Assessment of the Stabilization of Cu-, Pb-, and Zn-Contaminated Fine Soil Using Cockle Shells, Scallop Shells, and Starfish
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Collection of Contaminated Fine Soil
2.2. Stabilizing Agents
2.3. Stabilization Experiments
2.4. Heavy Metal Leaching Tests
2.5. X-ray Powder Diffraction (XRPD) Analysis
2.6. Scanning Electron Microscopy–Energy Dispersive X-ray Spectroscopy (SEM–EDX) Analysis
3. Results and Discussion
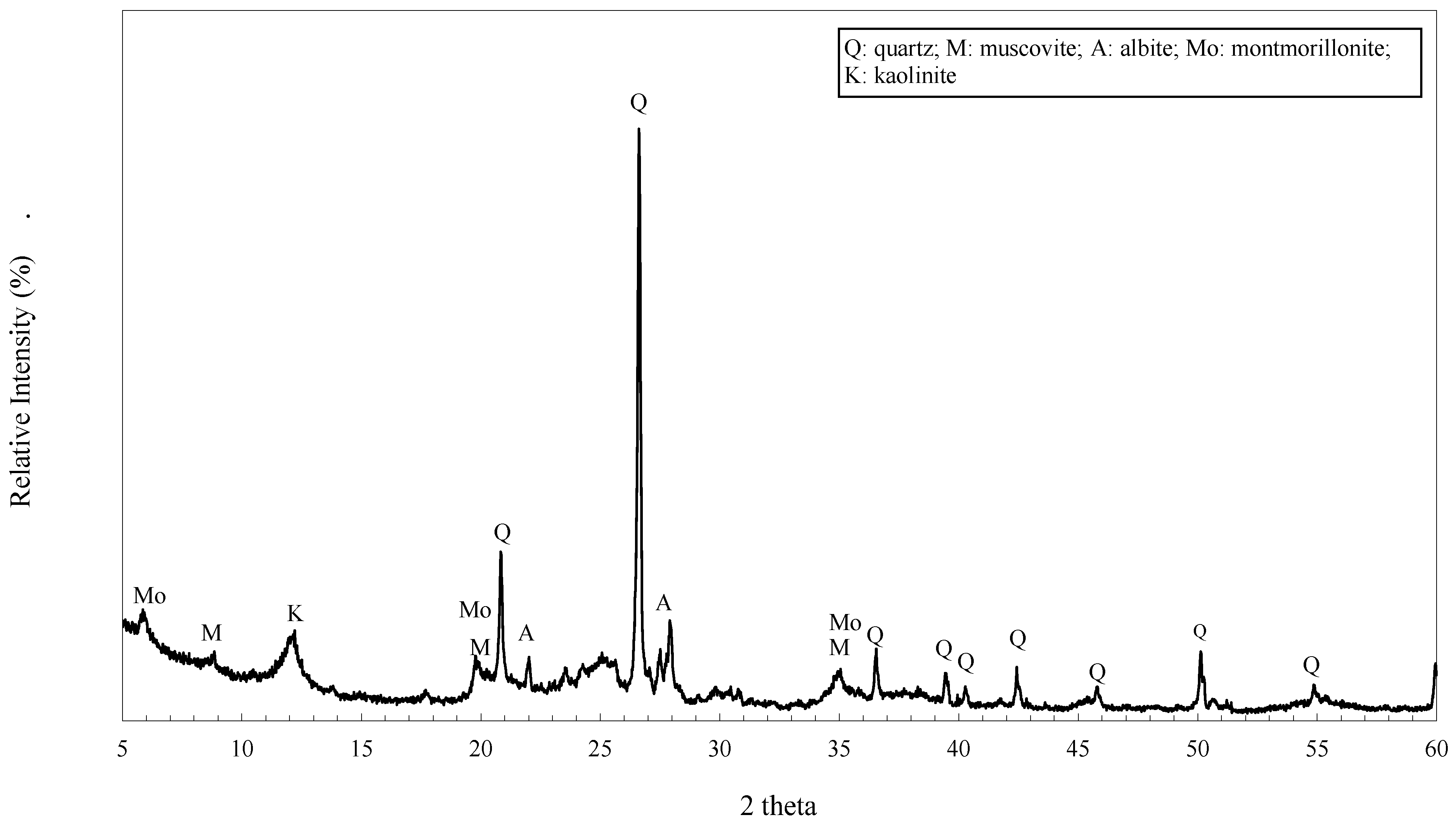
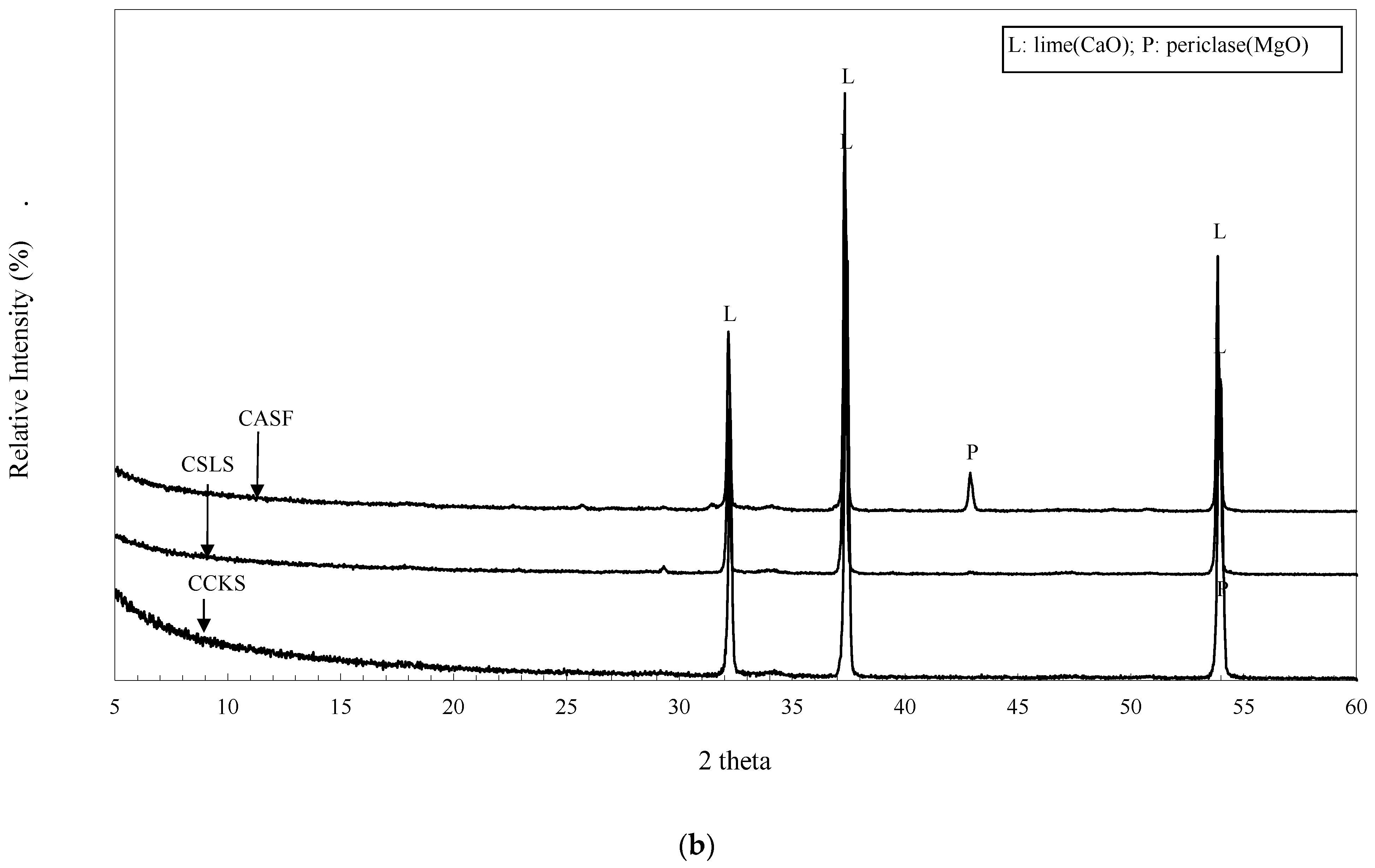
3.1. XRPD Analysis
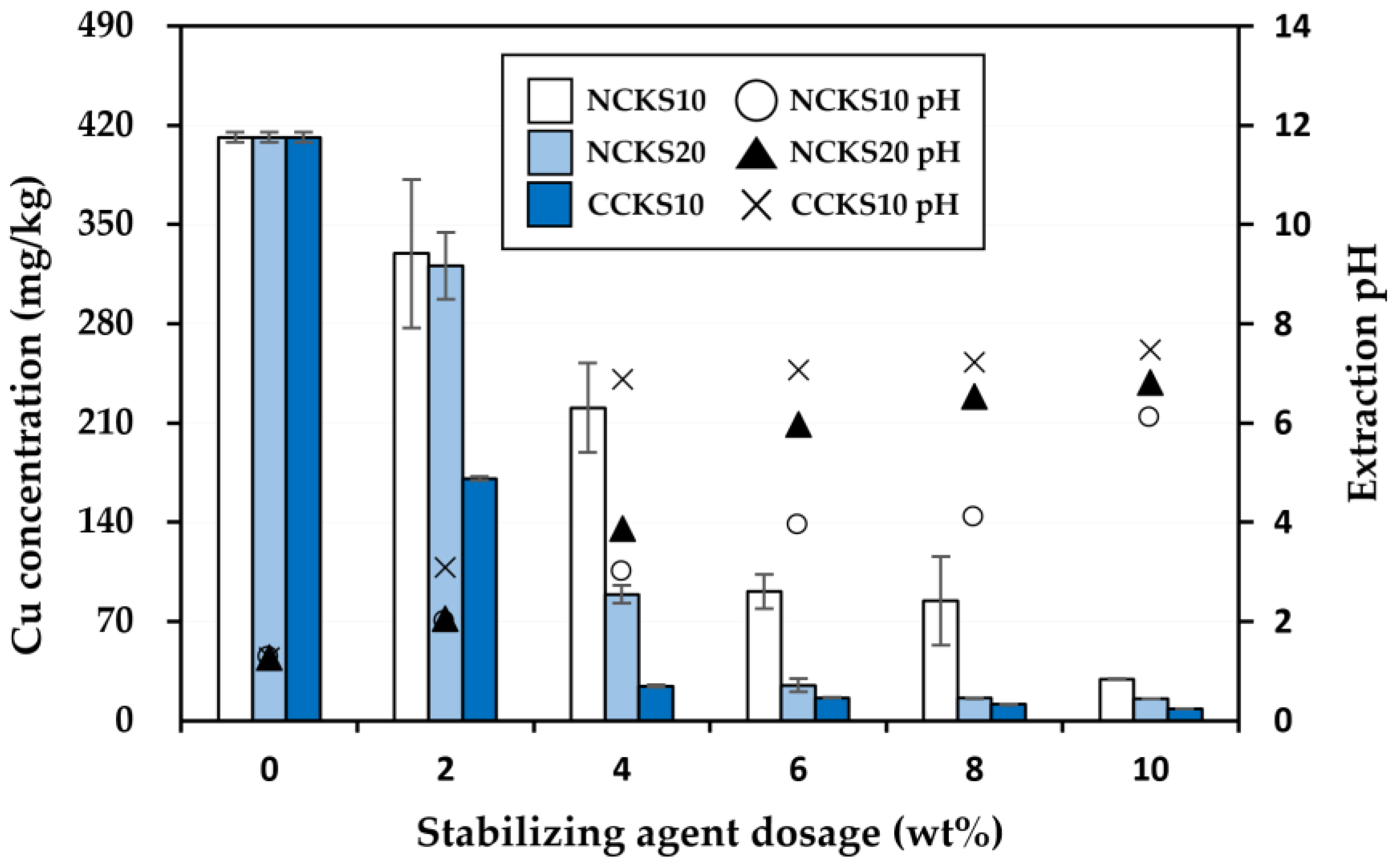
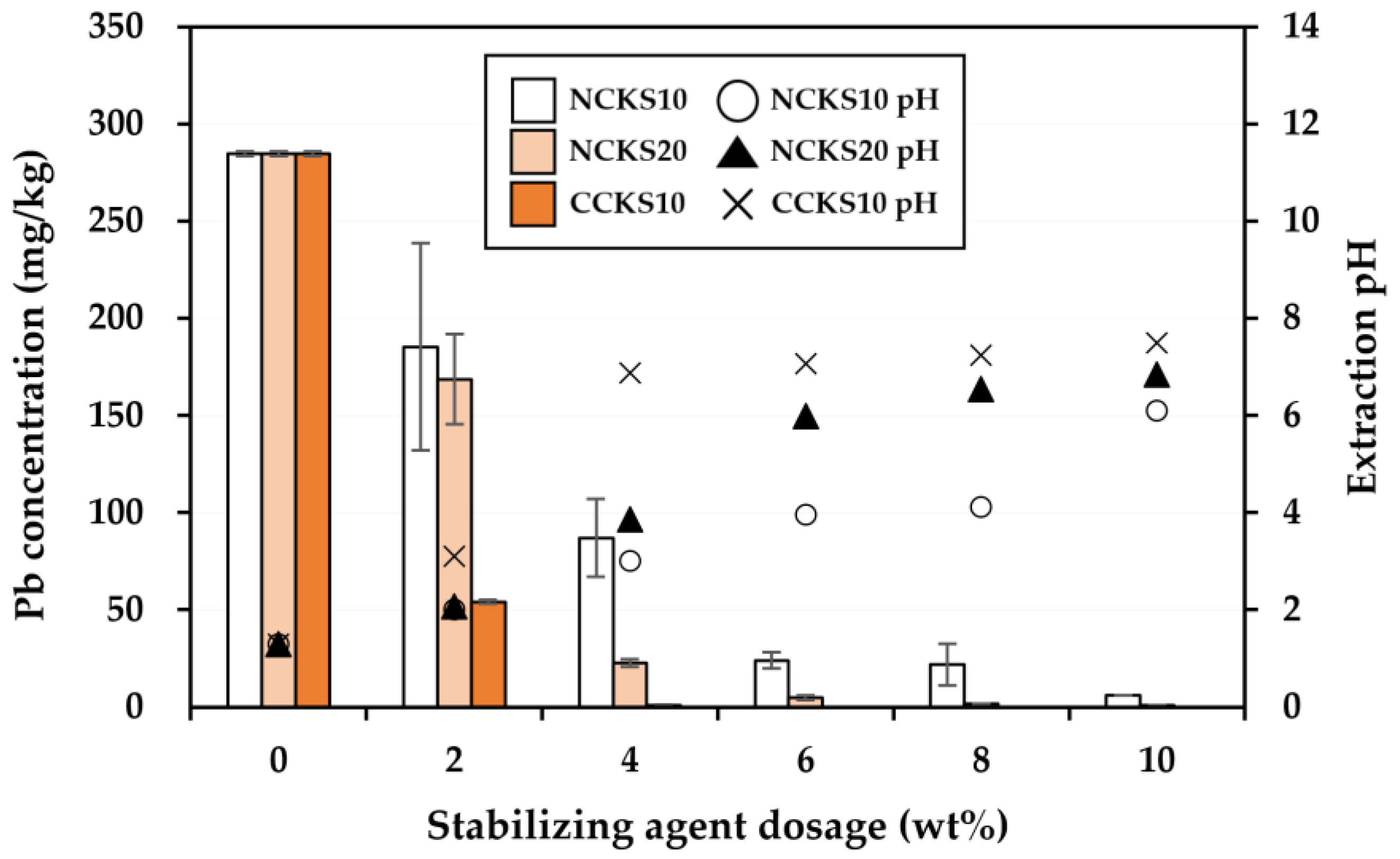
3.2. Effectiveness of the Stabilization Treatment
3.3. SEM–EDX Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pathak, S.; Agarwal, A.V.; Pandey, V.C. 1—Phytoremediation—A Holistic Approach for Remediation of Heavy Metals and Metalloids. In Bioremediation of Pollutants; Pandey, V.C., Singh, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–16. ISBN 978-0-12-819025-8. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil Washing for Metal Removal: A Review of Physical/Chemical Technologies and Field Applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Liu, Q.; Li, J.; Qiao, Z.; Sun, P.; Yang, Y. A Critical Review on Soil Washing during Soil Remediation for Heavy Metals and Organic Pollutants. Int. J. Environ. Sci. Technol. 2021, 19, 601–624. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, W.-C.; Lee, S.-H.; Kim, S.-O. Changes of Soil Properties through the Remediation Processes and Techniques for the Restoration of Remediated Soils. Econ. Environ. Geol. 2020, 53, 441–477. [Google Scholar]
- Moon, D.H.; Chang, Y.-Y.; Lee, M.; Koutsospyros, A.; Koh, I.-H.; Ji, W.H.; Park, J.-H. Assessment of Soil Washing for Heavy Metal Contaminated Paddy Soil Using FeCl 3 Washing Solutions. Environ. Geochem. Health 2021, 43, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.-H.; Kim, G.S.; Chang, Y.-Y.; Yang, J.-K.; Moon, D.H.; Choi, Y.; Ko, M.-S.; Ji, W.H. Characteristics of Agricultural Paddy Soil Contaminated by Lead after Bench-Scale In-Situ Washing with FeCl3. J. Soil Groundw. Environ. 2017, 22, 18–26. (In Korean) [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of Multiple Heavy Metal-Contaminated Soil through the Combination of Soil Washing and in Situ Immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Purev, O.; Choi, N.; Lee, J. Physical Separation of Contaminated Soil Using a Washing Ejector Based on Hydrodynamic Cavitation. Sustainability 2022, 14, 252. [Google Scholar] [CrossRef]
- Kim, H.; Cho, K.; Purev, O.; Choi, N.; Lee, J. Remediation of Toxic Heavy Metal Contaminated Soil by Combining a Washing Ejector Based on Hydrodynamic Cavitation and Soil Washing Process. Int. J. Environ. Res. Public Health 2022, 19, 786. [Google Scholar] [CrossRef]
- Park, S.H.; Koutsospyros, A.; Moon, D.H. Optimization of a High-Pressure Soil Washing System for Emergency Recovery of Heavy Metal-Contaminated Soil. Agriculture 2022, 12, 2054. [Google Scholar] [CrossRef]
- Lim, M.; Kim, M.-J. Stabilization of Residual Heavy Metals after Soil Washing of Mine Tailings Contaminated with Arsenic and Heavy Metals. J. Korean Soc. Environ. Eng. 2014, 36, 67–73. (In Korean) [Google Scholar] [CrossRef]
- USEPA. Engineering Bulletin: Technology Alternatives for the Remediation of Soils Contaminated with As, Cd, Cr, Hg, and Pb; USEPA: Washington, DC, USA, 1997.
- Moon, D.H.; Lee, J.-R.; Grubb, D.G.; Park, J.-H. An Assessment of Portland Cement, Cement Kiln Dust and Class C Fly Ash for the Immobilization of Zn in Contaminated Soils. Environ. Earth Sci. 2010, 61, 1745–1750. [Google Scholar] [CrossRef]
- Lim, J.-E.; Kim, K.-R.; Lee, S.-S.; Kwon, O.-K.; Yang, J.-E.; Ok, Y.-S. Stabilization of As (Arsenic (V) or Roxarsone) Contaminated Soils Using Zerovalent Iron and Basic Oxygen Furnace Slag. J. Korean Soc. Environ. Eng. 2010, 32, 631–638. (In Korean) [Google Scholar]
- Yoon, I.-H.; Moon, D.H.; Kim, K.-W.; Lee, K.-Y.; Lee, J.-H.; Kim, M.G. Mechanism for the Stabilization/Solidification of Arsenic-Contaminated Soils with Portland Cement and Cement Kiln Dust. J. Environ. Manag. 2010, 91, 2322–2328. [Google Scholar] [CrossRef]
- Park, Y.-J.; Shin, W.-S.; Choi, S.-J.; Lee, H.-H. Solidification/Stabilization of Heavy Metals in Sewage Sludge Prior to Use as a Landfill Cover Material. J. Korean Soc. Environ. Eng. 2010, 32, 665–675. (In Korean) [Google Scholar]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical Stabilization Remediation for Heavy Metals in Contaminated Soils on the Latest Decade: Available Stabilizing Materials and Associated Evaluation Methods—A Critical Review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Moon, D.-H.; Kim, K.-W.; Cheong, K.-H.; Kim, T.-S.; Khim, J.-H.; Moon, K.-R.; Choi, S.-B. Application of Waste Resources for the Stabilization of Heavy Metals (Pb, Cu) in Firing Range Soils. J. Korean Soc. Environ. Eng. 2011, 33, 71–76. (In Korean) [Google Scholar] [CrossRef]
- Lim, J.-E.; Moon, D.-H.; Kim, D.-J.; Kwon, O.-K.; Yang, J.-E.; Ok, Y.-S. Evaluation of the Feasibility of Oyster-Shell and Eggshell Wastes for Stabilization of Arsenic-Contaminated Soil. J. Korean Soc. Environ. Eng. 2009, 31, 1095–1104. (In Korean) [Google Scholar]
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and Opportunities of Bivalve Shells’ Waste Valorization in a Prospect of Circular Blue Bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.-H.; Kim, T.-S.; Khim, J.-H.; Choi, S.-B.; Moon, O.-R.; Ok, Y.-S. Stabilization of As in Soil Contaminated with Chromated Copper Arsenate (CCA) Using Calcinated Oyster Shells. Korean J. Environ. Agric. 2009, 28, 378–385. (In Korean) [Google Scholar] [CrossRef] [Green Version]
- Moon, D.H.; Cheong, K.-H.; Kim, T.-S.; Khim, J.-H.; Choi, S.-B.; Ok, Y.-S.; Moon, O.-R. Stabilization of Pb Contaminated Army Firing Range Soil Using Calcined Waste Oyster Shells. J. Korean Soc. Environ. Eng. 2010, 32, 185–192. (In Korean) [Google Scholar]
- Ahmad, M.; Hashimoto, Y.; Moon, D.H.; Lee, S.S.; Ok, Y.S. Immobilization of Lead in a Korean Military Shooting Range Soil Using Eggshell Waste: An Integrated Mechanistic Approach. J. Hazard. Mater. 2012, 209, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Dermatas, D.; Meng, X. Utilization of Fly Ash for Stabilization/Solidification of Heavy Metal Contaminated Soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Ministry of Environment (MOE). The Korean Standard Test (KST) Methods for Soils; Korean Ministry of Environment: Gwachun, Republic of Korea, 2002. (In Korean)
- Islam, M.N.; Taki, G.; Nguyen, X.P.; Jo, Y.-T.; Kim, J.; Park, J.-H. Heavy Metal Stabilization in Contaminated Soil by Treatment with Calcined Cockle Shell. Environ. Sci. Pollut. Res. 2017, 24, 7177–7183. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Sung, J.K.; Sarkar, B.; Wang, H.; Hashimoto, Y.; Tsang, D.C.; Ok, Y.S. Impact of Natural and Calcined Starfish (Asterina pectinifera) on the Stabilization of Pb, Zn and As in Contaminated Agricultural Soil. Environ. Geochem. Health 2017, 39, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; Cheong, K.H.; Khim, J.; Grubb, D.G.; Ko, I. Stabilization of Cu-Contaminated Army Firing Range Soils Using Waste Oyster Shells. Environ. Geochem. Health 2011, 33, 159–166. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Khim, J.; Wazne, M.; Hyun, S.; Park, J.-H.; Chang, Y.-Y.; Ok, Y.S. Stabilization of Pb2+ and Cu2+ Contaminated Firing Range Soil Using Calcined Oyster Shells and Waste Cow Bones. Chemosphere 2013, 91, 1349–1354. [Google Scholar] [CrossRef]
- Moon, D.H.; Hwang, I.; Koutsospyros, A.; Cheong, K.H.; Ok, Y.S.; Ji, W.H.; Park, J.-H. Stabilization of Lead (Pb) and Zinc (Zn) in Contaminated Rice Paddy Soil Using Starfish: A Preliminary Study. Chemosphere 2018, 199, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Ok, Y.S.; Oh, S.-E.; Ahmad, M.; Hyun, S.; Kim, K.-R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.-T.; Yang, J.E. Effects of Natural and Calcined Oyster Shells on Cd and Pb Immobilization in Contaminated Soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Moon, D.H.; Koutsospyros, A. Stabilization of Lead-Contaminated Mine Soil Using Natural Waste Materials. Agriculture 2022, 12, 367. [Google Scholar] [CrossRef]
- Moon, D.H.; Jung, S.P.; Koutsospyros, A. Assessment of the Stabilization of Mercury Contaminated Soil Using Starfish. Agriculture 2022, 12, 542. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Koutsospyros, A.; Chang, Y.-Y.; Hyun, S.; Ok, Y.S.; Park, J.-H. Assessment of Waste Oyster Shells and Coal Mine Drainage Sludge for the Stabilization of As-, Pb-, and Cu-Contaminated Soil. Environ. Sci. Pollut. Res. 2016, 23, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; Kim, K.-W.; Yoon, I.-H.; Grubb, D.G.; Shin, D.-Y.; Cheong, K.H.; Choi, H.-I.; Ok, Y.S.; Park, J.-H. Stabilization of Arsenic-Contaminated Mine Tailings Using Natural and Calcined Oyster Shells. Environ. Earth Sci. 2011, 64, 597–605. [Google Scholar] [CrossRef]
- Song, H.W.; Kim, J.M.; Kim, Y.H.; Kim, J.J. Mineralogical Properties and Heavy Metal Removal Efficiency of Shells. Mineral. Soc. Korea 2022, 35, 387–396. (In Korean) [Google Scholar]
- Hale, B.; Evans, L.; Lambert, R. Effects of Cement or Lime on Cd, Co, Cu, Ni, Pb, Sb and Zn Mobility in Field-Contaminated and Aged Soils. J. Hazard. Mater. 2012, 199–200, 119–127. [Google Scholar] [CrossRef]
- Paulose, B.; Datta, S.P.; Rattan, R.K.; Chhonkar, P.K. Effect of Amendments on the Extractability, Retention and Plant Uptake of Metals on a Sewage-Irrigated Soil. Environ. Pollut. 2007, 146, 19–24. [Google Scholar] [CrossRef]
- Moon, D.H.; Wazne, M.; Cheong, K.H.; Chang, Y.-Y.; Baek, K.; Ok, Y.S.; Park, J.-H. Stabilization of As-, Pb-, and Cu-Contaminated Soil Using Calcined Oyster Shells and Steel Slag. Environ. Sci. Pollut. Res. 2015, 22, 11162–11169. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D. An Evaluation of Lead Leachability from Stabilized/Solidified Soils under Modified Semi-Dynamic Leaching Conditions. Eng. Geol. 2006, 85, 67–74. [Google Scholar] [CrossRef]

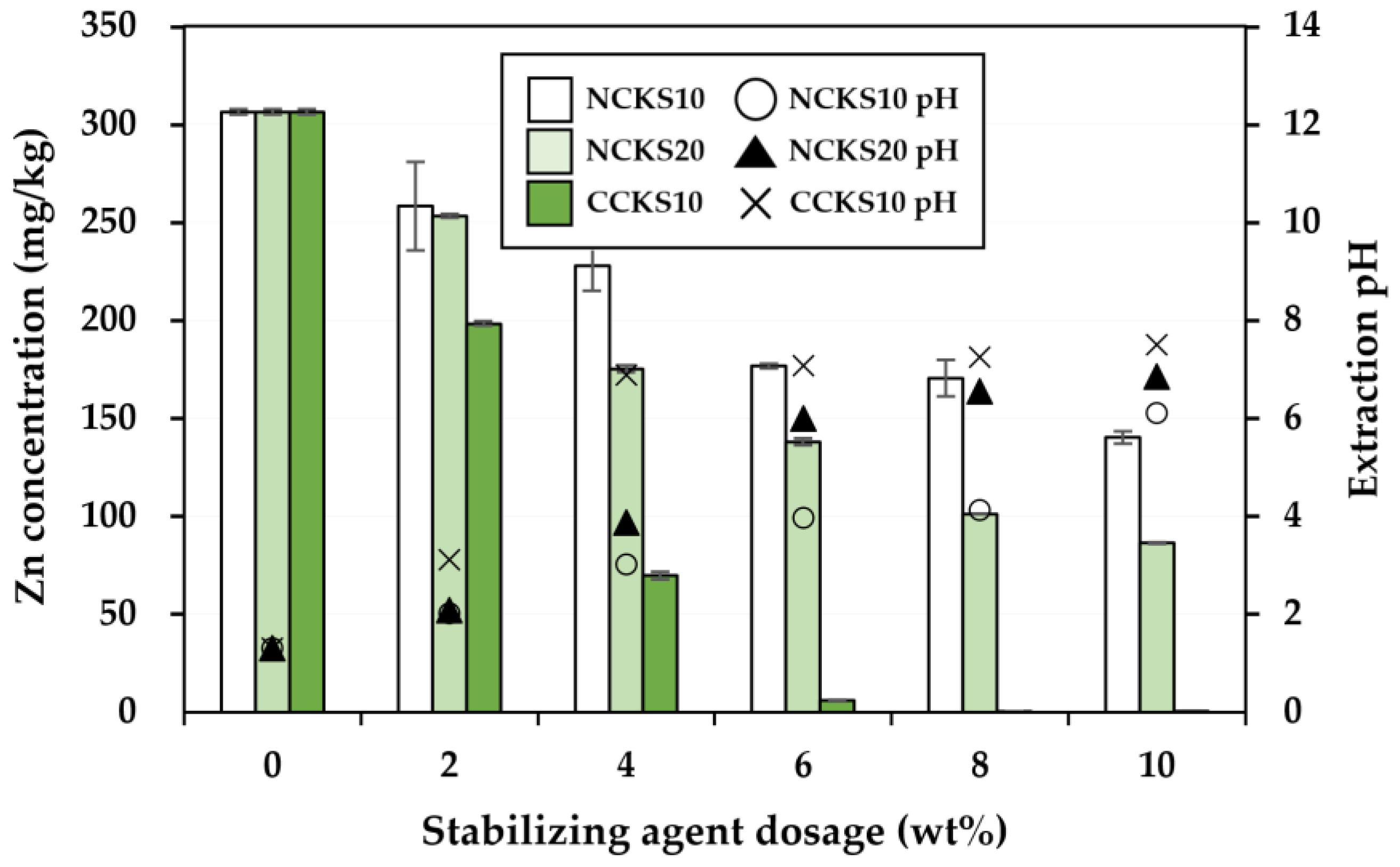
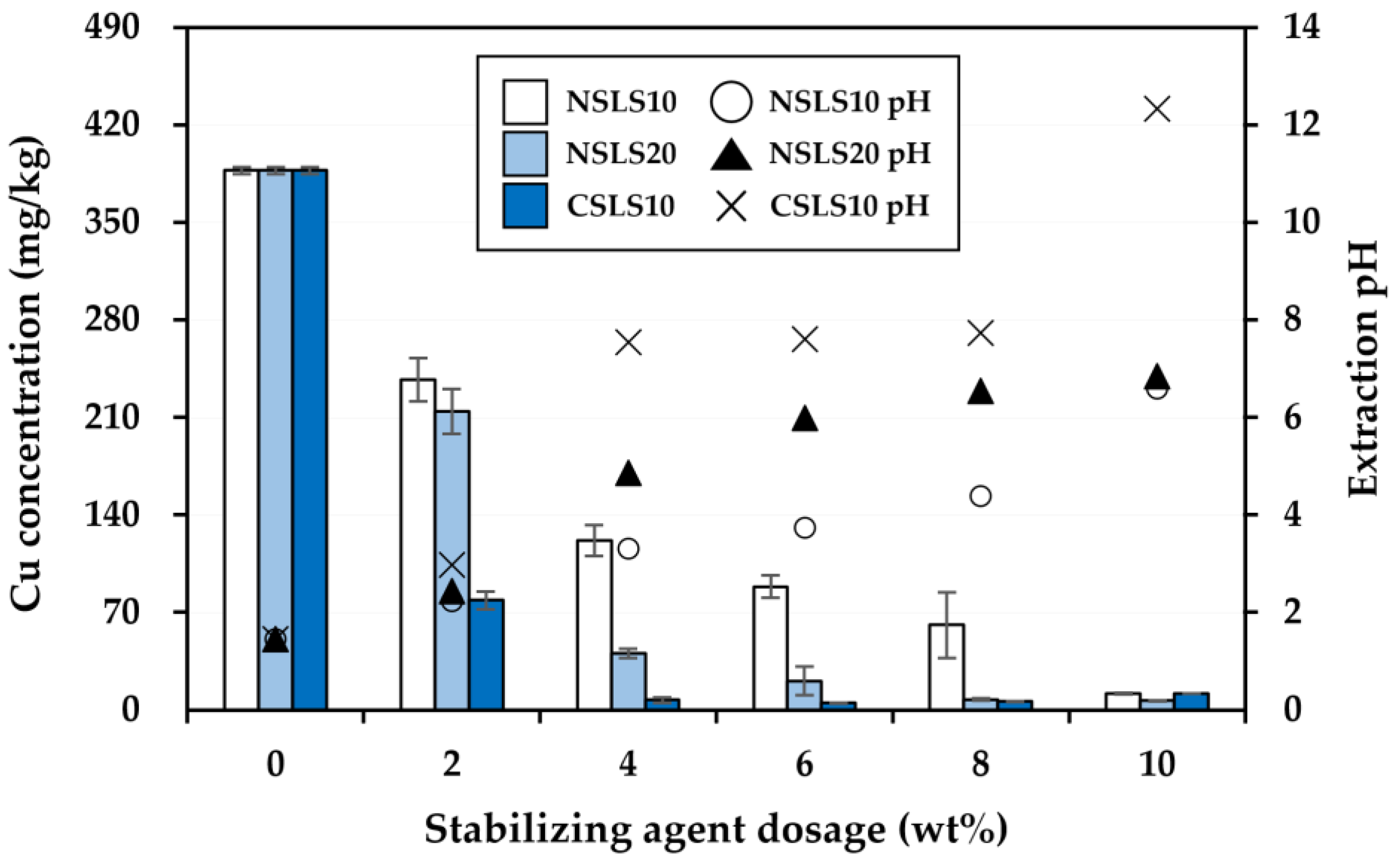
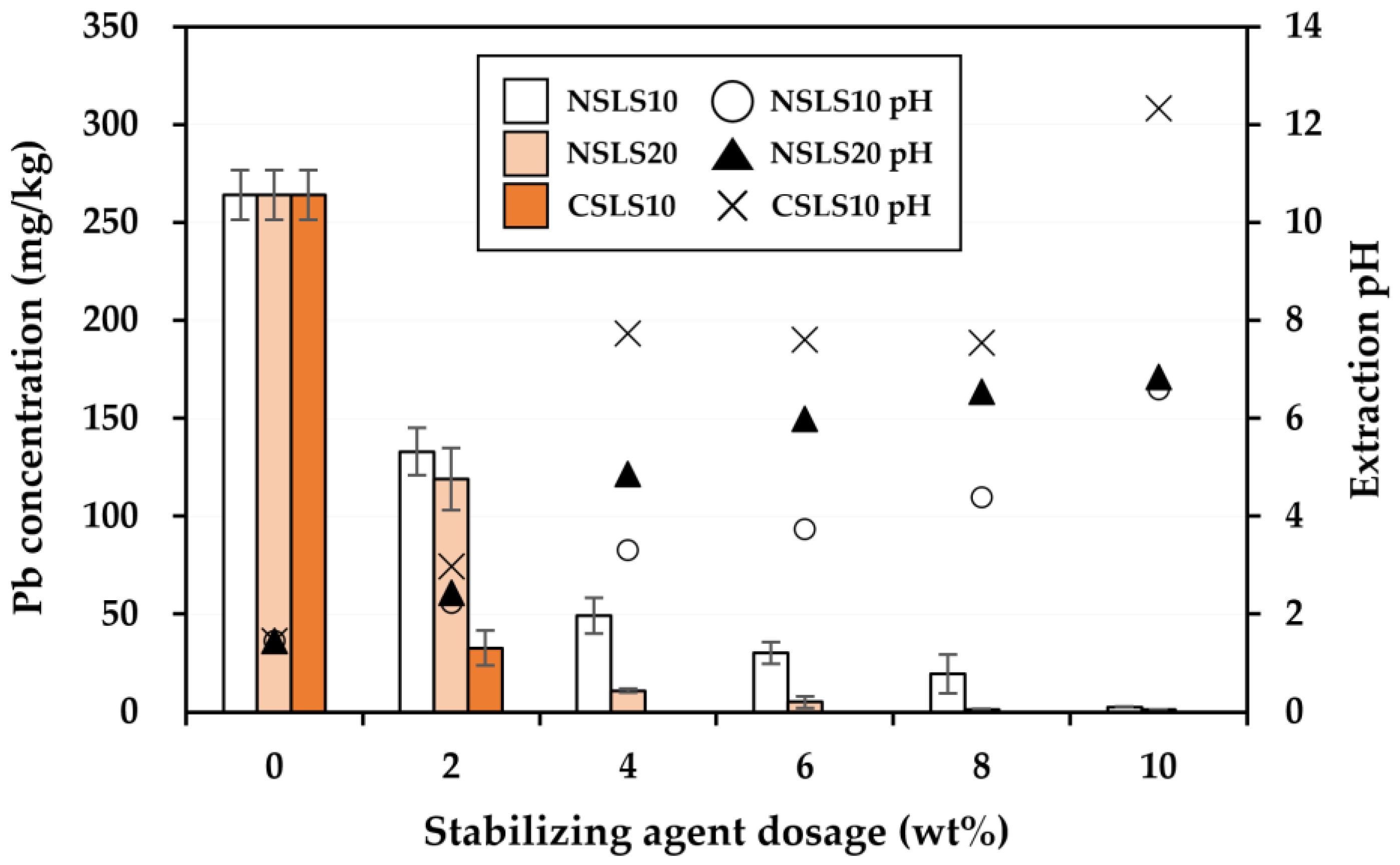
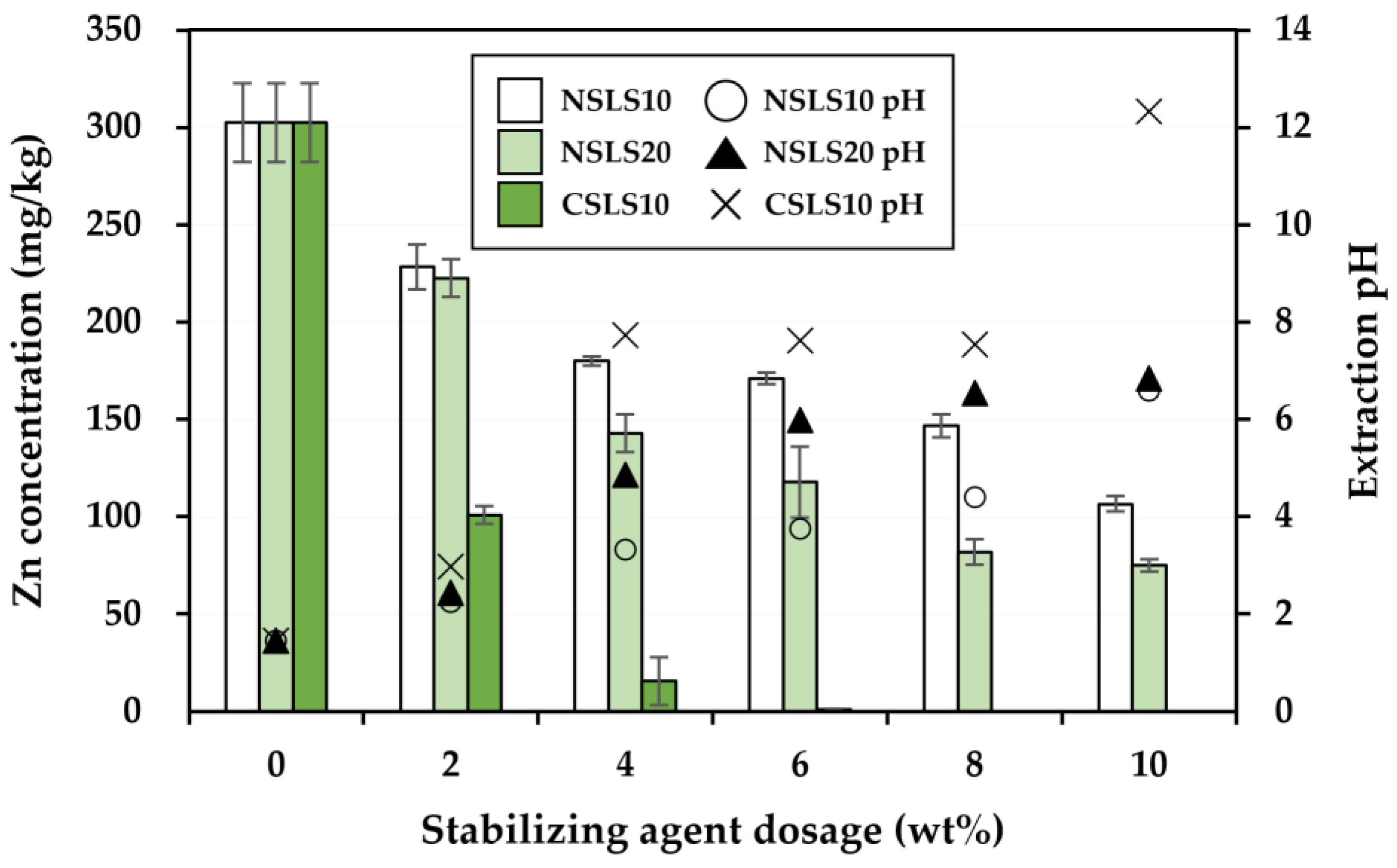
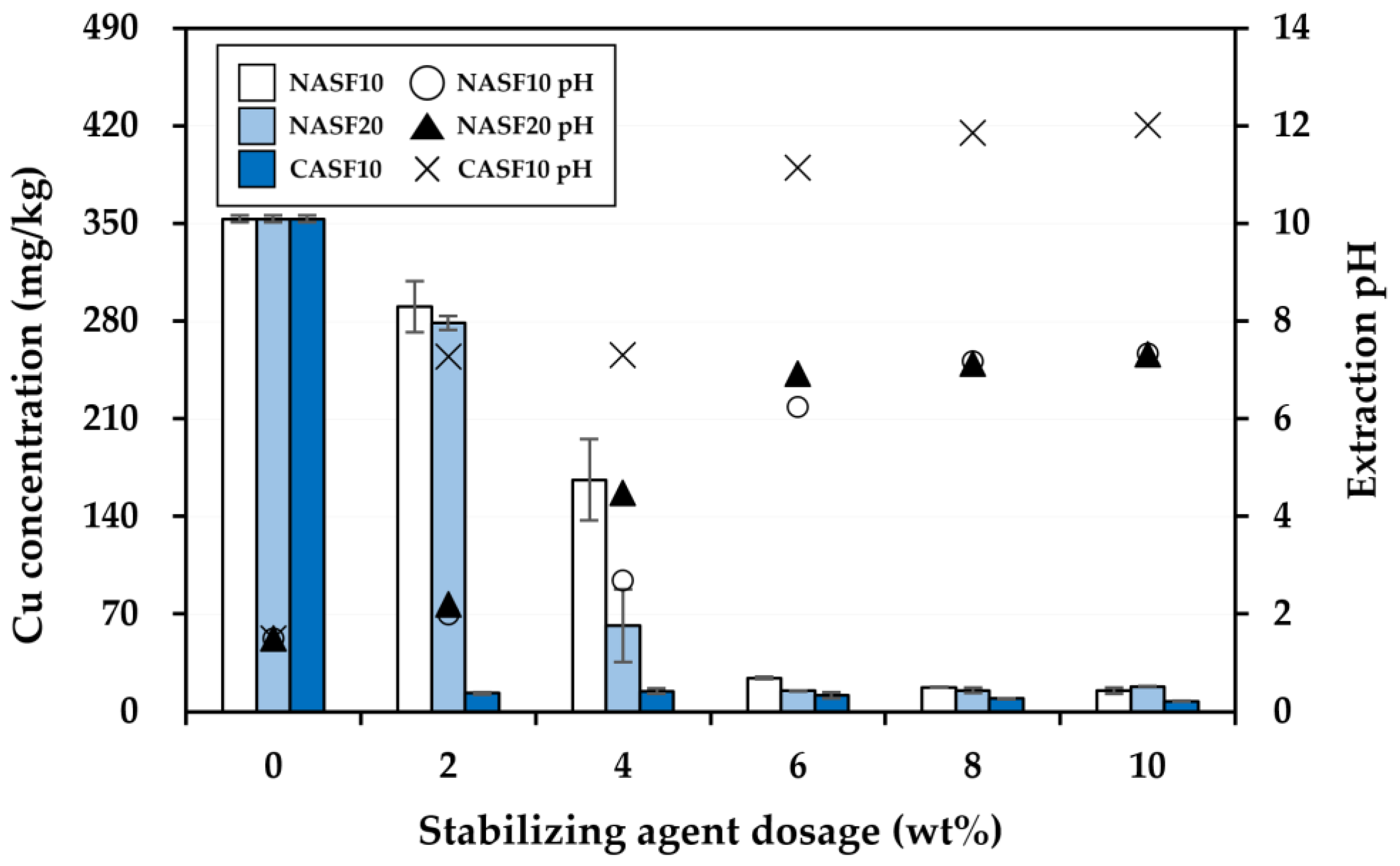
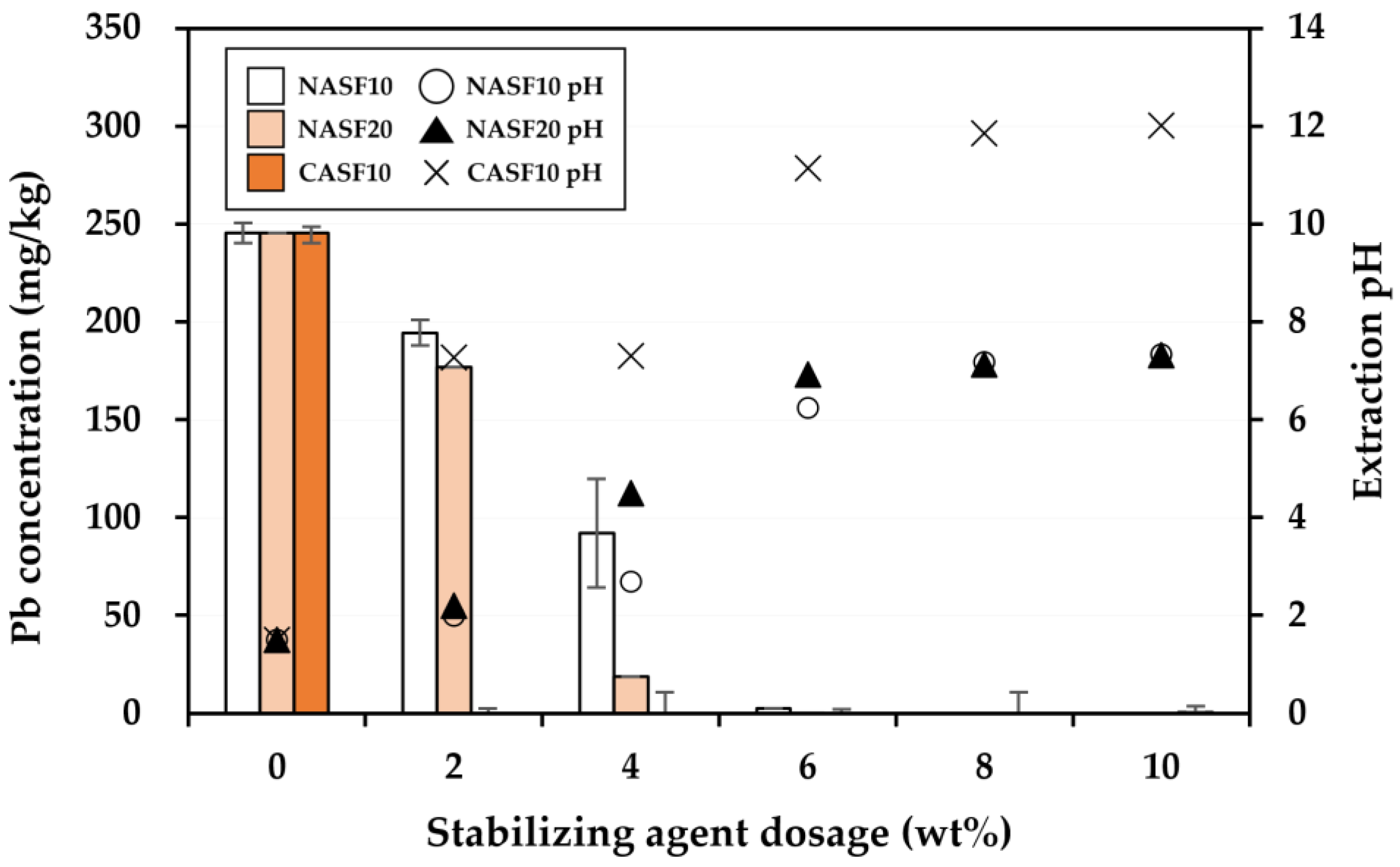
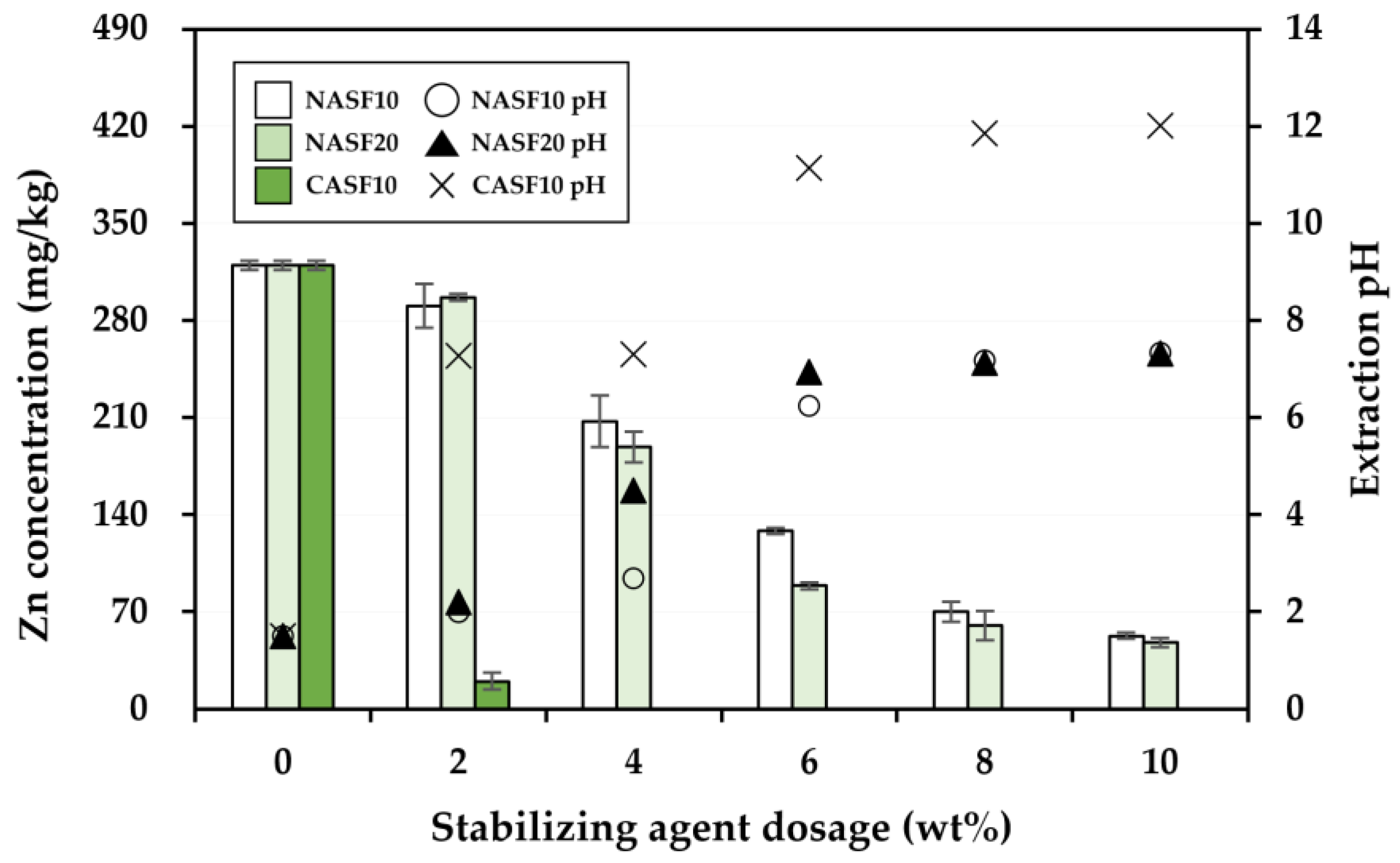
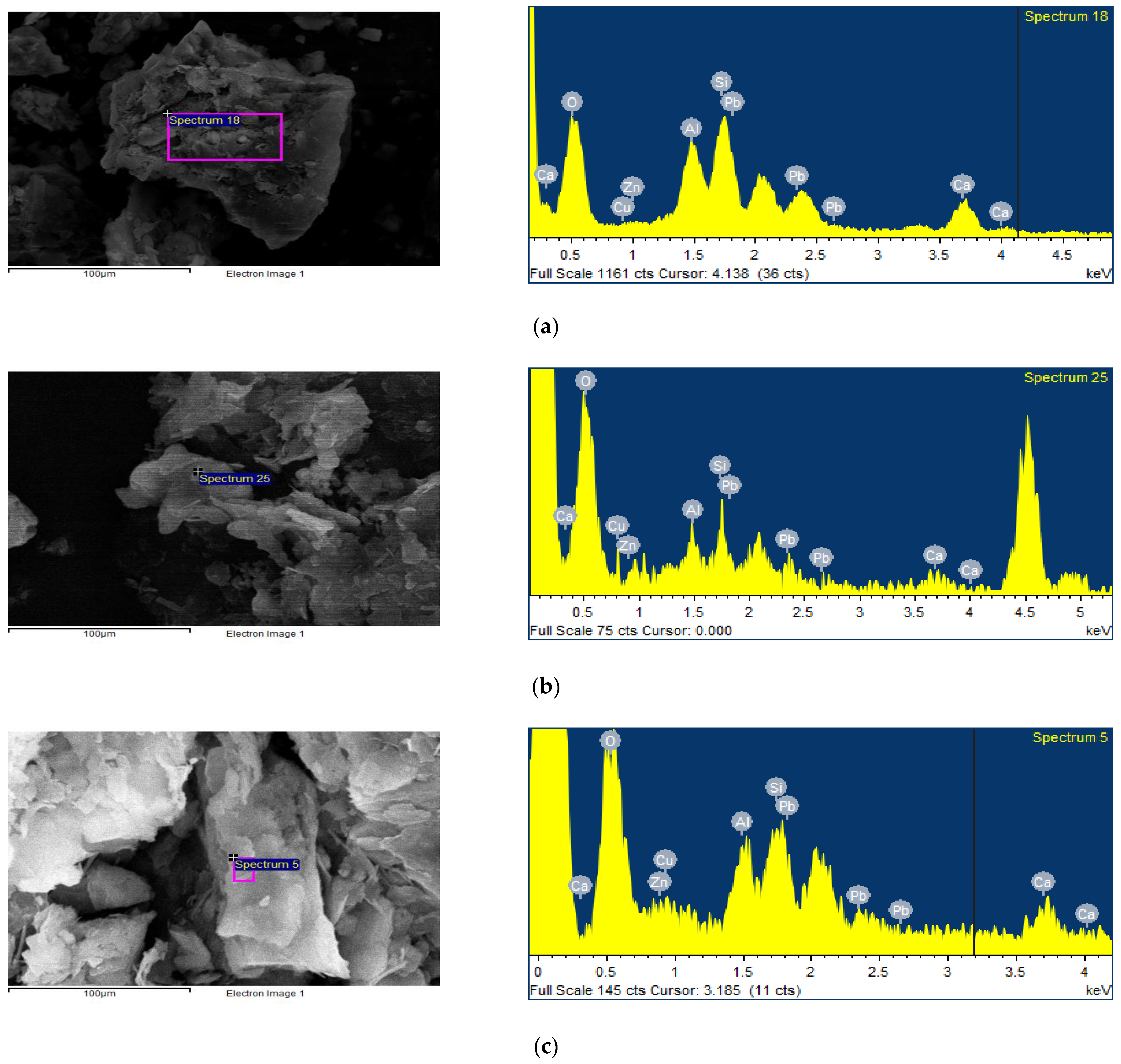
| Soil Properties | Contaminated Soil | Regulatory Limit a |
|---|---|---|
| Soil pH | 5.91 | |
| EC (µS/m) | 0.58 | |
| Organic matter content (%) b | 3.84 | |
| Composition (%) c | ||
| Sand | 54.1 | |
| Silt | 0.5 | |
| Clay | 45.4 | |
| Texture d | Sandy clay | |
| Heavy metals (mg∙kg−1) | ||
| Cu | 667.7 | 150 (Korean warning standard) |
| Pb | 514.6 | 200 (Korean warning standard) |
| Zn | 852.7 | 300 (Korean warning standard) |
| Major mineral compositions | Quartz, Muscovite, Albite, Montmorillonite, Kaolinite |
| Major Chemical Composition (%) | Contaminated Soil | NCKS | NSLS | NASF | CCKS | CSLS | CASF |
|---|---|---|---|---|---|---|---|
| SiO2 | 57.43 | 1.23 | 0.073 | 0.159 | 0.129 | 0.113 | 0.126 |
| Al2O3 | 26.74 | 0.475 | 0.209 | 0.061 | 0.041 | 0.154 | 0.130 |
| Na2O | 0.824 | 1.10 | 0.841 | 2.55 | 1.07 | 0.974 | 2.51 |
| K2O | 3.02 | 0.098 | 0.008 | 0.419 | 0.020 | 0.012 | 0.158 |
| CaO | 1.55 | 94.93 | 96.53 | 79.77 | 96.92 | 96.18 | 78.78 |
| Fe2O3 | 6.14 | 0.389 | 0.066 | 0.081 | 0.067 | 0.051 | 0.052 |
| SO3 | 0.187 | 0.704 | 0.609 | 6.47 | 0.261 | 0.580 | 2.45 |
| MnO | 0.109 | 0.157 | 0.007 | 0.009 | 0.024 | 0.007 | 0.004 |
| P2O5 | 0.484 | 0.172 | 0.124 | 1.42 | 0.045 | 0.129 | 0.633 |
| pH (1:5) | 5.91 | 9.40 | 9.42 | 7.37 | 12.45 | 12.51 | 12.29 |
| Sample ID | Contaminated Soil (wt%) | Stabilizing Agent Dosage (wt%) | L:S Ratio |
|---|---|---|---|
| CKS/SLS/ASF 0 wt% (Control) CCKS/CSLS/CASF 0 wt% | 100 | 0 | 30:1 |
| CKS/SLS/ASF 2 wt% CCKS/CSLS/CASF 2 wt% | 100 | 2 | 30:1 |
| CKS/SLS/ASF 4 wt% CCKS/CSLS/CASF 4 wt% | 100 | 4 | 30:1 |
| CKS/SLS/ASF 6 wt% CCKS/CSLS/CASF 6 wt% | 100 | 6 | 30:1 |
| CKS/SLS/ASF 8 wt% CCKS/CSLS/CASF 8 wt% | 100 | 8 | 30:1 |
| CKS/SLS/ASF 10 wt% CCKS/CSLS/CASF 10 wt% | 100 | 10 | 30:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; An, J.; Koutsospyros, A.; Moon, D.H. Assessment of the Stabilization of Cu-, Pb-, and Zn-Contaminated Fine Soil Using Cockle Shells, Scallop Shells, and Starfish. Agriculture 2023, 13, 1414. https://doi.org/10.3390/agriculture13071414
Park SH, An J, Koutsospyros A, Moon DH. Assessment of the Stabilization of Cu-, Pb-, and Zn-Contaminated Fine Soil Using Cockle Shells, Scallop Shells, and Starfish. Agriculture. 2023; 13(7):1414. https://doi.org/10.3390/agriculture13071414
Chicago/Turabian StylePark, Sang Hyeop, Jinsung An, Agamemnon Koutsospyros, and Deok Hyun Moon. 2023. "Assessment of the Stabilization of Cu-, Pb-, and Zn-Contaminated Fine Soil Using Cockle Shells, Scallop Shells, and Starfish" Agriculture 13, no. 7: 1414. https://doi.org/10.3390/agriculture13071414





