Effect of Duckweed (Spirodela polyrhiza)-Supplemented Semolina on the Production Parameters and Nutrient Composition of Yellow Mealworm (Tenebrio molitor)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Research Environment
2.2. Substrate Composition
2.3. Experimental Set-Up
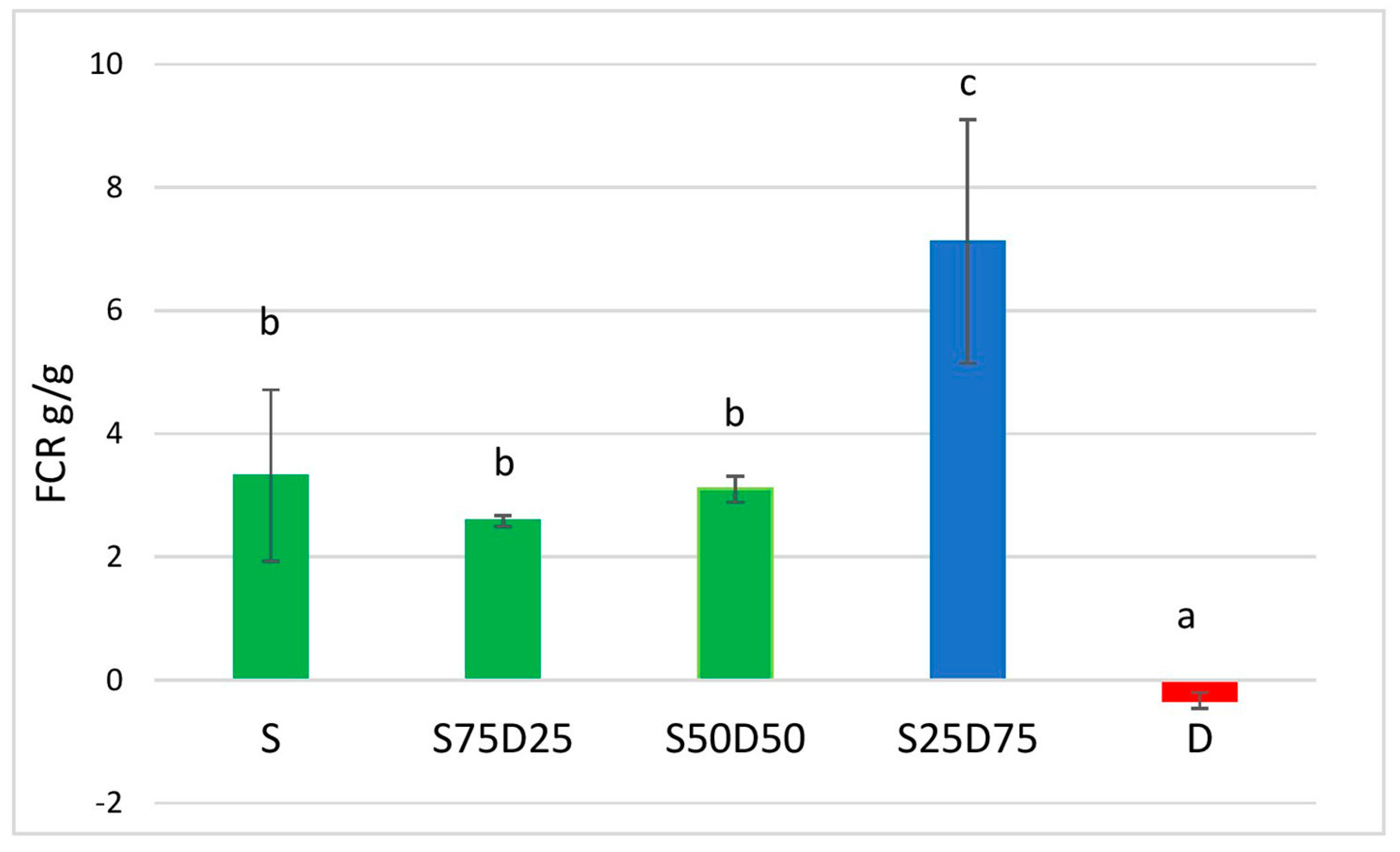
2.4. Feed Conversion Ratio
2.5. Proximate Analysis
2.6. Calculation of the Crude Protein Content of Individual Yellow Mealworms
2.7. Amino Acid Analysis
2.8. Fatty Acid Composition
2.9. Statistical Analysis
3. Results
3.1. Production Parameters
3.1.1. Growth Performance of Yellow Mealworm at Different Substrate Compositions
3.1.2. Feed Conversion Ratio
3.1.3. Survival Rate
3.2. Nutrient Composition
3.3. Amino Acid Composition of Yellow Mealworm at Different Substrate Compositions
3.4. Fatty Acid Composition of Yellow Mealworm at Different Substrate Compositions
4. Discussion
4.1. Production Parameters
4.2. Nutrient Composition
4.3. Amino Acid Composition of Yellow Mealworm
4.4. Fatty Acid Composition of Yellow Mealworm
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FCR | Feed conversion ratio |
| DM | Dry matter |
| CP | Crude protein |
| CFa | Crude fat |
| CF | Crude fiber |
| ADL | Acid detergent lignin |
| ADF | Acid detergent fiber |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| PUFA | Polyunsaturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| SFA | Saturated fatty acids |
| FA | Fatty acids |
| AA | Amino acid |
| NEAA | Non-essential amino acids |
| EAA | Essential amino acid |
| CTAB | Cetyltrimethylammonium bromide |
References
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will yellow mealworm become a source of safe proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Klejdysz, T.; Subramanyam, B.; Nawrot, J. Atlas of Stored-Product Insects and Mites; AACC International Inc.: Eagan, Minnesota, USA, 2013; Volume 589. [Google Scholar]
- Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other than fur Animals, with Protein Derived from Animals. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2021:295:TOC (accessed on 15 March 2023).
- Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 authorising the placing on the market of frozen, dried and powder forms of yellow mealworm (Tenebrio molitor larva) as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R0169&qid=1678903371451 (accessed on 15 March 2023).
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Kristensen, T.N.; Heckmann, L.H.; Sørensen, J.G. Breeding and maintaining high-quality insects. In Insects as Food and Feed: From Production to Consumption; Van Huis, A., Tomberlin, J.K., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 175–198. [Google Scholar]
- Heckmann, L.H.; Andersen, J.L.; Gianotten, N.; Calis, M.; Fischer, C.H.; Calis, H. Sustainable mealworm production for feed and food. Edible Insects Sustain. Food Syst. 2018, 321–328. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Van Broekhoven, S.; Oonincx, D.G.; Van Huis, A.; Van Loon, J.J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Morales-Ramos, J.; Rojas, M.; Shapiro-Ilan, D.; Tedders, W. Developmental plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of instar variation in number and development time under different diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Morales-Ramos, J.; Rojas, M.; Shapiro-Ilan, D.; Tedders, W. Self-selection of two diet components by Tenebrio molitor (Coleoptera: Tenebrionidae) larvae and its impact on fitness. Environ. Entomol. 2011, 40, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-llan, D.I.; Tedders, W.L. Use of nutrient self-selection as a diet refining tool in Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- Dragojlović, D.; Đuragić, O.; Pezo, L.; Popović, L.; Rakita, S.; Tomičić, Z.; Spasevski, N. Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals 2022, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.J. Increasing calcium levels in cultured insects. Zoo Biol.: Publ. Affil. Am. Zoo Aquar. Assoc. 2000, 19, 1–9. [Google Scholar] [CrossRef]
- Keil, C.; Maares, M.; Kröncke, N.; Benning, R.; Haase, H. Dietary zinc enrichment reduces the cadmium burden of mealworm beetle (Tenebrio molitor) larvae. Sci. Rep. 2020, 10, 20033. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Enrichment in specific fatty acids profile of Tenebrio molitor and Hermetia illucens larvae through feeding. Future Foods 2021, 3, 100016. [Google Scholar] [CrossRef]
- Landolt, E.; Kandeler, R. Biosystematic investigations in the family of duckweeds (Lemnaceae). Veroff. Geobot. Inst. ETH Zur. 1987, 2, 42–43. [Google Scholar]
- Leng, R.A.; Stambolie, J.H.; Bell, R. Duckweed—A potential high-protein feed resource for domestic animals and fish. Livest. Res. Rural. Dev. 1995, 7, 36. [Google Scholar]
- Anderson, K.E.; Lowman, Z.; Stomp, A.M.; Chang, J. Duckweed as a feed ingredient in laying hen diets and its effect on egg production and composition. Int. J. Poult. Sci. 2011, 10, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Shen, Y.; Zheng, Y.; Smith, G.; Sun, X.S.; Wang, D.; Zhao, Y.; Zhang, W.; Li, Y. Duckweed (Lemnaceae) for potentially nutritious human food: A review. Food Rev. Int. 2022, 1–15. [Google Scholar] [CrossRef]
- Rusoff, L.L.; Blakeney, E.W.; Culley, D.D. Duckweeds (Lemnaceae Family): A Potential Source of Protein and Amino Acids. J. Agric. Food Chem. 1980, 28, 848–850. [Google Scholar] [CrossRef]
- Islam, K.M.S. Feasibility of Duckweed as Poultry Feed—A Review. Ind. J. Animal. Sci. 2002, 72, 486–491. [Google Scholar]
- Hasan, M.R.; Rina, C. Use of Algae and Aquatic Macrophytes as Feed in Small-scale Aquaculture: A Review (No. 531); Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 5983-2:2009; Animal Feeding Stuffs—Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 2: Block Digestion and Steam Distillation Method. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Organization for Standardization: Geneva, Switzerland, 2000.
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New methods for high-accuracy insect chitin measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef]
- ISO 13906:2008; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. International Organization for Standardization: Geneva, Switzerland, 2008.
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef] [Green Version]
- Thévenot, A.; Rivera, J.L.; Wilfart, A.; Maillard, F.; Hassouna, M.; Senga-Kiesse, T.; Le Féon, S.; Aubin, J. Mealworm meal for animal feed: Environmental assessment and sensitivity analysis to guide future prospects. J. Clean. Prod. 2018, 170, 1260–1267. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Peni, D. Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture 2020, 10, 599. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.H.; Choi, W.H.; Hong, S.J.; Kim, N.J. Nutritional value of mealworm, Tenebrio Molitor as food source. Int. J. Ind. Entomol. 2012, 25, 93–9820. [Google Scholar] [CrossRef] [Green Version]
- Toviho, O.A.; Bársony, P. Nutrient Composition and Growth of Yellow Mealworm (Tenebrio molitor) at Different Ages and Stages of the Life Cycle. Agriculture 2022, 12, 1924. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Jajić, I.; Popović, A.; Urošević, M.I.; Krstović, S.; Petrović, M.; Guljaš, D.; Samardžić, M. Fatty and amino acid profile of mealworm larvae (Tenebrio molitor L.). Biotechnol. Anim. Husb. 2020, 36, 167–180. [Google Scholar] [CrossRef]
- Jajić, I.; Krstović, S.; Petrović, M.; Urošević, M.; Glamočić, D.; Samardžić, M.; Popović, A.; Guljaš, D. Changes in the chemical composition of the yellow mealworm (Tenebrio molitor L.) reared on different feedstuffs. J. Anim. Feed. Sci. 2022, 31, 191–200. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Green, J.A.; Hardy, R.W.; Brannon, E.L. The optimum dietary essential: Nonessential amino acid ratio for rainbow trout (Oncorhynchus mykiss), which maximizes nitrogen retention and minimizes nitrogen excretion. Fish Physiol. Biochem. 2002, 27, 109–115. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Münch, M.; Gropp, J.M. Non-essential amino acid sources in crystalline amino acid diets for trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 1995, 11, 317–321. [Google Scholar] [CrossRef]
- Mambrini, M.; Kaushik, S.J. Partial replacement of dietary protein nitrogen with dispensable amino acids in diets of Nile tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. 1994, 109, 469–477. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jedras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar]
- Kleber, M.E.; Delgado, G.E.; Lorkowski, S.; März, W.; von Schacky, C. Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2016, 252, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A low ω-6 to ω-3 PUFA ratio (n–6: N–3 PUFA) diet to treat fatty liver disease in obese youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]

| Dry Matter | Crude Protein | Crude Fat | Crude Fiber | Ash | Carbohydrate | |
|---|---|---|---|---|---|---|
| Semolina | 88.3 | 15.4 | 2.60 | 2.32 | 0.93 | 79.3 |
| Duckweed | 10.22 | 28.56 | 6.03 | 13.09 | 19.15 | 40.03 |
| Semolina (S) | Duckweed (D) | Total Weight of Substrate (g) | Label |
|---|---|---|---|
| 100% (20 g) | 0% | 20 g | S |
| 75% (15 g) | 25% (5 g) | 20 g | S75 D25 |
| 50% (10 g) | 50% (10 g) | 20 g | S50 D50 |
| 25% (5 g) | 75% (15 g) | 20 g | S25 D75 |
| 0% | 100% (20 g) | 20 g | D |
| Equipment | Varian CP 3800 |
|---|---|
| Detector | FID, 250 °C |
| Column | Restek Rt-2560, 100 m × 0.25 mm ID; 0.20 µm |
| Injector temperature | 250 °C |
| Split | 1:50 |
| Temperature program | 150° C (hold: 3 min), 2 °C/min, 240 °C (hold: 2 min) |
| Injected volume | 1 µl |
| Gas carrier | 1.9 mL/min helium (5.0), constant flow |
| S | S75D25 | S50D50 | S25D75 | D | |
|---|---|---|---|---|---|
| Weight (g) | 0.116 ± 0.005 b | 0.154 ± 0.008 d | 0.139 ± 0.002 c | 0.108 ± 0.002 b | 0.053 ± 0.004 a |
| Length (cm) | 2.081 ± 0.068 b | 2.349 ± 0.074 c | 2.385 ± 0.074 c | 1.930 ± 0.012 b | 1.673 ± 0.040 a |
| Width (cm) | 0.281 ± 0.006 ab | 0.296 ± 0.011 b | 0.286 ± 0.013 b | 0.278 ± 0.013 ab | 0.258 ± 0.014 a |
| S | S75D25 | S50D50 | S25D75 | D | |
|---|---|---|---|---|---|
| Dry Matter | 38.3 ± 0.26 c | 39 ± 0.36 d | 37.3 ± 0.10 a | 37.3 ± 0.02 a | 37.7 ± 0.17 b |
| Crude protein | 45.7 ± 0.18 a | 50.8 ± 0.22 b | 55.8 ± 0.18 c | 61.1 ± 0.18 d | 69 ± 0.22 e |
| Crude fat | 43.7 ± 0.22 e | 37.9 ± 0.28 d | 30.5 ± 0.27 c | 24.2 ± 0.14 b | 14.8 ± 0.20 a |
| Crude fiber | 7.4 ± 0.22 a | 7.9 ± 0.18 b | 9.4 ± 0.18 c | 10 ± 0.18 d | 11.1 ± 0.18 e |
| Crude ash | 2.8 ± 0.18 a | 3.4 ± 0.14 b | 4.2 ± 0.18 c | 4.6 ± 0.12 d | 5.0 ± 0.23 e |
| Chitin | 21.5 ± 0.17 b | 20.0 ± 0.12 a | 21.5 ± 0.28 b | 21.8 ± 0.20 b | 20.8 ± 0.12 a |
| S | S75D25 | S50D50 | S25D75 | D | |
|---|---|---|---|---|---|
| Average body weight (g) | 0.116 ± 0.005b | 0.154 ± 0.008 d | 0.139 ± 0.002 c | 0.108 ± 0.002 b | 0.053 ± 0.004 a |
| Crude protein (DM%) | 45.7 ± 0.18 a | 50.8 ± 0.22 b | 55.8 ± 0.18 c | 61.1 ± 0.18 d | 69 ± 0.22 e |
| Dry Matter | 38.3 ± 0.26 c | 39 ± 0.36 d | 37.3 ± 0.10 a | 37.3 ± 0.02 a | 37.7 ± 0.17 b |
| Average crude protein content/individual | 0.020 ± 0.001 b | 0.031 ± 0.001 d | 0.029 ± 0.002 d | 0.025 ± 0.001 c | 0.014 ± 0.001 a |
| S | S75D25 | S50D50 | S25D75 | D | |
|---|---|---|---|---|---|
| Asparagine | 1.23 ± 0.04 a | 1.38 ± 0.05 b | 1.22 ± 0.04 a | 1.70 ± 0.04 c | 1.74 ± 0.03 c |
| Threonine | 0.73 ± 0.02 a | 0.73 ± 0.01 a | 0.78 ± 0.01 b | 0.94± 0.01 c | 0.91 ± 0.01 c |
| Serine | 0.84 ± 0.07 ab | 0.79 ± 0.01 a | 0.88 ± 0.01 b | 1.08 ± 0.02 c | 1.02 ± 0.01 c |
| Glutamine | 2,14 ± 0.01 a | 2,26 ± 0.01 b | 2,25 ± 0.01 b | 2,64 ± 0.02 c | 2,66 ± 0.05 c |
| Proline | 1.20 ± 0.01 c | 0.93 ± 0.01 a | 1.15 ± 0.01 b | 1.56± 0.01 e | 1.52 ± 0.03 d |
| Glycine | 0.97 ± 0.01 a | 0.98 ± 0.01 a | 0.98 ± 0.01 a | 1.17 ± 0.02 c | 1.12 ± 0.02 b |
| Alanine | 1.60 ± 0.02 b | 1.36 ± 0.02 a | 1.57± 0.03 b | 1.74 ± 0.02 c | 1.61 ± 0.02 b |
| Valine | 1.13 ± 0.03 a | 1.20 ± 0.02 b | 1.20 ± 0.01 b | 1.49 ± 0.02 d | 1.39 ± 0.01 c |
| Methionine + cysteine | 0.32 ± 0.01 a | 0.3 ± 0.01 a | 0.3 ± 0.01 a | 0.42 ± 0.01 b | 0.43 ± 0.01 b |
| Isoleucine | 1.53 ± 0.02 b | 1.38 ± 0.0 a | 1.55 ± 0.04 b | 1.92 ± 0.01 d | 1.65 ± 0.02 c |
| Leucine | 1.32 ± 0.01 a | 1.38 ± 0.02 b | 1.40 ± 0.02 b | 1.63 ± 0.02 c | 1.61 ± 0.01 c |
| Phenylalanine + tyrosine | 1.57 ± 0.01 a | 1.86 ± 0.02 b | 1.730.03 b | 2.23 ± 0.03 c | 2.27 ± 0.01 c |
| Histidine | 0.60 ± 0.02 a | 0.64 ± 0.01 b | 0.63 ± 0.02 ab | 0.82 ± 0.01 d | 0.71 ± 0.01 c |
| Lysine | 1.27 ± 0.02 a | 1.27 ± 0.01 a | 1.31 ± 0.01 b | 1.51 ± 0.02 c | 1.60 ± 0.02 d |
| Arginine | 0.68 ± 0.01 b | 0.62 ± 0.01 a | 0.68 ± 0.02 b | 0.91 ± 0.03 c | 0.89 ± 0.02 c |
| EAA | 7.47 ± 0.04 a | 8.76 ± 0.04 b | 8.9 ± 0.49 b | 10.96 ± 0.04 c | 10.57 ± 0.05 c |
| EAA:NEAA | 0.86 ± 0.02 a | 1.05 ± 0.04 b | 1.02 ± 0.03 b | 1.01 ± 0.02 b | 1.00 ± 0.02 b |
| S | S75D25 | S50D50 | S25D75 | D | |
|---|---|---|---|---|---|
| Capric | 0.011 ± 0.00 a | 0.013 ± 0.00 a | 0.009 ± 0.00 ab | 0.019 ± 0.00 a | 0.014 ± 0.00 b |
| Lauric | 0.310 ± 0.01 bc | 0.260 ± 0.02 b | 0.210 ± 0.01 a | 0.300± 0.01 c | 0.240 ± 0.01 ab |
| Tridecylic | 0.038 ± 0.00 a | 0.046 ± 0.00 b | 0.038 ± 0.00 a | 0.052 ± 0.00 c | 0.047 ± 0.00 b |
| Myristic | 3.850 ± 0.02 a | 4.420 ± 0.01 c | 4.110 ± 0.01 b | 4.990 ± 0.01 d | 5.860 ± 0.01 e |
| Myristoleic | 0.005 ± 0.00 b | 0.008 ± 0.00 c | 0.000 ± 0.00 a | 0.000 ± 0.00 a | 0.020 ± 0.00 d |
| Pentadecylic | 0.067 ± 0.00 a | 0.104 ± 0.00 b | 0.139 ± 0.00 c | 0.237 ± 0.00 d | 0.138 ± 0.00 c |
| Palmitic | 14.770 ± 0.01 b | 16.770 ± 0.02 c | 18.260 ± 0.01 d | 20.180 ± 0.01 e | 12.430 ± 0.1 a |
| Palmitoleic | 1.790 ± 0.01 d | 1.547 ± 0.01 c | 1.350 ± 0.01 b | 1.290 ± 0.01 a | 2.490 ± 0.01 e |
| Magaric | 0.054 ± 0.00 a | 0.083 ± 0.00 b | 0.083 ± 0.00 b | 0.185 ± 0.00 d | 0.115 ± 0.00 c |
| Stearic | 2.830 ± 0.01 a | 4.630 ± 0.01 d | 4.510 ± 0.01 c | 5.420 ± 0.01 e | 3.310 ± 0.01 b |
| Elaidic | 0.003 ± 0.00 a | 0.004 ± 0.00 a | 0.009 ± 0.00 c | 0.018 ± 0.00 d | 0.006 ± 0.00 b |
| Oleic | 51.900 ± 0.04 c | 51.700 ± 0.02 b | 53.330 ± 0.01 d | 47.890 ± 0.01 a | 47.900 ± 0.01 a |
| Linoleic | 22.970 ± 0.01 d | 19.270 ± 0.01 c | 16.860 ± 0.01 a | 18.020 ± 0.01 b | 25.320 ± 0.01 e |
| Arachidic | 0.230 ± 0.01 a | 0.3800 ± 0.01 c | 0.400 ± 0.01 d | 0.370 ± 0.01 c | 0.310 ± 0.01 b |
| Γlinolenic | 0.000 ± 0.00 a | 0.017 ± 0.00 c | 0.013 ± 0.00 b | 0.000 ± 0.00 a | 0.000 ± 0.00 a |
| Gondonic | 0.035 ± 0.00 d | 0.008 ± 0.00 a | 0.013 ± 0.00 b | 0.019 ± 0.00 c | 0.020 ± 0.00 c |
| Alinolenic | 1.020 ± 0.01 d | 0.641 ± 0.00 b | 0.590 ± 0.00 a | 0.706 ± 0.00 c | 1.552 ± 0.00 e |
| Behenic | 0.107 ± 0.00 c | 0.083 ± 0.0 b | 0.054 ± 0.00 a | 0.238 ± 0.0 e | 0.189 ± 0.00 d |
| Eicosenoic | 0.026 ± 0.00 a | 0.029 ± 0.00 b | 0.036 ± 0.00 c | 0.082 ± 0.00 e | 0.067 ± 0.00 d |
| Nervonic | 0.005 ± 0.00 b | 0.000 ± 0.00 a | 0.006 ± 0.00 b | 0.000 ± 0.00 a | 0.01 ± 0.00 b |
| SFA | 22.25 ± 0.02 a | 26.78 ± 0.02 c | 27.79 ± 0.01 d | 31.98 ± 0.01 e | 22.63 ± 0.02 b |
| MUFA | 53.73 ± 0.02 d | 53.26 ± 0.01 c | 54.70 ± 0.01 e | 49.21 ± 0.01 a | 50.43 ± 0.01 b |
| PUFA N-6 | 22.97 ± 0.02 d | 19.29 ± 0.01 c | 16.88 ± 0.01 a | 18.02 ± 0.02 b | 25.32 ± 0.02 e |
| PUFA N-3 | 1.04 ± 0.01 c | 0.67 ± 0.01 a | 0.63 ± 0.00 a | 0.79 ± 0.01 b | 1.62 ± 0.02 d |
| PUFA N-6/ N-3 | 22.08 ± 0.01 b | 28.79 ± 0.01 e | 26.79 ± 0.01 d | 22.81 ± 0.01 c | 15.63 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toviho, O.A.; Imane, M.; Tünde, P.; Péter, B. Effect of Duckweed (Spirodela polyrhiza)-Supplemented Semolina on the Production Parameters and Nutrient Composition of Yellow Mealworm (Tenebrio molitor). Agriculture 2023, 13, 1386. https://doi.org/10.3390/agriculture13071386
Toviho OA, Imane M, Tünde P, Péter B. Effect of Duckweed (Spirodela polyrhiza)-Supplemented Semolina on the Production Parameters and Nutrient Composition of Yellow Mealworm (Tenebrio molitor). Agriculture. 2023; 13(7):1386. https://doi.org/10.3390/agriculture13071386
Chicago/Turabian StyleToviho, Odunayo A., Moutia Imane, Pusztahelyi Tünde, and Bársony Péter. 2023. "Effect of Duckweed (Spirodela polyrhiza)-Supplemented Semolina on the Production Parameters and Nutrient Composition of Yellow Mealworm (Tenebrio molitor)" Agriculture 13, no. 7: 1386. https://doi.org/10.3390/agriculture13071386
APA StyleToviho, O. A., Imane, M., Tünde, P., & Péter, B. (2023). Effect of Duckweed (Spirodela polyrhiza)-Supplemented Semolina on the Production Parameters and Nutrient Composition of Yellow Mealworm (Tenebrio molitor). Agriculture, 13(7), 1386. https://doi.org/10.3390/agriculture13071386









