Activity of α-d-Galactosidase in Long-Stored Seeds of Vicia hirsuta
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Germination Tests and Assay of Seed Vigour Parameters
2.3. α-d-Galactosidase Activity Assays
2.4. Statistical Analysis
3. Results and Discussion
3.1. Germination and Vigour Parameters of Long-Stored Seeds
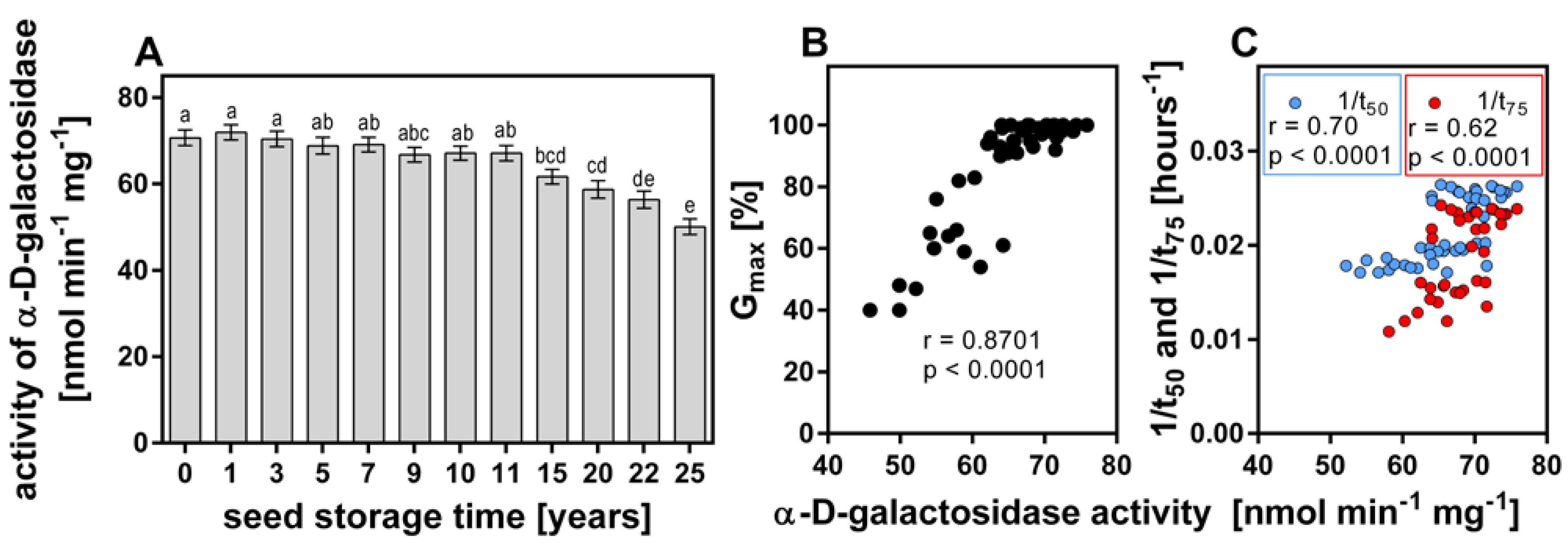
3.2. Activity of α-d-Galactosidase of Long-Stored Seeds
3.3. Correlation between α-d-Galactosidase Activity and Germination of Long-Stored Seeds
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walters, C. Understanding the mechanisms and kinetics of seed ageing. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Fleming, M.B.; Hill, L.M.; Walters, C. The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann. Bot. 2019, 123, 133–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. ZmAGA1 Hydrolyzes RFOs Late during the Lag Phase of Seed Germination, Shifting Sugar Metabolism toward Seed Germination Over Seed Ageing Tolerance. J. Agric. Food Chem. 2021, 69, 11606–11615. [Google Scholar] [CrossRef] [PubMed]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Basserdorf, Switzerland, 2015. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Ramtekey, V.; Cherukuri, S.; Kumar, S.; V, S.K.; Sheoran, S.; K, U.B.; K, B.N.; Singh, A.N.; Singh, H.V. Seed Longevity in Legumes: Deeper Insights into Mechanisms and Molecular Perspectives. Front. Plant Sci. 2022, 13, 918206. [Google Scholar] [CrossRef]
- Ewart, A.J. On the longevity of seeds. Proc. R. Soc. Vic. 1908, 21, 1–210. [Google Scholar] [CrossRef]
- Priestley, D.A.; Cullinan, V.I.; Wolfe, J. Differences in seed longevity at the species level. Plant Cell Environ. 1985, 8, 557–562. [Google Scholar] [CrossRef]
- Roos, E.E.; Davidson, D.A. Record longevities of vegetable seeds in storage. Hort. Sci. 1992, 27, 393–396. [Google Scholar] [CrossRef]
- Solberg, S.Ø.; Yndgaard, F.; Andreasen, C.; Von Bothmer, R.; Loskutov, I.G.; Asdal, Å. Long-term storage and longevity of orthodox seeds: A systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Lee, J.S.; Velasco-Punzalan, M.; Pacleb, M.; Valdez, R.; Kretzschmar, T.; McNally, K.L.; Ismail, A.M.; Cruz, P.C.S.; Sackville, H.N.R.; Hay, F.R. Variation in seed longevity among diverse Indica rice varieties. Ann. Bot. 2019, 124, 447–460. [Google Scholar] [CrossRef]
- Nagel, M.; Borner, A. The longevity of crop seeds stored under ambient conditions. Seed Sci. Res. 2010, 20, 1–12. [Google Scholar] [CrossRef]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Corbineau, F. Markers of seed quality: From present to future. Seed Sci. Res. 2012, 22, 61–68. [Google Scholar] [CrossRef]
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Vandecasteele, C.; Teulat-Merah, B.; Morère-Le Paven, M.C.; Leprince, O.; Ly Vu, B.; Viau, L.; Ledroit, L.; Pelletier, S.; Payet, N.; Satour, P.; et al. Quantitative trait loci analysis reveals a correlation between the ratio of sucrose/raffinose family oligosaccharides and seed vigour in Medicago truncatula. Plant Cell Environ. 2011, 34, 1473–1487. [Google Scholar] [CrossRef]
- El-Kassaby, Y.A.; Moss, I.; Kolotelo, D.; Stoehr, M. Seed germination: Mathematical representation and parameters extraction. For. Sci. 2008, 54, 220–227. [Google Scholar]
- Joosen, R.V.; Kodde, J.; Willems, L.A.; Ligterink, W.; van der Plas, L.H.; Hilhorst, H.W. GERMINATOR: A software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 2010, 62, 148–159. [Google Scholar] [CrossRef]
- Bello, P.; Bradford, K.J. Single-seed oxygen consumption measurements and population-based threshold models link respiration and germination rates under diverse conditions. Seed Sci. Res. 2016, 26, 199–221. [Google Scholar] [CrossRef]
- Bradford, K.J.; Tarquis, A.M.; Durán, J.M. A population-based threshold model describing the relationship between germination rates and seed deterioration. J. Exp. Bot. 1993, 44, 1225–1234. [Google Scholar] [CrossRef]
- Stegner, M.; Wagner, J.; Roach, T. Antioxidant depletion during seed storage under ambient conditions. Seed Sci. Res. 2022, 32, 150–156. [Google Scholar] [CrossRef]
- Abdul Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Chatelain, E.; Hundertmark, M.; Leprince, O.; Le Gall, S.; Satour, P.; Deligny-Penninck, S.; Rogniaux, H.; Buitink, J. Temporal profiling of the heat-stable proteome during late maturation of Medicago truncatula seeds identifies a restricted subset of late embryogenesis abundant proteins associated with longevity. Plant Cell Environ. 2012, 35, 1440–1455. [Google Scholar] [CrossRef]
- Leprince, O.; Buitink, J. Desiccation tolerance: From genomics to the field. Plant Sci. 2010, 179, 554–564. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Wang, D.; Liu, Y.; Dirk, L.M.A.; Goodman, J.; Downie, A.B.; Wang, J.; Wang, G.; Zhao, T. Regulation of Seed Vigor by Manipulation of Raffinose Family Oligosaccharides in Maize and Arabidopsis thaliana. Mol. Plant 2017, 10, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Cueff, G.; Hegedus, D.D.; Rajjou, L.; Bentsink, L. A role for seed storage proteins in Arabidopsis seed longevity. J. Exp. Bot. 2015, 66, 6399–6413. [Google Scholar] [CrossRef] [PubMed]
- Pereira Lima, J.J.; Buitink, J.; Lalanne, D.; Rossi, R.F.; Pelletier, S.; da Silva, E.A.A.; Leprince, O. Molecular characterization of the acquisition of longevity during seed maturation in soybean. PLoS ONE 2017, 12, e0180282. [Google Scholar] [CrossRef]
- Petla, B.P.; Kamble, N.U.; Kumar, M.; Verma, P.; Ghosh, S.; Singh, A.; Rao, V.; Salvi, P.; Kaur, H.; Saxena, S.C.; et al. Rice PROTEIN l-ISOASPARTYL METHYLTRANSFERASE isoforms differentially accumulate during seed maturation to restrict deleterious isoAsp and reactive oxygen species accumulation and are implicated in seed vigor and longevity. New Phytol. 2016, 211, 627–645. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E.; Kalemba, E. Non-reducing sugar levels in beech (Fagus sylvatica) seeds as related to withstanding desiccation and storage. J. Plant Physiol. 2009, 166, 1381–1390. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Righetti, K.; Vu, J.L.; Pelletier, S.; Vu, B.L.; Glaab, E.; Lalanne, D.; Pasha, A.; Patel, R.V.; Provart, N.J.; Verdier, J.; et al. Inference of Longevity-Related Genes from a Robust Coexpression Network of Seed Maturation Identifies Regulators Linking Seed Storability to Biotic Defense-Related Pathways. Plant Cell 2015, 27, 2692–2708. [Google Scholar] [CrossRef] [PubMed]
- Rosnoblet, C.; Aubry, C.; Leprince, O.; Vu, B.L.; Rogniaux, H.; Buitink, J. The regulatory gamma subunit SNF4b of the sucrose non-fermenting-related kinase complex is involved in longevity and stachyose accumulation during maturation of Medicago truncatula seeds. Plant J. 2007, 51, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Varshney, V.; Majee, M. Raffinose family oligosaccharides (RFOs): Role in seed vigor and longevity. Biosci. Rep. 2022, 42, BSR20220198. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A Regulatory Network-Based Approach Dissects Late Maturation Processes Related to the Acquisition of Desiccation Tolerance and Longevity of Medicago truncatula Seeds. Plant Physiol. 2013, 163, 757–774. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Luo, X.; Dai, Y.; Yang, Y.; Zheng, C.; Yang, W.; Shu, K. A matter of life and death: Molecular, physiological, and environmental regulation of seed longevity. Plant Cell Environ. 2020, 43, 293–302. [Google Scholar] [CrossRef]
- Chhabra, R.; Shabnam, S.T. Seed Ageing, Storage and Deterioration: An Irresistible Physiological Phenomenon. Agric. Rev. 2019, 40, 234–238. [Google Scholar]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late seed maturation: Drying without dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and environmental factors regulating seed longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Raffinose family oligosaccharides and sucrose contents in 13 Spanish lupin cultivars. Food Chem. 2005, 91, 645–649. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Alpha-galactosides: Antinutritional factors or functional ingredients? Crit. Rev. Food Sci. Nutr. 2008, 48, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Obendorf, R.L.; Górecki, R.J. Soluble carbohydrates in legume seeds. Seed Sci. Res. 2012, 22, 219–242. [Google Scholar] [CrossRef]
- Xiang, X.; Yang, L.; Hua, S.; Li, W.; Sun, Y.; Ma, H.; Zhang, J.; Zeng, X. Determination of oligosaccharide contents in 19 cultivars of chickpea (Cicer arietinum L.) seeds by high performance liquid. Food Chem. 2008, 111, 215–219. [Google Scholar]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; et al. Raffinose Family Oligosaccharides: Friend or Foe for Human and Plant Health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef]
- Sanyal, R.; Kumar, S.; Pattanayak, A.; Kar, A.; Bishi, S.K. Optimizing raffinose family oligosaccharides content in plants: A tightrope walk. Front. Plant Sci. 2023, 14, 1134754. [Google Scholar] [CrossRef]
- Bailly, C.; Audigier, C.; Ladonne, F.; Wagner, M.H.; Coste, F.; Corbineau, F.; Côme, D. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J. Exp. Bot. 2001, 52, 701–708. [Google Scholar] [CrossRef]
- Gojło, E.; Pupel, P.; Lahuta, L.B.; Podliński, P.; Kucewicz, M.; Górecki, R.J. The acquisition of desiccation tolerance in developing Vicia hirsuta seeds coincides with an increase in galactinol synthase expression and soluble α-D-galactosides accumulation. J. Plant Physiol. 2015, 184, 37–48. [Google Scholar] [CrossRef]
- Jing, Y.; Lang, S.; Wang, D.; Xue, H.; Wang, X.F. Functional characterization of galactinol synthase and raffinose synthase in desiccation tolerance acquisition in developing Arabidopsis seeds. J. Plant Physiol. 2018, 230, 109–121. [Google Scholar] [CrossRef]
- Arunraj, R.; Skori, L.; Kumar, A.; Hickerson, N.M.N.; Shoma, N.; Vairamani, M.; Samuel, M.A. Spatial regulation of alpha-galactosidase activity and its influence on raffinose family oligosaccharides during seed maturation and germination in Cicer arietinum. Plant Signal Behav. 2020, 15, 1709707. [Google Scholar] [CrossRef] [PubMed]
- Gangl, R.; Tenhaken, R. Raffinose Family Oligosaccharides Act As Galactose Stores in Seeds and Are Required for Rapid Germination of Arabidopsis in the Dark. Front. Plant Sci. 2016, 7, 1115. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, M.S.; Dietrich, S.M. Mobilisation of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth. (Leguminosae-Faboideae). Plant Sci. 1996, 117, 33–43. [Google Scholar] [CrossRef]
- He, F.; Huang, F.; Wilson, K.A.; Tan-Wilson, A. Protein storage vacuole acidification as a control of storage protein mobilization in soybeans. J. Exp. Bot. 2007, 58, 1059–1070. [Google Scholar] [CrossRef]
- Peterbauer, T.; Richter, A. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci. Res. 2001, 11, 185–197. [Google Scholar]
- Blöchl, A.; Peterbauer, T.; Richter, A. Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. J. Plant Physiol. 2007, 164, 1093–1096. [Google Scholar] [CrossRef]
- Blöchl, A.; Peterbauer, T.; Hofmann, J.; Richter, A. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 2008, 228, 99–110. [Google Scholar] [CrossRef]
- Dey, P.M.; Pridham, J.B. Purification and properties of α-D-galactosidases from Vicia faba seeds. Biochem. J. 1969, 113, 49–55. [Google Scholar] [CrossRef]
- Guimarães, V.M.; de Rezende, S.T.; Moreira, M.A.; de Barros, E.G.; Felix, C.R. Characterization of alpha-galactosidases from germinating soybean seed and their use for hydrolysis of oligosaccharides. Phytochemistry 2001, 58, 67–73. [Google Scholar] [CrossRef]
- Ataíde, G.D.M.; Borges, E.E.D.L.; Gonçalves, J.F.D.C.; Guimarães, V.M.; Bicalho, E.M.; Flores, A.V. Activities of α-galactosidase and polygalacturonase during hydration of Dalbergia nigra ((Vell.) Fr All. ex Benth.) seeds at different temperatures. J. Seed Sci. 2013, 35, 92–98. [Google Scholar] [CrossRef]
- Feurtado, J.A.; Banik, M.; Bewley, J.D. The cloning and characterization of alpha-galactosidase present during and following germination of tomato (Lycopersicon esculentum Mill.) seed. J. Exp. Bot. 2001, 52, 1239–1249. [Google Scholar] [PubMed]
- Herman, E.M.; Shannon, L.M. Accumulation and subcellular localization of α-galactosidase-hemagglutinin in developing soybean cotyledons. Plant Physiol. 1985, 77, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Marraccini, P.; Rogers, W.J.; Caillet, V.; Deshayes, A.; Granato, D.; Lausanne, F.; Lechat, S.; Pridmore, D.; Pétiard, V. Biochemical and molecular characterization of α-D-galactosidase from coffee beans. Plant Physiol. Biochem. 2005, 43, 909–920. [Google Scholar] [CrossRef]
- Obendorf, R.L. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci. Res. 1997, 7, 63–74. [Google Scholar] [CrossRef]
- Reid, S.G.; Meier, H. Enzymatic activities and galactomannan mobilization in germinating seeds of fenugreek (Trigonella foenum-graecum L. Leguminosae). Secretion of a-galactosidase and b-mannosidase by the aleurone layer. Planta 1973, 112, 301–308. [Google Scholar] [CrossRef]
- Yasui, T.; Endo, Y.; Ohashi, H. Infrageneric variation of the low molecular weight carbohydrate composition of the seeds of the genus Vicia (Leguminosae). Bot. Mag. 1987, 100, 255–272. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Górecki, R.J.; Gojło, E.; Horbowicz, M. Differences in accumulation of soluble α-galactosides during seed maturation of several Vicia species. Acta Physiol. Plant. 2005, 27, 163–171. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Goszczyńska, J.; Horbowicz, M. Seed α-D-galactosides of selected Vicia species and enzymes involved in their biosynthesis. Acta Biol. Crac. Ser. Bot. 2010, 52, 27–35. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kucewicz, M.; Maćkiewicz, K.; Źróbek-Sokolnik, A. Selected aspects of tiny vetch (Vicia hirsuta (L.) GRAY S.F.) seed ecology: Generative reproduction and effects of seed maturity and seed storage on seed germination. Acta Agrobot. 2010, 63, 205–212. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Longevity, Storage, and Deterioration. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 341–376. [Google Scholar]
- Colville, L.; Pritchard, H.W. Seed life span and food security. New Phytol. 2019, 224, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Justice, O.L.; Bass, L.N. Principles and Practices of Seed Storage (No. 506); US Department of Agriculture Press: Washington, DC, USA, 1978.
- Desheva, G. The Longevity of Crop Seeds Stored Under Long-term Condition in the National Gene Bank of Bulgaria. Agriculture 2016, 62, 90–100. [Google Scholar] [CrossRef]
- Shenzao, F.; Guangkun, Y.; Xia, X.; Shuhua, W.; Xinghua, W.; Xinxiong, L. Levels of crotonaldehyde and 4-hydroxy-(E)-2-nonenal and expression of genes encoding carbonyl-scavenging enzyme at critical node during rice seed aging. Rice Sci. 2018, 25, 152–160. [Google Scholar] [CrossRef]
- Yin, G.; Whelan, J.; Wu, S.; Zhou, J.; Chen, B.; Chen, X.; Zhang, J.; He, J.; Xin, X. Comprehensive Mitochondrial Metabolic Shift during the Critical Node of Seed Ageing in Rice. PLoS ONE 2016, 11, e0148013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, L.; Chen, C.; Zhou, T.; Wu, Q.; Wen, F.; Chen, J.; Pritchard, H.W.; Peng, C.; Pei, J.; et al. Comparative changes in sugars and lipids show evidence of a critical node for regeneration in safflower seeds during aging. Front. Plant Sci. 2022, 13, 1020478. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, A.J.; Ellis, R.H. Longevity, Viability and Dormancy. In Seeds: The ecology of Regeneration in Plant Comunities; Fenner, M., Ed.; CABI: Wallingford, UK, 1992; pp. 193–229. [Google Scholar]
- Pukacka, S.; Hoffmann, S.K.; Goslar, J.; Pukacki, P.M.; Wójkiewicz, E. Water and lipid relations in beech (Fagus sylvatica L.) seeds and its effect on storage behaviour. Biochim. Biophys. Acta 2003, 1621, 48–56. [Google Scholar] [CrossRef]
- Colville, L.; Bradley, E.L.; Lloyd, A.S.; Pritchard, H.W.; Castle, L.; Kranner, I. Volatile fingerprints of seeds of four species indicate the involvement of alcoholic fermentation, lipid peroxidation, and Maillard reactions in seed deterioration during ageing and desiccation stress. J. Exp. Bot. 2012, 63, 6519–6530. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Pukacka, S. Carbonylated proteins accumulated as vitality decreases during long-term storage of beech (Fagus sylvatica L.) seeds. Trees 2014, 28, 503–515. [Google Scholar] [CrossRef]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed Longevity—The Evolution of Knowledge and a Conceptual Framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef]
- Veselova, T.V.; Veselovsky, V.A.; Obroucheva, N.V. Deterioration mechanisms in air-dry pea seeds during early aging. Plant Physiol. Biochem. 2015, 87, 133–139. [Google Scholar] [CrossRef]
- Xin, X.; Tian, Q.; Yin, G.; Chen, X.; Zhang, J.; Ng, S.; Lu, X. Reduced mitochondrial and ascorbate-glutathione activity after artificial ageing in soybean seed. J. Plant Physiol. 2014, 171, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.X.; Fu, H.; Gao, J.D.; Zhang, Y.X.; Huang, W.J.; Chen, Z.J.; Qi, Z.; Yan, S.J.; Liu, J. Identification of metabolomic biomarkers of seed vigor and aging in hybrid rice. Rice. 2022, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Silvestroni, A.; Connes, C.; Juillard, V.; de Giori, G.S.; Piard, J.C.; Sesma, F. Reduction of non-digestible oligosaccharides in soymilk: Application of engineered lactic acid bacteria that produce alpha-galactosidase. Genet. Mol. Res. 2004, 30, 432–440. [Google Scholar]
- Lahuta, L.B.; Goszczyńska, J. Inhibition of raffinose family oligosaccharides and galactosyl pinitols breakdown delays germination of winter vetch [Vicia villosa Roth.] seeds. Acta Soc. Bot. Pol. 2009, 78, 203–208. [Google Scholar] [CrossRef]
- Polowick, P.L.; Polowick, D.S.; Baliski, D.S.; Bock, C.; Ray, H.; Fawzy, G. Over-expression of α-galactosidase in pea seeds to reduce raffinose oligosaccharide content. Botany 2009, 87, 526–532. [Google Scholar] [CrossRef]
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef]
- Tetreault, H.; Fleming, M.; Hill, L.; Dorr, E.; Yeater, K.; Richards, C.; Walters, C. A Power Analysis for Detecting Aging of Dry-stored Soybean Seeds: Germination versus RNA Integrity Assessments. Crop Sci. 2023, 63, 1481–1493. [Google Scholar] [CrossRef]
- Pereira Neto, L.G.; Rossini, B.C.; Marino, C.L.; Toorop, P.E.; Silva, E.A.A. Comparative Seeds Storage Transcriptome Analysis of Astronium fraxinifolium Schott, a Threatened Tree Species from Brazil. Int. J. Mol. Sci. 2022, 23, 13852. [Google Scholar] [CrossRef]



| Trait | Gmax | 1/t50 | 1/t75 | t50 | t75 | U8020 | EL | RL | SL | SDW | SVI 1 | SVI 2 | AGAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gmax | 1 | 0.82 **** | 0.88 **** | −0.80 **** | −0.87 **** | −0.86 **** | 0.84 **** | 0.88 **** | 0.89 **** | 0.83 **** | 0.96 **** | 0.95 **** | 0.87 **** |
| 1/t50 | 1 | 0.99 **** | −0.98 **** | −0.97 **** | −0.89 **** | 0.70 **** | 0.70 **** | 0.72 **** | 0.67 **** | 0.77 **** | 0.76 **** | 0.70 **** | |
| 1/t75 | 1 | −0.99 **** | −0.98 **** | −0.92 **** | 0.67 **** | 0.65 **** | 0.75 **** | 0.57 **** | 0.92 **** | 0.82 **** | 0.62 **** | ||
| t50 | 1 | 0.98 **** | 0.91 **** | −0.68 **** | −0.68 **** | −0.70 **** | −0.65 **** | −0.75 **** | −0.75 **** | −0.67 **** | |||
| t75 | 1 | 0.97 **** | −0.66 **** | −0.71 **** | −0.77 **** | −0.62 **** | −0.95 **** | −0.87 **** | −0.65 **** | ||||
| U8020 | 1 | −0.63 **** | −0.74 **** | −0.77 **** | −0.63 **** | −0.94 **** | −0.87 **** | −0.68 **** | |||||
| EL | 1 | 0.87 **** | 0.98 **** | 0.95 **** | 0.91 **** | 0.91 **** | 0.83 **** | ||||||
| RL | 1 | 0.96 **** | 0.90 **** | 0.94 **** | 0.93 **** | 0.85 **** | |||||||
| SL | 1 | 0.96 **** | 0.95 **** | 0.95 **** | 0.87 **** | ||||||||
| SDW | 1 | 0.90 **** | 0.94 **** | 0.82 **** | |||||||||
| SVI 1 | 1 | 0.99 **** | 0.89 **** | ||||||||||
| SVI 2 | 1 | 0.87 **** | |||||||||||
| AGAL | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gojło, E. Activity of α-d-Galactosidase in Long-Stored Seeds of Vicia hirsuta. Agriculture 2023, 13, 1306. https://doi.org/10.3390/agriculture13071306
Gojło E. Activity of α-d-Galactosidase in Long-Stored Seeds of Vicia hirsuta. Agriculture. 2023; 13(7):1306. https://doi.org/10.3390/agriculture13071306
Chicago/Turabian StyleGojło, Ewa. 2023. "Activity of α-d-Galactosidase in Long-Stored Seeds of Vicia hirsuta" Agriculture 13, no. 7: 1306. https://doi.org/10.3390/agriculture13071306
APA StyleGojło, E. (2023). Activity of α-d-Galactosidase in Long-Stored Seeds of Vicia hirsuta. Agriculture, 13(7), 1306. https://doi.org/10.3390/agriculture13071306






