Comparative Study of Isoflavone Synthesis Genes in Two Wild Soybean Varieties Using Transcriptomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Determination of Isoflavones Contents of Seeds
2.4. Total RNA Extraction, Library Construction, and Sequencing
2.5. Transcriptomic Data Analysis
2.6. Principal Component Analysis
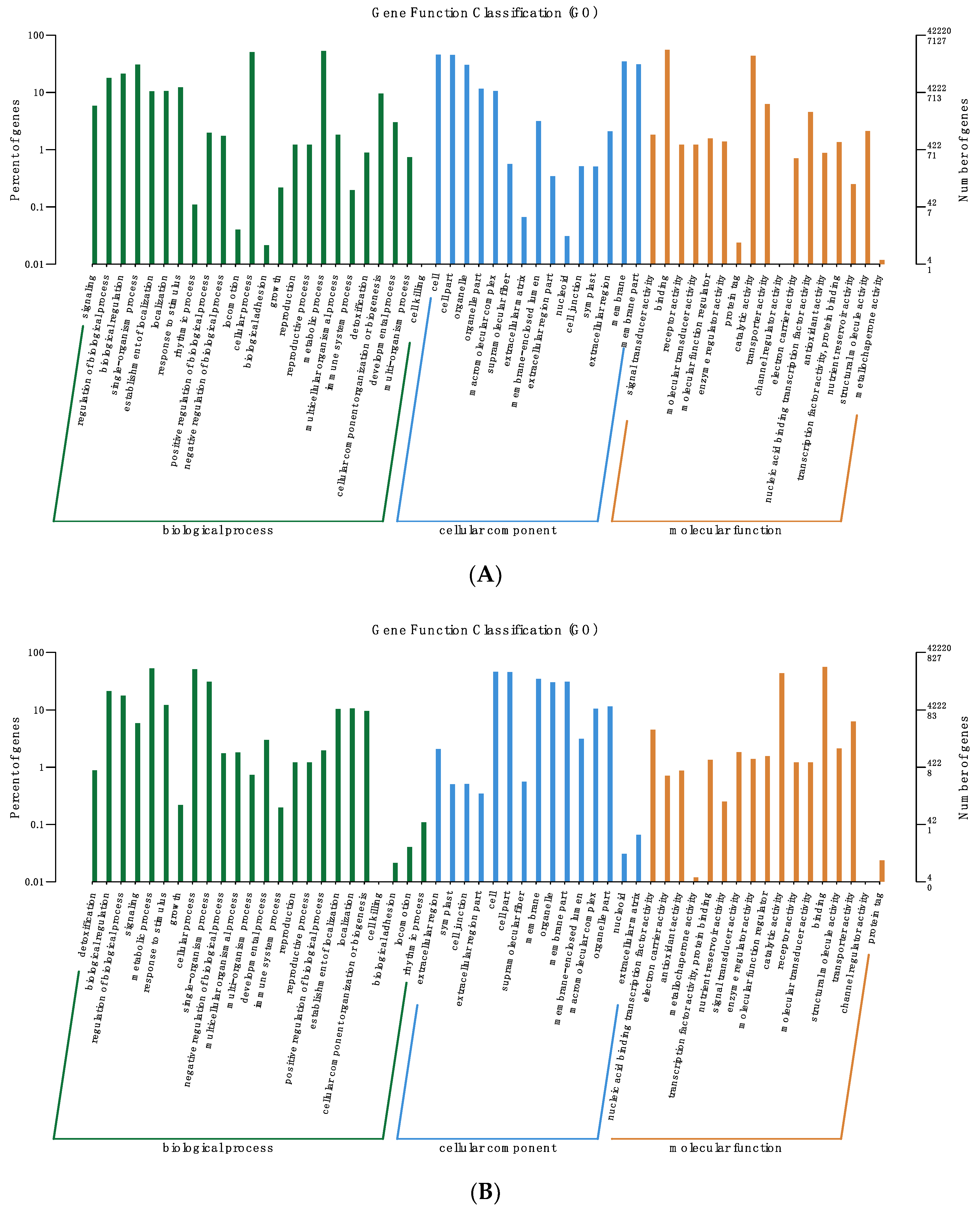
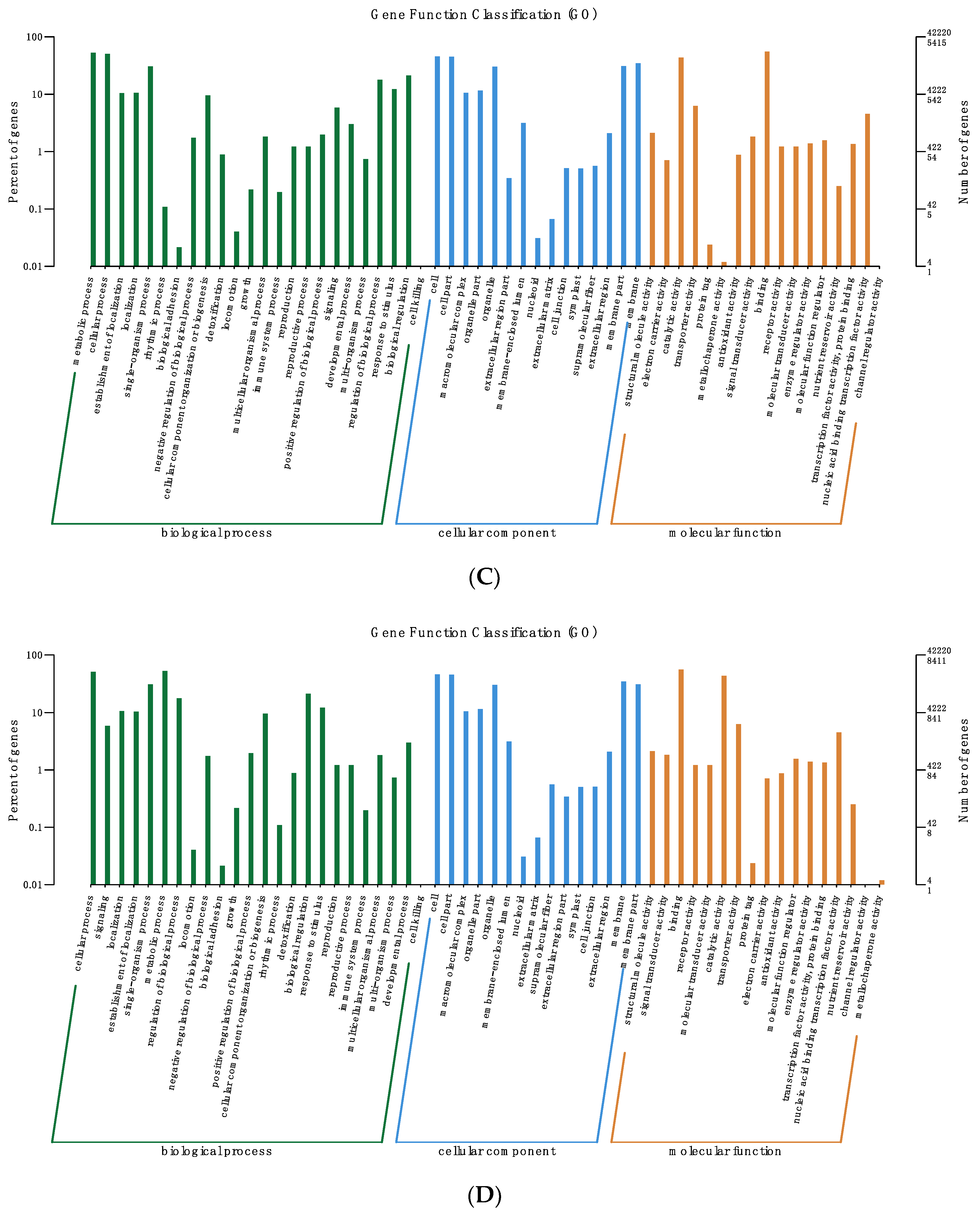
2.7. Functional Classification of DEGs
2.8. Transcription Factor Analysis
2.9. Quantitative Real-Time PCR (qRT–PCR) Verification
3. Results
3.1. Isoflavone Content in Seeds of ZYD7068 and ZYD7194
3.2. Transcriptome Profiles of ZYD7068 and ZYD7194 at R6 and R8 Stages
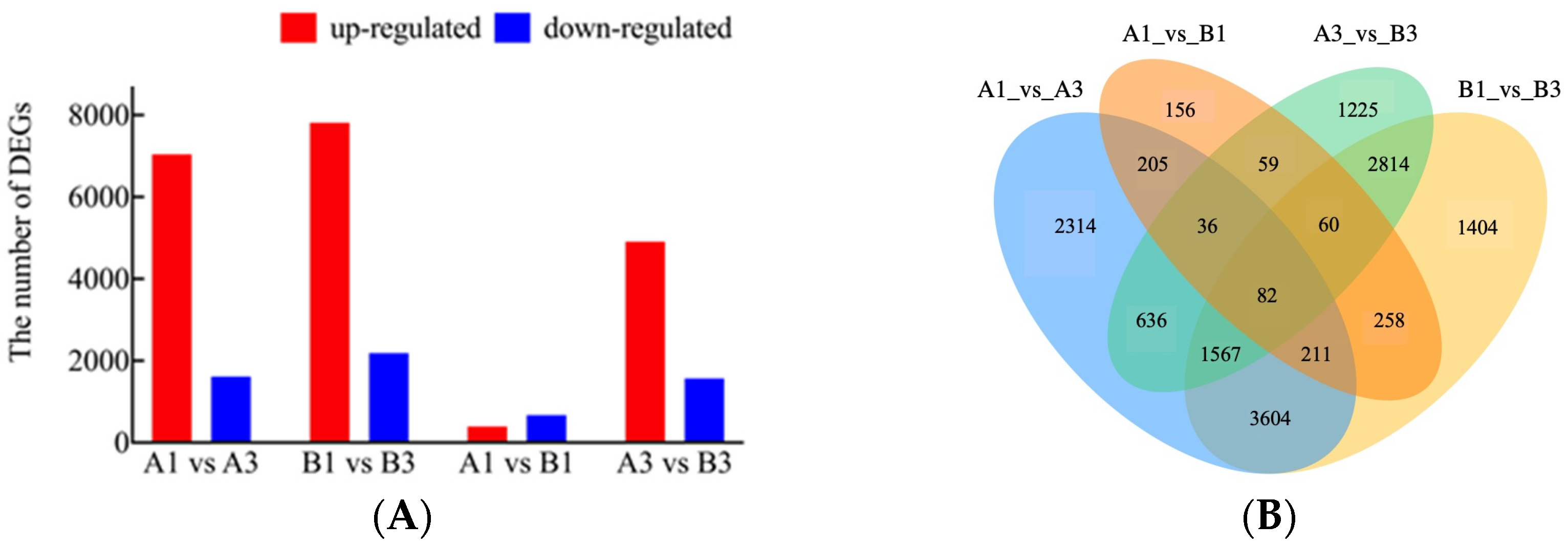
3.3. Identification of DEGs of ZYD7068 and ZYD7194 at Different Stages
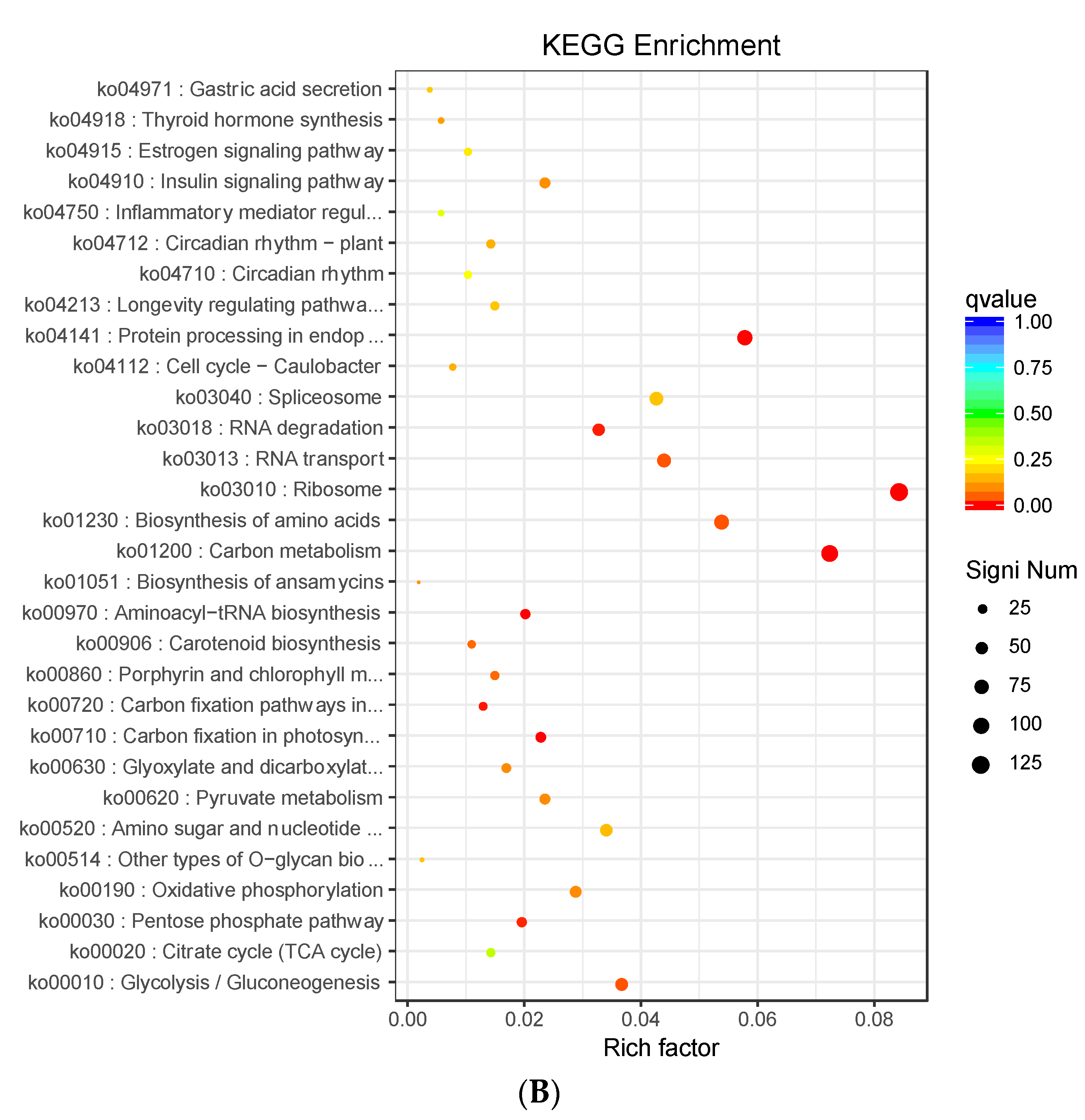
3.4. Analysis of DEGs Clustering and Pathway Enrichment
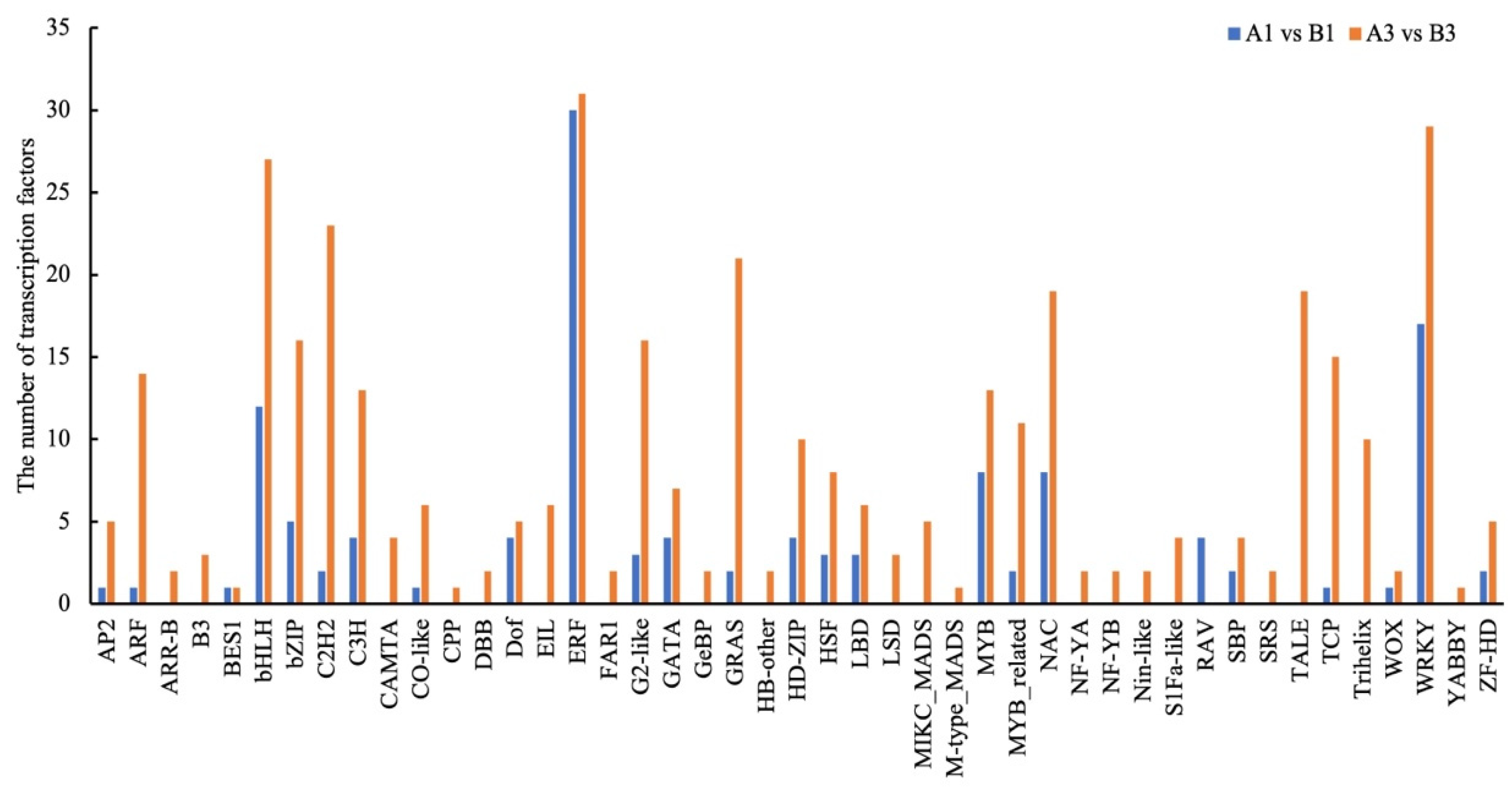
3.5. Different Expression Patterns of Transcription Factors in Two Genotypes at Different Stages
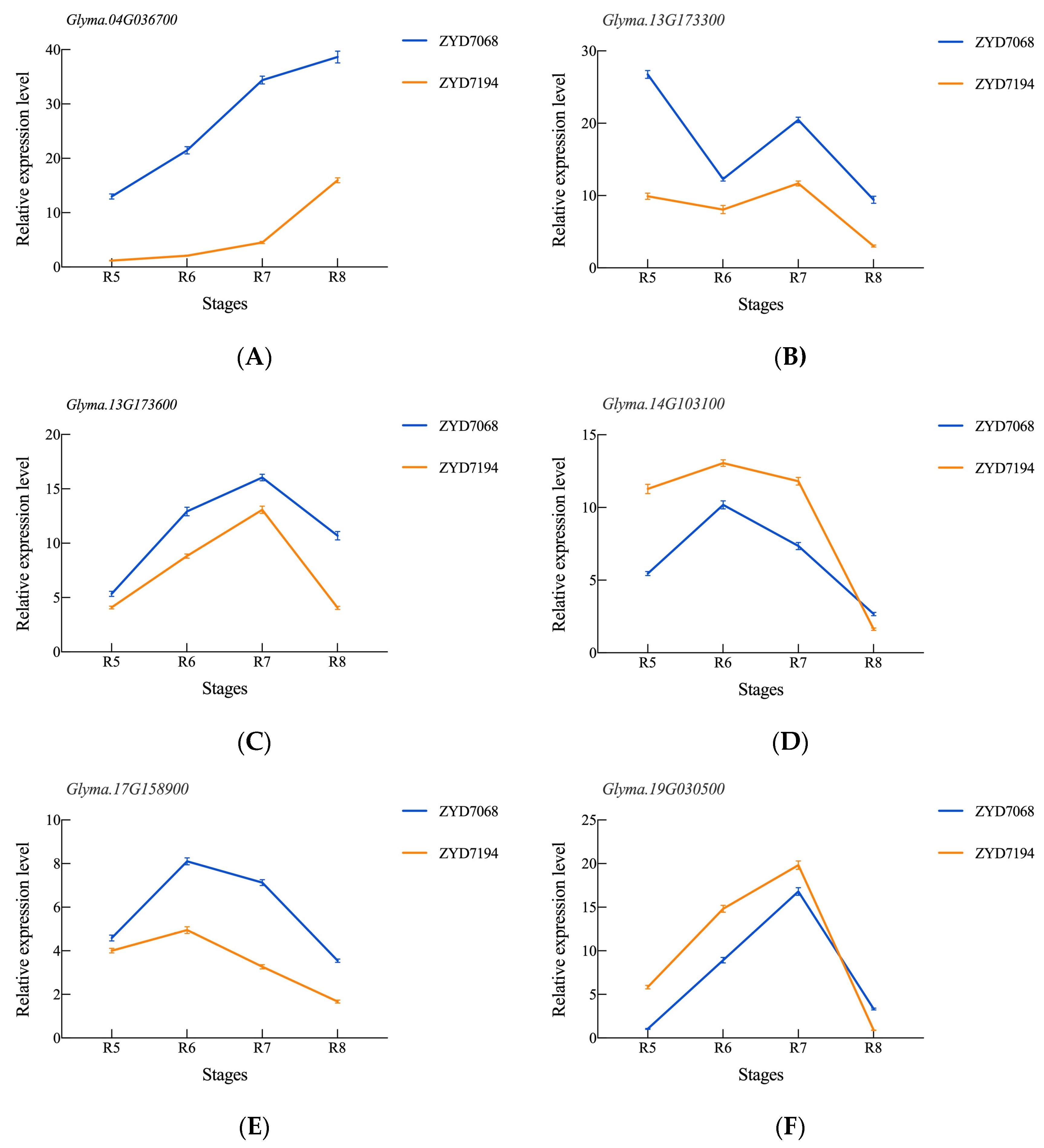
3.6. Validation of RNA-Seq Expression Levels by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.O.; Yu, O.; Heatherly, L.G.; Krishnan, H.B. Accumulation of Genistein and Daidzein, Soybean Isoflavones Implicated in Promoting Human Health, Is Significantly Elevated by Irrigation. J. Agric. Food Chem. 2004, 52, 7574–7579. [Google Scholar] [CrossRef]
- Hyten, D.L.; Song, Q.; Zhu, Y.; Choi, I.-Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef] [PubMed]
- Stupar, R.M. Into the wild: The soybean genome meets its undomesticated relative. Proc. Natl. Acad. Sci. USA 2010, 107, 21947–21948. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-J.; Xing, L.-L.; Guo, Y.; Wang, J.; Jackson, S.A.; Chang, R.-Z. A platform for soybean molecular breeding: The utilization of core collections for food security. Plant Mol. Biol. 2013, 83, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, M.-W.; Xie, M.; Liu, X.; Ni, M.; Shao, G.; Song, C.; Kay-Yuen Yim, A.; Tao, Y.; Wong, F.-L.; et al. Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat. Commun. 2014, 5, 4340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, Q.; Griffin, J.D.; Song, B.-H. Genetic architecture of wild soybean (Glycine soja) response to soybean cyst nematode (Heterodera glycines). Mol. Genet. Genom. MGG 2017, 292, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hyten, D.L.; Niblack, T.L.; Diers, B.W. Stacking Resistance Alleles from Wild and Domestic Soybean Sources Improves Soybean Cyst Nematode Resistance. Crop Sci. 2011, 51, 934–943. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Davis, E.L.; Wang, J.; Griffin, J.D.; Kofsky, J.; Song, B.-H. Genome-Wide Association Study of Resistance to Soybean Cyst Nematode (Heterodera glycines) HG Type 2.5.7 in Wild Soybean (Glycine soja). Front. Plant Sci. 2016, 7, 1214. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, T.; Gai, J. Genetic Variability and Evolutionary Peculiarity of Isoflavone Content and Its Components in Soybean Germplasm from China. Sci. Agric. Sin. 2010, 44, 3919–3929. [Google Scholar]
- Li, L.; Hur, M.; Lee, J.-Y.; Zhou, W.; Song, Z.; Ransom, N.; Demirkale, C.Y.; Nettleton, D.; Westgate, M.; Arendsee, Z.; et al. A systems biology approach toward understanding seed composition in soybean. BMC Genom. 2015, 16, S9. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Girke, T.; Benning, C.; Ohlrogge, J.B. Contrapuntal Networks of Gene Expression during Arabidopsis Seed Filling [W]. Plant Cell 2002, 14, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis Seed Development and Germination Is Associated with Temporally Distinct Metabolic Switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Furutani, I.; Sukegawa, S.; Kyozuka, J. Genome-wide analysis of spatial and temporal gene expression in rice panicle development. Plant J. 2006, 46, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.-J.; Zhang, J.-J.; Xue, H.-W. Genome-Wide Analysis of the Complex Transcriptional Networks of Rice Developing Seeds. PLoS ONE 2012, 7, e31081. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.-W.; Dong, Y.-Y.; Wang, N.; Sun, Y.-P.; Li, X.-Y.; Liu, L.; Fan, X.-D.; Yin, H.-L.; Jing, Y.-Y.; et al. Sequence mining and transcript profiling to explore differentially expressed genes associated with lipid biosynthesis during soybean seed development. BMC Plant Biol. 2012, 12, 122. [Google Scholar] [CrossRef]
- Collakova, E.; Aghamirzaie, D.; Fang, Y.; Klumas, C.; Tabataba, F.; Kakumanu, A.; Myers, E.; Heath, L.S.; Grene, R. Metabolic and Transcriptional Reprogramming in Developing Soybean (Glycine max) Embryos. Metabolites 2013, 3, 347–372. [Google Scholar] [CrossRef]
- Han, T.; Wu, C.; Tong, Z.; Mentreddy, R.S.; Tan, K.; Gai, J. Postflowering photoperiod regulates vegetative growth and reproductive development of soybean. Environ. Exp. Bot. 2006, 55, 120–129. [Google Scholar] [CrossRef]
- Kim, J.-A.; Chung, I.-M. Change in isoflavone concentration of soybean (Glycine max L.) seeds at different growth stages. J. Sci. Food Agric. 2007, 87, 496–503. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Dixit, A.K.; Bhatnagar, D.; Chauhan, G.S. Relative Changes in Tocopherols, Isoflavones, Total Phenolic Content, and Antioxidative Activity in Soybean Seeds at Different Reproductive Stages. J. Agric. Food Chem. 2009, 57, 2705–2710. [Google Scholar] [CrossRef]
- Dhaubhadel, S.; Gijzen, M.; Moy, P.; Farhangkhoee, M. Transcriptome Analysis Reveals a Critical Role of CHS7 and CHS8 Genes for Isoflavonoid Synthesis in Soybean Seeds. Plant Physiol. 2007, 143, 326–338. [Google Scholar] [CrossRef]
- Fehr, W.; Caviness, C.E. Stages of Soybean Development. Spec. Rep. 1977, 17, 929–931. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression of RNA-Seq data at the gene level—The DESeq package. EMBL Eur. Mol. Biol. Lab. 2013, 24, 413–426. [Google Scholar]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-△△Ct Method. Methods San. Diego. Calif. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Fan, C.; Li, H.; Zhang, Q.; Fu, Y.-F. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol. Biol. 2009, 10, 93. [Google Scholar] [CrossRef]
- Qiu, L.; Li, Y.; Guan, R.-X.; Liu, Z.-X.; Wang, L.-X.; Chang, R.-Z. Establishment, Representative Testing and Research Progress of Soybean Core Collection and Mini Core Collection: Establishment, Representative Testing and Research Progress of Soybean Core Collection and Mini Core Collection. Acta Agron. Sin. 2009, 35, 571–579. [Google Scholar] [CrossRef]
- Pei, R.; Zhang, J.; Tian, L.; Zhang, S.; Han, F.; Yan, S.; Wang, L.; Li, B.; Sun, J. Identification of novel QTL associated with soybean isoflavone content. Crop J. 2018, 6, 244–252. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Han, F.; Li, B.; Yan, S.; Sun, J.; Wang, L. Isoflavone Content of Soybean Cultivars from Maturity Group 0 to VI Grown in Northern and Southern China. J. Am. Oil Chem. Soc. 2014, 91, 1019–1028. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Huai, Y.; Abdelghany, A.M.; Shaibu, A.S.; Qi, J.; Feng, Y.; Liu, Y.; Li, J.; Qiu, L.; et al. Identification of genes for seed isoflavones based on bulk segregant analysis sequencing in soybean natural population. Theor. Appl. Genet. 2023, 136, 13. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-S.; Huang, L.-J.; Lai, Y.-H.; Chang, J.-C.; Lee, R.-S.; Chiou, R.Y.-Y. Solvent effects on extraction and HPLC analysis of soybean isoflavones and variations of isoflavone compositions as affected by crop season. J. Agric. Food Chem. 2007, 55, 7712–7715. [Google Scholar] [CrossRef] [PubMed]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Dhaubhadel, S. Soybean chalcone isomerase: Evolution of the fold, and the differential expression and localization of the gene family. Planta 2015, 241, 507–523. [Google Scholar] [CrossRef]
- Mameda, R.; Waki, T.; Kawai, Y.; Takahashi, S.; Nakayama, T. Involvement of chalcone reductase in the soybean isoflavone metabolon: Identification of GmCHR5, which interacts with 2-hydroxyisoflavanone synthase. Plant J. 2018, 96, 56–74. [Google Scholar] [CrossRef]
- Jiang, Y.N.; Wang, B.; Li, H.; Yao, L.M.; Wu, T.L. Flavonoid Production Is Effectively Regulated by RNAi Interference of Two Flavone Synthase Genes from Glycine max. J. Plant Biol. 2010, 53, 425–432. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Chu, S.; Yan, H.-L.; Yu, D. Diversifying Selection on Flavanone 3-Hydroxylase and Isoflavone Synthase Genes in Cultivated Soybean and Its Wild Progenitors. PLoS ONE 2013, 8, e54154. [Google Scholar] [CrossRef]
- Yan, F.; Di, S.; Rojas Rodas, F.; Rodriguez Torrico, T.; Murai, Y.; Iwashina, T.; Anai, T.; Takahashi, R. Allelic variation of soybean flower color gene W4 encoding dihydroflavonol 4-reductase 2. BMC Plant Biol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their Glycosides and Glycoconjugates. Synthesis and Biological Activity. Curr. Org. Chem. 2016, 21, 218–235. [Google Scholar] [CrossRef]
- Yi, J.; Derynck, M.R.; Li, X.; Telmer, P.; Marsolais, F.; Dhaubhadel, S. A single-repeat MYB transcription factor, GmMYB176, regulates CHS8 gene expression and affects isoflavonoid biosynthesis in soybean: GmMYB176 and isoflavonoid biosynthesis. Plant J. 2010, 62, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, J.; Zhu, Y.; Liu, S.; Zhou, X.; Zhang, H.; Wang, C.; Yang, W.; Tian, Z.; Cheng, H.; et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017, 13, e1006770. [Google Scholar] [CrossRef]
- Matus, J.T.; Poupin, M.J.; Cañón, P.; Bordeu, E.; Alcalde, J.A.; Arce-Johnson, P. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol. Biol. 2010, 72, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Lu, G.-H.; Huep, G.; Schmelzer, E.; Weisshaar, B.; Sagasser, M. TRANSPARENT TESTA1 interacts with R2R3-MYB factors and affects early and late steps of flavonoid biosynthesis in the endothelium of Arabidopsis thaliana seeds. Plant J. 2011, 67, 406–419. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.-M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, H.; Jiang, X.; Wang, P.; Dai, X.; Chen, W.; Gao, L.; Xia, T. A WD40 Repeat Protein from Camellia sinensis Regulates Anthocyanin and Proanthocyanidin Accumulation through the Formation of MYB–bHLH–WD40 Ternary Complexes. Int. J. Mol. Sci. 2018, 19, 1686. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Y.; Fang, Z.-T.; Lin, J.-K.; Sun, Y.; Du, Z.-Z.; Guo, Y.-L.; Liu, J.-H.; Liang, Y.-R.; Ye, J.-H. Complementary iTRAQ Proteomic and Transcriptomic Analyses of Leaves in Tea Plant (Camellia sinensis L.) with Different Maturity and Regulatory Network of Flavonoid Biosynthesis. J. Proteome Res. 2019, 18, 252–264. [Google Scholar] [CrossRef] [PubMed]



| Sample | A1 | B1 | A3 | B3 |
|---|---|---|---|---|
| Raw reads | 61,567,635 | 50,030,576 | 55,995,015 | 71,934,601 |
| Raw bases | 9,235,145,300 | 7,504,586,400 | 8,399,252,300 | 10,790,190,200 |
| Raw reads Q30 (%) | 92.17 | 92.04 | 92.48 | 93.54 |
| Clean reads | 58,030,861 | 47,092,579 | 53,356,499 | 69,751,187 |
| Clean bases | 8,564,826,368 | 6,949,538,189 | 7,867,510,069 | 10,141,929,545 |
| Clean reads Q30 (%) | 94.60 | 94.55 | 94.60 | 96.65 |
| GC Bases Count (bp) | 3,995,773,891 | 3,264,362,061 | 3,748,642,305 | 4,851,047,168 |
| GC Bases Ratio (%) | 46.66 | 46.98 | 47.64 | 47.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhao, K.; Ren, H.; Lamlom, S.F.; Liu, X.; Wang, X.; Zhang, F.; Yuan, R.; Wang, J. Comparative Study of Isoflavone Synthesis Genes in Two Wild Soybean Varieties Using Transcriptomic Analysis. Agriculture 2023, 13, 1164. https://doi.org/10.3390/agriculture13061164
Zhang B, Zhao K, Ren H, Lamlom SF, Liu X, Wang X, Zhang F, Yuan R, Wang J. Comparative Study of Isoflavone Synthesis Genes in Two Wild Soybean Varieties Using Transcriptomic Analysis. Agriculture. 2023; 13(6):1164. https://doi.org/10.3390/agriculture13061164
Chicago/Turabian StyleZhang, Bixian, Kezhen Zhao, Honglei Ren, Sobhi F. Lamlom, Xiulin Liu, Xueyang Wang, Fengyi Zhang, Rongqiang Yuan, and Jiajun Wang. 2023. "Comparative Study of Isoflavone Synthesis Genes in Two Wild Soybean Varieties Using Transcriptomic Analysis" Agriculture 13, no. 6: 1164. https://doi.org/10.3390/agriculture13061164






