | 0.645 | 0.3706 | 0.3709 | 15.107 | 15.112 | 99.94 |
| Moisture content (%) | 7.23 | 12.21 | 9.21 | 0.995 | 0.6960 | 0.7103 | 9.058 | 9.151 | 97.99 |
| Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | |
|---|---|---|---|---|---|
| Fat content (%) | 0.476 ** | ||||
| Ash content (%) | −0.423 ** | −0.315 ** | |||
| Starch content (%) | 0.200 ** | 0.126 * | −0.151 ** | ||
| Crude fiber content (%) | −0.588 ** | −0.305 ** | 0.369 ** | −0.038 | |
| Moisture content (%) | −0.576 ** | −0.312 ** | 0.365 ** | −0.712 ** | 0.114 * |
| Source of Variation | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) |
|---|---|---|---|---|---|---|
| m.s. | m.s. | m.s. | m.s. | m.s. | m.s. | |
| Environments (E) | 1.433 ** | 0.146 ** | 0.990 ** | 3.144 ** | 0.146 ** | 2.271 ** |
| REPS/Environments | 0.082 ns | 0.001 ns | 0.02 ns | 0.069 ns | 0.03 ns | 0.031 ns |
| Varieties (G) | 79.791 ** | 0.933 ** | 1.959 ** | 77.020 ** | 1.982 ** | 29.241 ** |
| Environments × Varieties (G × E) | 1.079 ** | 0.211 ** | 0.070 ** | 2.840 ** | 0.397 ** | 1.903 ** |
| Error | 0.063 | 0.001 | 0.001 | 0.078 | 0.003 | 0.025 |
| Environments | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) | |
|---|---|---|---|---|---|---|---|
| Conventional | Giannitsa | 1094 | 26 | 344 | 1547 | 287 | 94 |
| Florina | 231 | 77 | 159 | 1598 | 294 | 327 | |
| Trikala | 380 | 57 | 277 | 1589 | 236 | 245 | |
| Kalambaka | 599 | 78 | 135 | 881 | 163 | 87 | |
| Low-Input | Giannitsa | 381 | 31 | 800 | 1470 | 345 | 144 |
| Florina | 327 | 96 | 240 | 3001 | 294 | 303 | |
| Trikala | 623 | 61 | 573 | 2135 | 710 | 74 | |
| Kalambaka | 347 | 30 | 489 | 1250 | 724 | 76 | |
| Conventional and Low-Input | Giannitsa | 572 | 26 | 476 | 1507 | 316 | 115 |
| Florina | 262 | 84 | 194 | 2066 | 291 | 289 | |
| Trikala | 476 | 59 | 378 | 1802 | 319 | 111 | |
| Kalambaka | 432 | 32 | 215 | 1046 | 255 | 82 |
| Genotypes | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) | |
|---|---|---|---|---|---|---|---|
| Conventional | Olympos | 2448 | 59 | 4043 | 4526 | 280 | 328 |
| Pisso | 2487 | 70 | 2357 | 2727 | 534 | 1274 | |
| Livioletta | 1556 | 64 | 526 | 5288 | 324 | 531 | |
| Vermio | 2027 | 33 | 193 | 4037 | 206 | 347 | |
| Dodoni | 794 | 35 | 3369 | 5531 | 186 | 83 | |
| Low-Input | Olympos | 1384 | 40 | 1318 | 5721 | 568 | 62 |
| Pisso | 2160 | 133 | 683 | 2536 | 915 | 1429 | |
| Livioletta | 1932 | 284 | 553 | 2609 | 1856 | 944 | |
| Vermio | 4109 | 26 | 682 | 3486 | 1395 | 407 | |
| Dodoni | 1763 | 126 | 1489 | 2170 | 403 | 151 | |
| Conventional and Low-Input | Olympos | 1774 | 44 | 1243 | 4957 | 377 | 101 |
| Pisso | 1973 | 79 | 600 | 2586 | 664 | 1368 | |
| Livioletta | 1224 | 106 | 548 | 3128 | 385 | 688 | |
| Vermio | 2592 | 28 | 304 | 3790 | 349 | 313 | |
| Dodoni | 1109 | 55 | 1172 | 2927 | 251 | 106 |
| Genotypes | Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | Moisture (%) | |
|---|---|---|---|---|---|---|---|
| Giannitsa | |||||||
| Conventional | Olympos | 8913 | 1680 | 4026 | 8194 | 2970 | 1092 |
| Pisso | 6910 | 1651 | 4041 | 8715 | 2440 | 1830 | |
| Livioletta | 5924 | 1294 | 175 | 9084 | 3105 | 1140 | |
| Vermio | 9798 | 1853 | 3545 | 8532 | 3406 | 1464 | |
| Dodoni | 8170 | 1532 | 4431 | 9545 | 3475 | 1784 | |
| Low-Input | Olympos | 7345 | 1537 | 3181 | 9267 | 2966 | 1308 |
| Pisso | 8548 | 1496 | 3652 | 9780 | 3701 | 1674 | |
| Livioletta | 7339 | 1279 | 3099 | 9583 | 3877 | 1902 | |
| Vermio | 8174 | 1374 | 4262 | 9313 | 2944 | 1465 | |
| Dodoni | 8487 | 1143 | 3497 | 9823 | 3467 | 1426 | |
| Conventional and Low-Input | Olympos | 2019 | 1719 | 3440 | 8151 | 540 | 138 |
| Pisso | 7686 | 28 | 613 | 6045 | 2205 | 1663 | |
| Livioletta | 6216 | 115 | 260 | 9799 | 747 | 1296 | |
| Vermio | 2805 | 1035 | 2425 | 5221 | 220 | 1061 | |
| Dodoni | 5466 | 638 | 1827 | 2210 | 1831 | 241 | |
| Florina | |||||||
| Conventional | Olympos | 9404 | 1730 | 4594 | 10663 | 3087 | 1287 |
| Pisso | 8576 | 1365 | 3392 | 9042 | 2604 | 1209 | |
| Livioletta | 8644 | 1672 | 4445 | 9290 | 2849 | 1285 | |
| Vermio | 8277 | 1198 | 3773 | 9118 | 2831 | 1640 | |
| Dodoni | 6057 | 1527 | 4301 | 8599 | 3113 | 1731 | |
| Low-Input | Olympos | 6212 | 1574 | 4049 | 8424 | 3799 | 1112 |
| Pisso | 5950 | 1292 | 3792 | 9431 | 3963 | 1957 | |
| Livioletta | 6190 | 1162 | 4069 | 8594 | 2832 | 1226 | |
| Vermio | 9595 | 1174 | 4184 | 8700 | 3165 | 1334 | |
| Dodoni | 8561 | 1420 | 4813 | 9163 | 2891 | 1465 | |
| Conventional and Low-Input | Olympos | 7767 | 164 | 839 | 9219 | 2411 | 85 |
| Pisso | 1146 | 522 | 877 | 6427 | 345 | 1514 | |
| Livioletta | 1213 | 153 | 3263 | 4882 | 333 | 1078 | |
| Vermio | 7584 | 633 | 4196 | 4924 | 1092 | 1116 | |
| Dodoni | 1584 | 185 | 790 | 5548 | 2731 | 1653 | |
| Trikala | |||||||
| Conventional | Olympos | 5230 | 1505 | 4523 | 9582 | 2978 | 1800 |
| Pisso | 6855 | 1671 | 3520 | 9339 | 3038 | 1849 | |
| Livioletta | 7769 | 1534 | 4014 | 8412 | 2928 | 1438 | |
| Vermio | 8877 | 1362 | 3987 | 8922 | 3424 | 1378 | |
| Dodoni | 6791 | 1198 | 4064 | 8892 | 2865 | 1340 | |
| Low-Input | Olympos | 6293 | 1607 | 3991 | 9703 | 3039 | 1448 |
| Pisso | 7670 | 1321 | 3497 | 9087 | 3857 | 1684 | |
| Livioletta | 6668 | 1508 | 4066 | 9224 | 3334 | 1890 | |
| Vermio | 9118 | 1779 | 3759 | 8987 | 3315 | 1576 | |
| Dodoni | 8218 | 1184 | 3466 | 8610 | 2851 | 1545 | |
| Conventional and Low-Input | Olympos | 5670 | 306 | 1617 | 10287 | 2621 | 365 |
| Pisso | 5222 | 311 | 432 | 9502 | 1692 | 1881 | |
| Livioletta | 1718 | 636 | 1849 | 4738 | 2282 | 1410 | |
| Vermio | 8794 | 207 | 1805 | 9339 | 1071 | 518 | |
| Dodoni | 7721 | 429 | 3501 | 6251 | 241 | 527 | |
| Kalambaka | |||||||
| Conventional | Olympos | 7291 | 1308 | 3240 | 10337 | 4048 | 1321 |
| Pisso | 8298 | 1323 | 4687 | 9149 | 2556 | 1149 | |
| Livioletta | 8904 | 1791 | 3692 | 9251 | 2661 | 1564 | |
| Vermio | 8800 | 1343 | 3613 | 9060 | 3281 | 1351 | |
| Dodoni | 8476 | 1449 | 4122 | 9923 | 3896 | 1454 | |
| Low-Input | Olympos | 6106 | 1506 | 3580 | 9752 | 2956 | 1335 |
| Pisso | 9598 | 1351 | 3366 | 8140 | 3069 | 1105 | |
| Livioletta | 6695 | 1697 | 3975 | 2355 | 2695 | 1668 | |
| Vermio | 8238 | 1194 | 3542 | 7049 | 2772 | 1532 | |
| Dodoni | 7157 | 1461 | 3841 | 8397 | 3872 | 1421 | |
| Conventional and Low-Input | Olympos | 6903 | 673 | 2105 | 4010 | 1072 | 534 |
| Pisso | 3140 | 113 | 4117 | 9151 | 1810 | 1056 | |
| Livioletta | 1731 | 311 | 2016 | 3783 | 593 | 1719 | |
| Vermio | 6479 | 27 | 578 | 8353 | 386 | 240 | |
| Dodoni | 6016 | 676 | 1365 | 9742 | 1979 | 697 | |
| Traits | Min. | Max. | Mean | sd | GCV (%) | PCV (%) | H2 (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Crude protein content (%) | 20.13 | 25.08 | 22.85 | 1.15 | 1.2299 | 1.2467 | 4.8530 | 4.8861 | 98.65 |
| Fat content (%) | 1.11 | 2.10 | 1.56 | 0.24 | 0.0113 | 0.0146 | 6.8011 | 7.7313 | 77.38 |
| Ash content (%) | 2.94 | 3.99 | 3.29 | 0.21 | 0.0295 | 0.0306 | 5.2142 | 5.3099 | 96.43 |
| Starch content (%) | 47.02 | 52.83 | 49.68 | 1.31 | 1.1591 | 1.2034 | 2.1671 | 2.2082 | 96.31 |
| Crude fiber content (%) | 5.02 | 6.53 | 5.61 | 0.33 | 0.0248 | 0.0310 | 2.8041 | 3.1357 | 79.97 |
| Moisture content (%) | 7.74 | 12.25 | 9.51 | 0.92 | 0.4272 | 0.4569 | 6.8741 | 7.1094 | 93.49 |
| Crude Protein (%DM) | Fat (%DM) | Ash (%DM) | Starch (%DM) | Crude Fiber (%DM) | |
|---|---|---|---|---|---|
| Fat (%DM) | 0.271 ** | ||||
| Ash (%DM) | 0.653 ** | 0.174 ** | |||
| Starch (%DM) | 0.449 ** | 0.173 ** | 0.527 ** | ||
| Crude fiber (%DM) | 0.343 ** | 0.069 | 0.357 ** | 0.373 ** | |
| Moisture (%) | −0.540 ** | −0.377 ** | −0.396 ** | −0.619 ** | −0.390 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Kantas, D.; Ipsilandis, C.G. A Comparative Study on Stability of Seed Characteristics in Vetch and Pea Cultivations. Agriculture 2023, 13, 1092. https://doi.org/10.3390/agriculture13051092
Greveniotis V, Bouloumpasi E, Zotis S, Korkovelos A, Kantas D, Ipsilandis CG. A Comparative Study on Stability of Seed Characteristics in Vetch and Pea Cultivations. Agriculture. 2023; 13(5):1092. https://doi.org/10.3390/agriculture13051092
Chicago/Turabian StyleGreveniotis, Vasileios, Elisavet Bouloumpasi, Stylianos Zotis, Athanasios Korkovelos, Dimitrios Kantas, and Constantinos G. Ipsilandis. 2023. "A Comparative Study on Stability of Seed Characteristics in Vetch and Pea Cultivations" Agriculture 13, no. 5: 1092. https://doi.org/10.3390/agriculture13051092
APA StyleGreveniotis, V., Bouloumpasi, E., Zotis, S., Korkovelos, A., Kantas, D., & Ipsilandis, C. G. (2023). A Comparative Study on Stability of Seed Characteristics in Vetch and Pea Cultivations. Agriculture, 13(5), 1092. https://doi.org/10.3390/agriculture13051092






