Developing a Portable Spectrometer to Detect Chemical Contaminants in Irrigation Water
Abstract
1. Introduction
2. Theoretical Study
2.1. Optical Dispersion
2.2. Light Absorbance and Transmittance Based on Beer–Lambert Law
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Ammonium Nitrate (NH4NO3)
3.1.2. Organic Pesticides
3.1.3. Zinc Oxide
3.1.4. Water Sources from Irrigation System
3.2. Spectroscopy Techniques
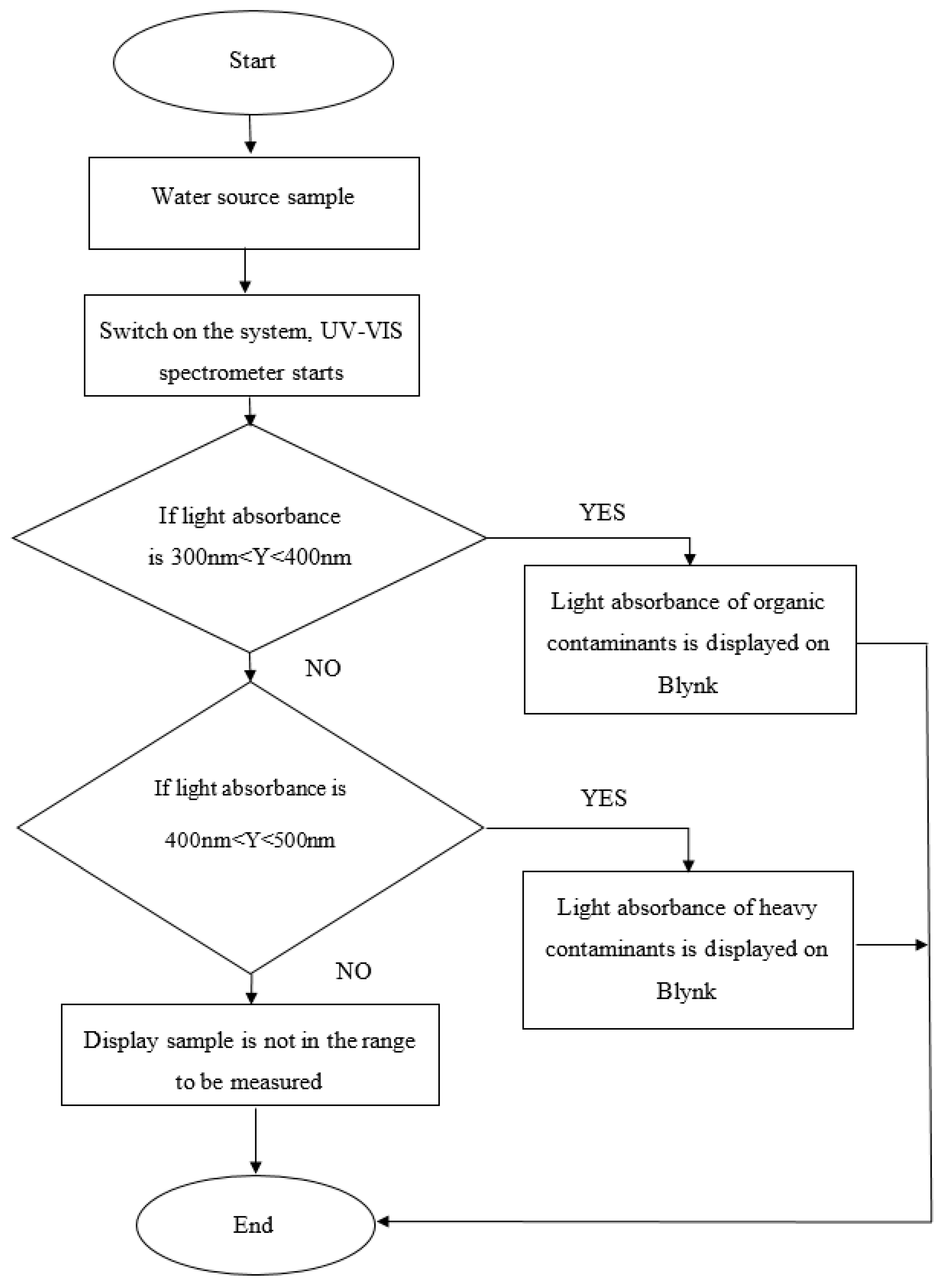
3.2.1. Measuring Light Absorbance Using a Portable Spectroscopy Technique (ESP32-Based Spectrometer)
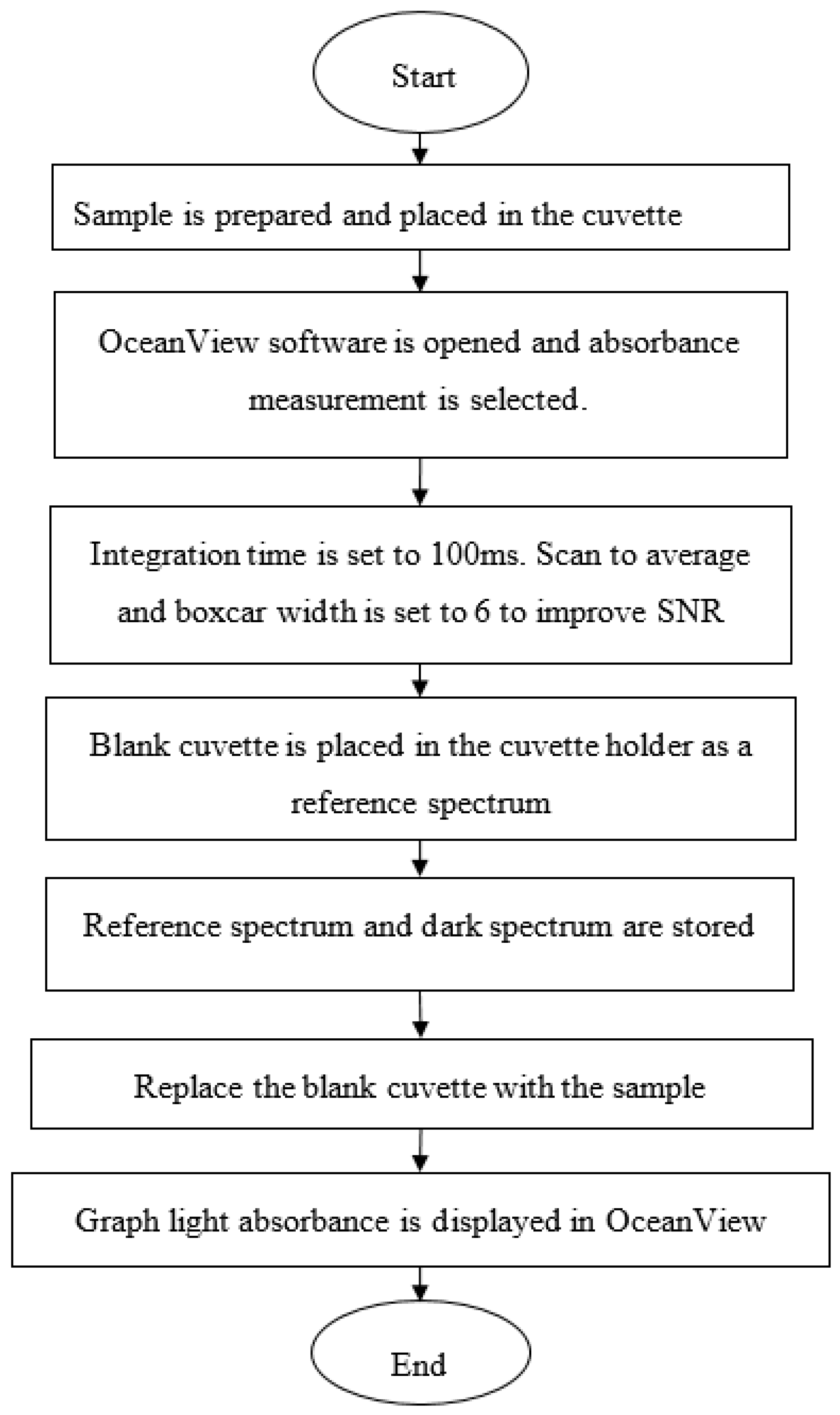
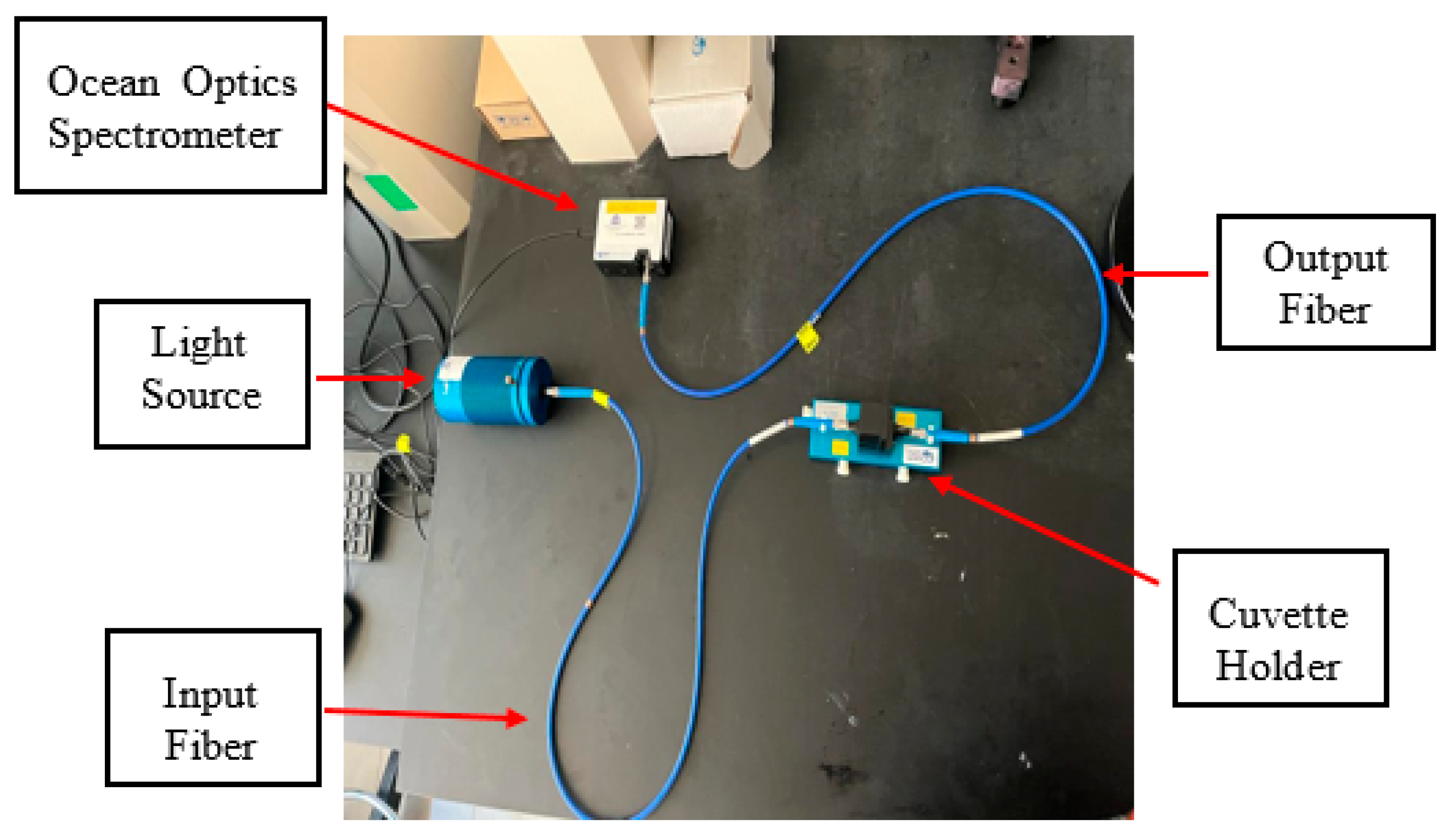
3.2.2. Measuring Light Absorbance Using a Lab-Based Spectroscopy Technique (Ocean Optics Spectrometer)
4. Results
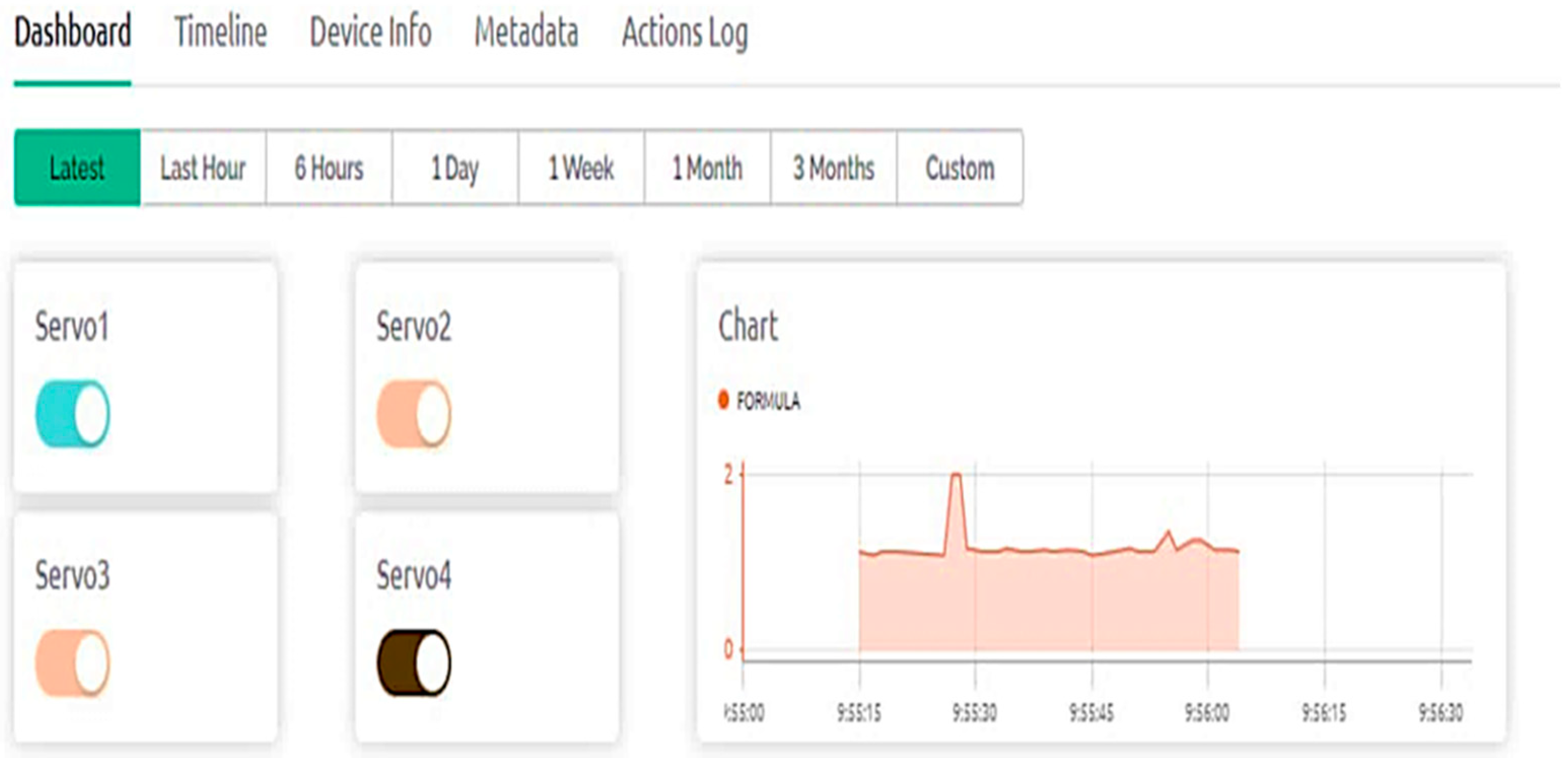
4.1. Real-Time Monitoring Using Blynk Application
4.2. Light Absorbance from ESP32-Based Spectrometer
4.3. Light Absorbance from Ocean Optics Spectrometer
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Water for Sustainable Food and Agriculture: A report produced for the G20 Presidency of Germany. 2017. Available online: https://www.fao.org/3/i7959e/i7959e.pdf (accessed on 10 April 2023).
- Food and Agriculture Organization of the United Nations. The State of the World’s Land and Water Resources for Food and Agriculture 2021—Systems at Breaking Point. 2022. Available online: https://www.fao.org/land-water/solaw2021/en/ (accessed on 10 April 2023).
- Ciaramella, B.R.; Corinzia, S.A.; Cosentino, S.L.; Testa, G. Phytoremediation of Heavy Metal Contaminated Soils Using Safflower. Agronomy 2022, 12, 2302. [Google Scholar] [CrossRef]
- Hernández-Pitalúa, D.; Hernández-Orduña, M.G.; Martínez-Escalante, G.A.; Lagunes-Gómez, I. Influence of Lead (Pb) and Its Relationship with the pH of Water on the Growth of Creole Maize (Zea mays L.). Agriculture 2022, 12, 749. [Google Scholar] [CrossRef]
- Hashem, I.A.; Abbas, A.Y.; Abd El, A.E.-N.H.; Salem, H.M.; El-Hosseiny, O.E.; Abdel-Salam, M.A.; Saleem, M.H.; Zhou, W.; Hu, R. Potential of rice straw biochar, sulfur and ryegrass (Lolium perenne L.) in remediating soil contaminated with nickel through irrigation with untreated wastewater. PeerJ 2020, 6, 2020. [Google Scholar] [CrossRef]
- Hall, L.W.; Anderson, R.D. A Comparison of Sediment Metal Concentrations as Potential Stressors to Resident Benthic Communities in an Agricultural Waterbody. Agriculture 2022, 12, 1029. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on water quality detection by uv-vis spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Hashimi, M.H.; Hashimi, R.; Ryan, Q. Toxic Effects of Pesticides on Humans, Plants, Animals, Pollinators and Beneficial Organisms. Asian Plant Res. J. 2020, 5, 37–47. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.; Chang, H.; Li, Z.; Xue, J. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Chandini, R.; Kumar, R.; Prakash, O. The Impact of Chemical Fertilizers on our Environment and Ecosystem. Res. Trends Environ. Sci. 2019, 4, 69–86. Available online: https://www.researchgate.net/publication/331132826 (accessed on 5 April 2023).
- Zainurin, S.N.; Ismail, W.Z.W.; Mahamud, S.N.I.; Ismail, I.; Jamaludin, J.; Ab. Aziz, N.A. Integration of Sensing Framework with A Decision Support System for Monitoring Water Quality in Agriculture. Agriculture 2023, 13, 1000. [Google Scholar] [CrossRef]
- Razman, N.A.; Wan Ismail, W.Z.; Razak, M.H.A.; Ismail, I.; Jamaludin, J. Design and analysis of water quality monitoring and filtration system for different types of water in Malaysia. Int. J. Environ. Sci. Technol. 2023, 20, 3789–3800. [Google Scholar] [CrossRef]
- Sevda, T. Estimation of groundwater quality using an integration of water quality index, artificial intelligence methods and GIS: Case study, Central Mediterranean Region of Turkey. Appl. Water Sci. 2023, 13, 15. [Google Scholar]
- Wan Ismail, W.Z.; Goldys, E.M.; Dawes, J.M. Extended emission wavelength of random dye lasers by exploiting radiative and non-radiative energy transfer. App. Phys. B 2016, 122, 40. [Google Scholar] [CrossRef]
- Peteinatos, G.G.; Korsaeth, A.; Berge, T.W.; Gerhards, R. Using optical sensors to identify water deprivation, nitrogen shortage, weed presence and fungal infection in wheat. Agriculture 2016, 6, 24. [Google Scholar] [CrossRef]
- Laganovska, K.; Zolotarjovs, A.; Vázquez, M.; Mc Donnell, K.; Liepins, J.; Ben-Yoav, H.; Karitans, V.; Smits, K. Portable low-cost open-source wireless spectrophotometer for fast and reliable measurements. HardwareX 2020, 7, e00108. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Wang, S.; Zhang, L.S. Online water quality monitoring based on UV–Vis spectrometry and artificial neural networks in a river confluence near Sherfield-on-Loddon. Environ. Monit. Assess. 2022, 194, 9. [Google Scholar] [CrossRef]
- Kamil, N.A.I.M.; Nor’aini, Z.S.; Wan Ismail, W.Z.; Balakrishnan, S.R.; Juliza Jamaludin, J.; Ismail, I. Investigating the Quality of Milk using Spectrometry Technique and Scattering Theory. Eng. Technol. Appl. Sci. Res. 2021, 11, 7111–7117. [Google Scholar] [CrossRef]
- Kamil, N.A.I.M.; Ismail, W.Z.; Wan Ismail, I.; Jamaludin, J.; Hanasil, N.S.; Ibrahim, R.K.R. Analysis of Milk from Different Sources Based on Light Propagation and Random Laser Properties. Photonics 2021, 8, 486. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-Based Food Diagnostic Technologies: A Review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef]
- Transportation, L.F.S. Chapter 12 Linear Fiber-Optic Signal Transportation. In Modern Cable Television Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 509–557. [Google Scholar]
- Listiaji, P.; Suparta, G.B. Low-cost imaging spectrophotometer system for absorbance measurement. J. Phys. Conf. Ser. 2020, 1567, 042093. [Google Scholar] [CrossRef]
- Wilson, K.; Walker, J. Principles and Techniques of Biochemistry and Molecular Biology, 7th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; Volume 39. [Google Scholar]
- Agilent Technologies. The Basics of UV-Vis Spectrophotometry A Primer. 2021. Available online: https://www.agilent.com/cs/library/primers/public/primer-uv-vis-basics-5980-1397en-agilent.pdf (accessed on 10 April 2023).
- Tzanetou, E.N.; Karasali, H. A Comprehensive Review of Organochlorine Pesticide Monitoring in Agricultural Soils: The Silent Threat of a Conventional Agricultural Past. Agriculture 2022, 12, 728. [Google Scholar] [CrossRef]
- Hamid, N.; Suhaimi, S.; Othman, M.Z.; Wan Ismail, W.Z. A Review on Thermal Evaporation Method to Synthesis Zinc Oxide as Photocatalytic Material. Nano Hybrids Compos. 2021, 31, 55–63. [Google Scholar] [CrossRef]
- Mlangeni, A.T.; Raab, A.; Kumambala, P.; Monjerezi, M.; Matumba, L.; Feldmann, J. Evaluation of Metal (loids) Concentrations in Soils of Selected Rice Paddy Fields in Malawi. Agronomy 2022, 12, 2349. [Google Scholar] [CrossRef]
- Cooper, M.; Michael, K. Chapter 2: Spectroscopy: How we know what we know about the structure of matter in OCLUE. In Organic Chemistry, Life, the Universe & Everything; Michigan State University Libraries: East Lansing, MI, USA, 2020; ISBN 978-1-62610-102-9. [Google Scholar]
- The Royal Society of Chemistry. Ultraviolet/visible spectroscopy. In Modern Chemical Techniques; The Royal Society of Chemistry: London, UK, 2015; pp. 92–115. [Google Scholar]
- Rohit, J.V.; Kailasa, S.K. Cyclen dithiocarbamate-functionalized silver nanoparticles as a probe for colorimetric sensing of thiram and paraquat pesticides via host–guest chemistry. J. Nanoparticle Res. 2014, 16, 2585. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Bagheri, H.; Safari, R. Effect of electrical discharge in water on concentration of nitrate solution. Chin. Phys. B 2017, 26, 025101. [Google Scholar] [CrossRef]
- Keen, O.S.; Love, N.G.; Linden, K.G. Nitrate Photochemistry in the Context of Water Reclamation. In Water Reclamation and Sustainability; Elsevier: Amsterdam, The Netherlands, 2014; pp. 229–246. [Google Scholar] [CrossRef]
- Yeshno, E.; Arnon, S.; Dahan, O. Real-time monitoring of nitrate in soils as a key for optimization of agricultural productivity and prevention of groundwater pollution. Hydrol. Earth Syst. Sci. 2019, 23, 3997–4010. [Google Scholar] [CrossRef]
- Manyasree, D.; Kiranmayi, P.; Kolli, V.R. Characterization and antibacterial activity of zno nanoparticles synthesized by co precipitation method. Int. J. Appl. Pharm. 2018, 10, 224–228. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.H.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef]
- Schaller, C. Structure & Reactivity in Organic, Biological and Inorganic Chemistry; College Saint Benedict/Saint John’s University: Queens, NY, USA, 2014. [Google Scholar]




| ESP32-Based Spectrometer | Ocean Optic Spectrometer |
|---|---|
| Portable | Non-portable |
| On-site real-time monitoring | Lab based monitoring |
| Low cost | High cost |
| Less accurate | More accurate |
| Monitor and display result via mobile phone and computer | Monitor and display via computer |
| Non-invasive | Non-invasive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainurin, S.N.; Wan Ismail, W.Z.; Wan Azlan, W.A.N.; Zainul Ariffin, K.N.; Wan Ahmad Kamil, W.M. Developing a Portable Spectrometer to Detect Chemical Contaminants in Irrigation Water. Agriculture 2023, 13, 1202. https://doi.org/10.3390/agriculture13061202
Zainurin SN, Wan Ismail WZ, Wan Azlan WAN, Zainul Ariffin KN, Wan Ahmad Kamil WM. Developing a Portable Spectrometer to Detect Chemical Contaminants in Irrigation Water. Agriculture. 2023; 13(6):1202. https://doi.org/10.3390/agriculture13061202
Chicago/Turabian StyleZainurin, Siti Nadhirah, Wan Zakiah Wan Ismail, Wan Aina Nadhirah Wan Azlan, Khairul Nabilah Zainul Ariffin, and Wan Maryam Wan Ahmad Kamil. 2023. "Developing a Portable Spectrometer to Detect Chemical Contaminants in Irrigation Water" Agriculture 13, no. 6: 1202. https://doi.org/10.3390/agriculture13061202
APA StyleZainurin, S. N., Wan Ismail, W. Z., Wan Azlan, W. A. N., Zainul Ariffin, K. N., & Wan Ahmad Kamil, W. M. (2023). Developing a Portable Spectrometer to Detect Chemical Contaminants in Irrigation Water. Agriculture, 13(6), 1202. https://doi.org/10.3390/agriculture13061202







